Abstract
Objective:
To determine the free amino acid contents of chamomile flowers using reverse-phase high-performance column chromatography preceded by pre-column derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC), and to determine the reliability of this method.
Materials and Methods:
Derivatization with reconstituted AQC was used to prepare the samples and standards for injection into the chromatography column. The peaks were analyzed by fluorescence detection (λ excitation, 250 nm; λ emission, 395 nm.
Results:
Alanine, proline, and leucine were the most abundant amino acids, whereas tyrosine and methionine were the least abundant. The linearity of the method was found to be good with amino acid concentrations of 0.012-0.36 μM. The precision was 0.05-1.36%; average recovery, 91.12-129.41%; and limit of detection, 0.006-0.058 μM.
Conclusion:
The method is reliable for determining the free amino acid content of different types of chamomile flowers.
Keywords: Amino acids, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, Matricaria chamomilla, Matricaria recutita, reversed-phase high performance liquid chromatography
INTRODUCTION
Matricaria chamomilla (Matricaria recutita) and Chamaemelum nobile (Anthemis nobilis), particularly the dried flower heads of these plants, are widely used in traditional and herbal medicine in European countries.[1,2,3,4] When used as an infusion, their extracts have remarkable anti-inflammatory, antipeptic, antibacterial, and antifungal effects.[1,5,6] So far, the main components of the extracts have been identified as volatile derivatives and flavonoid compound.[2,3,5,7,8] Nonetheless, the mechanisms responsible for the therapeutic properties and other important components of these plants have still not been elucidated clearly.
Amino acids are an important component of herbal plants and are involved in many activities,[9,10,11,12] but analytical methods for qualitative and quantitative assessment of M. chamomilla extracts have seldom been published before. Considering the medicinal value of these plant extracts, it is important to investigate the other nutrient constituents and, therefore, better understand the value of the chamomile flower.
We have presented a method for determining the free amino acid content of different types of chamomile flowers. Water extracts were derivatized using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC), which reacts with primary and secondary amino acids to yield fluorescent derivates. The fluorescent derivatives were analyzed by reverse-phase high performance chromatography (RP-HPLC) to determine the amino acid content.
EXPERIMENTAL
Sample preparation
The dried head flowers of M. chamomilla and C. nobile were purchased from the local market (Wurumqi, Xinjiang, China). After being air-dried and crushed into a powder with a mixer/grinder, 2.0 g of the sample were accurately weighed and quantitatively transferred into a beaker. The beaker was placed in a water bath at 40°C to melt the mass. Then, the digested sample was transferred into a 50 mL calibrated flask; the volume was made up by adding enough water to the mark and the beaker was kept in an ultrasonic bath for 30 min at 40°C. The extract was centrifuged and 30 ml of water was added to the residue, which was then sonicated for 30 min. The solution was then passed through a 0.22 μm Millipore membrane (SHIMADZU, Japan), and the filtrate was transferred to a 100 mL volumetric flask and diluted with water to make up the volume. This solution was passed through a 0.22 μm millipore membrane, and the filtrate was used for the following experiments.
Derivatization procedure for amino acid analysis
Preparation of AccQ-Fluor derivatizing agent
One milliliter of AccQ Fluor Reagent Diluent (AccQ-Tag Reagent kit; Waters, Milford, Massachusetts, USA) was transferred to a vial containing AccQ-Fluor reagent powder (Waters). This closed vial was mixed by vortexing (SK-1, Jintan, China) for 10 s and heated on a heating block at 55°C until it dissolved, but for no longer than 10 min. The reconstituted reagent can be stored in a refrigerator at 4°C for up to 2 weeks.
Preparation of standard amino acid solutions
Standard solutions of the following amino acids (Sigma) were prepared: Aspartic acid (Asp), glutamic acid (Glu), serine (Ser), glycine (Gly), histidine (His), arginine (Arg), threonine (Thr), alanine (Ala), proline (Pro), tyrosine (Tyr), valine (Val), methionine (Met), isoleucine (Ile), leucine (Leu), phenylalanine (Phe), and lysine (Lys). The solutions were prepared by adding 0.1 M HCl. The concentration of the standard solutions was 25 nM. They were stored in a freezer at 4°C till use. The mixed standard solution contained 25 pmol of each amino acid derivative.
