Abstract
Background:
Agrimonia Pilosa Ledeb (APL), a traditional Chinese medicine, has been reported a variety of biological activities, including treating T2DM.
Objective:
Triterpenoid compound (TC) was collected from APL. The aim of this study was to investigate the effects of TC on 3T3-L1 preadipocytes differentiation and genes related to differentiation and IR.
Materials and Methods:
Column chromatography was used to collect TC from ALP. 3T3-L1 cell differentiation was induced typically in the presence of various concentrations of TC or pioglitazone. Oil red O staining and measurement of intracellular TG content were performed on the seventh day of differentiation. Then quantitative polymerase chain reaction (Q-PCR) was used to test the expressions of three transcription factors (PPARγ, CCAAT enhancer binding protein-α (C/EBP-α), and sterol regulatory element-binding protein 1 (SREBP-1)) and the target genes of PPARγ including glucose transporter (GLUT4), lipoprotein lipase (LPL), fat acid binding protein (AP2), and adiponectin in 3T3-L1 cells.
Results:
At the concentration of 5, 25 and 125 μg/mL, TC significantly promoted triglyceride accumulation. Further study showed that TC could promote the expression of PPARγ, C/EBPα and ADD1/SREBP1 significantly at 125 μg/mL. As for downstream genes controlled by PPARγ, TC at 25 and 125 μg/mL could significantly promote the expression of GLUT4 and adiponectin. However, the expression of aP2 related to lipid metabolism and adiposity in the TC group was significantly lower than that in the pioglitazone group.
Conclusion:
TC could promote preadipocytes differentiation through activating PPARγ and downstream controlled genes. TC has the ideal insulin sensitization with lower adipogenic action than classical TZDs in vitro. So TC from Agrimonia Pilosa Ledeb has a good prospect as a natural drug for IR and T2DM.
Keywords: Agrimonia Pilosa Ledeb, aP2, PPARγ, triterpenoid compound, Type 2 diabetes mellitus
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a systemic metabolic disease, whose occurrence and development involved in many factors and signal pathways including hyperglycaemia, dyslipidaemia and oxidative damage. Although the etiology mechanism is complex, the main pathological features are insulin resistance (IR) and β-cell function damage.[1] IR, a physiological condition, is that cells fail to respond to the normal actions of insulin. In muscle and fat cells, IR reduces the uptake of glucose and the storage of muscle glycogens and triglycerides, while IR in liver cells results in reduced liver glycogen synthesis and storage.
Now the treatments of T2DM mainly focus on improving IR. The most effective drugs which improve IR are thiazolidinedione (TZDs), such as rosiglitazone, pioglitazone and so on.[2] As a ligand of peroxisome proliferator-activated receptor gamma (PPARγ), TZDs can promote the transcriptional regulation of downstream genes related to IR selectively, and suppress inflammatory factors, such as TNF-α, NF-kB and so on. Though promoting differentiation of preadipocytes, they gather more intracellular lipids. So TZDs are associated with safety issues including weight gain, edema and cardiovascular disease while in treatment.[3,4] In 2010, the use of rosiglitazone had been restricted by the Food and Drug Administration and European Medicines Evaluation Agency. In order to resolve the current predicament of T2DM treatment, some alternative cure measures should be considered. There is a growing need in searching for natural bioactive components with benefits of improving IR or treating T2DM from vegetables, fruits, tea, spice, and medical herbs.[5,6]
Agrimonia Pilosa Ledeb (APL), belonging to Rosaceae, is a traditional Chinese medicine and a wild vegetable for tonic function. It is used to treat tumor, T2DM and blood, gastrointestinal, genitourinary, and gynecological diseases in traditional Chinese medicine. Now clinical practices had shown that Agrimonia Pilosa Ledeb had good therapeutic effect on treating T2DM. A growing body of research found that the major chemical components in Agrimonia Pilosa Ledeb are triterpenoids,[7] flavonoids,[8,9,10] coumarin compounds and agrimonolides. Triterpenoids from some plants have been confirmed to promote differentiation of 3T3-L1 preadipocytes and to improve IR,[11,12] retinopathy, nephropathy and diabetic vascular dysfunction.[13] In previous study, we found that triterpenoids was one of the most abundant components in Agrimonia Pilosa Ledeb. In order to clarify whether TC from Agrimonia Pilosa Ledeb is the component with therapeutic effect on IR or T2DM, we collected TC from Agrimonia Pilosa Ledeb, and then studied its effects on the differentiation of 3T3-L1 preadipocytes and the expressions of genes related to preadipocytes differentiation and IR, including PPARγ, C/EBPs, adipocyte determination differentiation dependent factor1/sterol regulatory element binding protein1 (ADD1/SREBP1), aP2, GLUT4, LPL and adiponectin. Our study will lay the foundation for developing drugs for IR and T2DM from Agrimonia Pilosa Ledeb.
MATERIALS AND METHODS
Materials
3T3-L1 preadipocytes were purchased from ATCC (American Type Culture Collection). The dried entire plants of Agrimonia Pilosa Ledeb purchased from Western Medicine City (Chongqing, China) in 2011. Pioglitazone was purchased from Chongqing Taiji Industry (Group) Co