Derivatization of the samples
The mixed amino acid solution or filtered sample (10 μL) was transferred to a full recovery autosampler, to which 70 μL of AccQ-Fluor borate buffer (Waters) was added; the solution was vortexed briefly and then 20 μL of reconstituted AccQ Fluor reagent (Waters), and the solution was mixed by vortexing for several seconds. It was then heated on a heating block at 55°C for 10 min. Derivatives were stable at room temperature for up to 1 week.
Chromatographic conditions and procedure
The HPLC system used was a Waters Alliance consisting of a 2695 separation model and a 2475 scanning fluorescence detector (Waters; Millipore, Milford, MA, USA). Data were collected and analyzed with the Empower 3 system (Waters Corporation, Milford).
Chromatographic separation was carried out on a Shimpack column (250 mm × 4.6 mm; I.D., 5 μm) fitted with a pre-column safeguard containing the same packing material (12.5 mm × 4.6 mm, 4 μm). The column was thermostatted at 37°C; the flow rate was 1.0 mL/min; and the injection volume was 5 μL. Mobile phase A consisted of 0.3 M sodium acetate containing 5% acetonitrile (pH 6.5); mobile phase B comprised acetonitrile and methanol (both were purchased from Sigma) and Milli-Q water (20:60:20, v/v/v; Milli-Q plus system, Millipore Corporation, USA). The gradient profile is shown in Table 1. In terms of retention time, the composition of each peak was confirmed and determined in accordance with the external standard method. Before the gradient was started, the column was equilibrated in 100% A for 10 min. Fluorescence detection was carried out by λ irradiation at 250 nm excitation and 395 nm emission wavelength.
Table 1.
Gradient profile
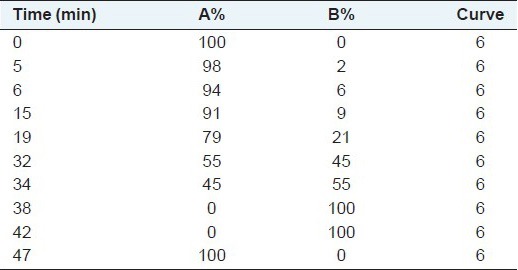
Chromatographic standards were prepared by mixing the standard amino acid solutions with the appropriate amount of water to obtain the required concentration. (The mixed standard solution contained 25 pmol of each amino acid derivative).
RESULTS AND DISCUSSION
Qualitative and quantitative analysis
The tested amino acids were identified by comparing their retention times with those of available standards. Identified peaks were confirmed by spiking samples with standard mixtures and subjecting them to chromatographic evaluation. Quantitative data were obtained by plotting the peak concentrations of the standard solutions against the peak concentrations of the sample solution (data not shown). Chromatograms showing the amino acid peaks of the standard and sample solutions are shown in Figure 1. As can be seen, alanine, proline, and leucine were the most abundant amino acids, whereas the amount of tyrosine and methionine was low.
Figure 1.
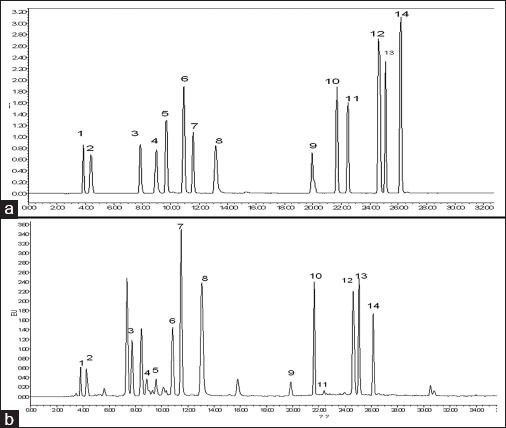
HPLC chromatogram of the standard solution of mixed amino acids (a) and the sample (b)1. Asp 2. Glu 3. Ser 4. Gly 5. His 6. Arg+Thr 7. Ala 8. Pro 9. Tyr 10. Val 11. Met 12. Ileu+leu 13. Lys 14. Phe
Validation of the analysis
Linearity and limit of detection
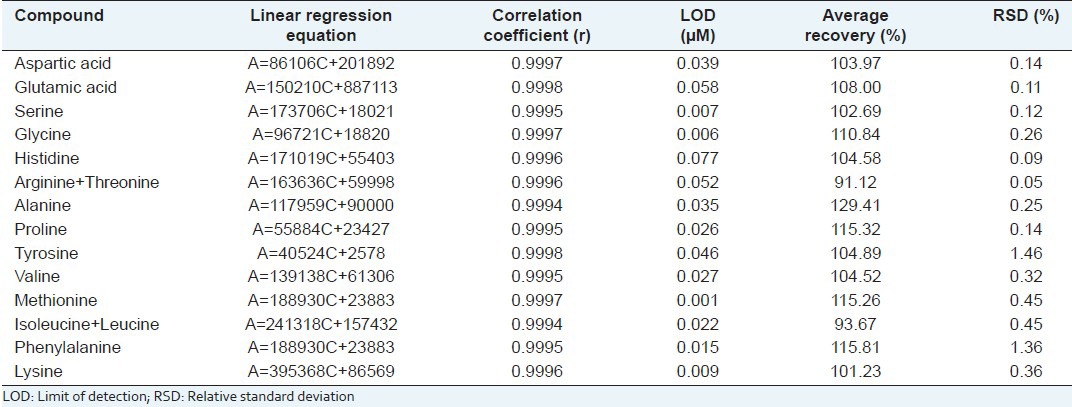
Linearity was tested by chromatographic analysis of standards containing 0.012, 0.024, 0.06, 0.12, 0.20, and 0.36 μM of each amino acid according to the procedure described before. The detection limits for each amino acid at a signal-to-noise ratio of 3 were calculated. Linearity data were calculated by examining the area vs. concentration of amino acid plots. The results (regression equations and correlation coefficients) obtained are reported in Table 2. The linearity between peak areas and concentrations was good, and the regression coefficients (r) were greater than 0.9994 for all the curves, which demonstrates an excellent linearity of the calibrations.
Table 2.
Linear regression equation, correlation coefficient, and recovery rate
Precision and accuracy
Precision was expressed in terms of coefficients of variation [Table 2]: The data presented indicate good agreement among the individual test results. The replicability was determined by chromatographic analysis of six replicate samples of the extracts on the same day. Repeatability was estimated by triplicate injection of the water extract on three consecutive days. Triplicate analysis of amino acids in the sample provided a good coefficient of variation. In all cases, relative standard deviation (RSD) was lower than 2.0%, which indicates that the derivatization procedure is reliable.
In order to examine the accuracy of the quantitative determination, recovery tests were performed by adding known amounts of the mixed standard into the samples and analyzing them. Amino acids were measured in the spiked and unspiked samples and the recovery rates were calculated. The assay was carried out in triplicate. The average recovery was between 91.12% and 129.41%.
Application of the method for the analysis of amino acids
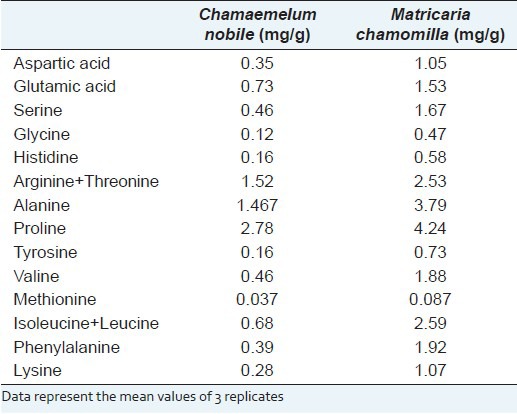
The amino acid contents (mg/g) of the analyzed samples are reported in Table 3. Values shown represent the mean of three determinations.
Table 3.
Free amino acid content in the analyzed sample
CONCLUSIONS
We have presented an optimized analytical method for the determination of free amino acids in M. chamomilla. The method has sufficient reproducibility and accuracy to allow the determination of amino acid content in M. chamomilla. M. chamomilla was characterized by higher amino acid content in comparison to C. nobile. These differences probably contribute to the specific taste properties of chamomile and are probably influenced by the raw materials, yeast strain, and technological procedures used for preparing the extracts.
ACKNOWLEDGMENTS
This research was supported by a grant from the National Natural Science Foundation of China (No. 81260144). We also thank Professor Xinxia Li for her technical assistance and support during the course of this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mulinacci N, Romania A, Pinelli E. Characterization of Matricaria recutita L. flower extracts by HPLC-MS and HPLC-DAD analysis. J Chromatogr. 2000:301–7. [Google Scholar]
- 2.Guimaraes R, Barros L, Duenas M, Calhelha RC, Carvalho AM, Santos-Buelga C, et al. Infusion and decoction of wild German chamomile: Bioactivity and characterization of organic acids and phenolic compounds. Food Chem. 2013;136:947–54. doi: 10.1016/j.foodchem.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Singh O, Khanam Z, Misra N, Srivastava MK. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev. 2011;5:82–95. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matic IZ, Juranic Z, Savikin K, Zdunic G, Nadvinski N, Godevac D. Chamomile and marigold tea: Chemical characterization and evaluation of anticancer activity. Phytother Res. 2012;10:4807–12. doi: 10.1002/ptr.4807. [DOI] [PubMed] [Google Scholar]
- 5.Cemek M, Kaga S, Simsek N, Büyükokuroğlu ME, Konuk M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J Nat Med. 2008;62:284–93. doi: 10.1007/s11418-008-0228-1. [DOI] [PubMed] [Google Scholar]
- 6.Curra M, Martins MA, Lauxen IS, Pellicioli AC, Sant’ana Filho M, Pavesi VC, et al. Effect of topical chamomile on immunohistochemical levels of IL-1beta and TNF-alpha in 5-fluorouracil-induced oral mucositis in hamsters. Cancer Chemother Pharmacol. 2012;71:293–9. doi: 10.1007/s00280-012-2013-9. [DOI] [PubMed] [Google Scholar]
- 7.Pereira RP, Fachinetto R, de Souza Prestes A, Puntel RL, Santos da Silva GN, Heinzmann BM, et al. Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citrates. Neurochem Res. 2009;34:973–83. doi: 10.1007/s11064-008-9861-z. [DOI] [PubMed] [Google Scholar]
- 8.Bernal JL, Nozal MJ, Toribio L, Diego JC, Ruiz A. A comparative study of several HPLC methods for determining free amino acid profiles in honey. J Sep Sci. 2005;28:1039–47. doi: 10.1002/jssc.200500008. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Yang Z, Xie B, Shan L, Dong J. Determination of 21 free amino acids in beer by HPLC with AQC pre-column derivatization. Se Pu. 2007;25:939–41. [PubMed] [Google Scholar]
- 10.Amino acid determination in grape juices and wines by hplc using a modification of the 6-Aminoquinolyl-N-ydroxysuccinimidyl Carbamate (AQC) method. Chromatographia. 2003:29–35. [Google Scholar]
- 11.Determination of amino acid in flower of Lilium brownii var. Viridulumby Pre-column derivation OPA-HPLC. J Med Plant. 2011;4:25–7. [Google Scholar]
- 12.Yu H, Ding YS, Mou SF. Direct and simultaneous determination of amino acids and sugars in rice wine by high-performance anion-exchange chromatography with integrated pulsed amperometric detection. Chromatographia. 2003:11–12. [Google Scholar]


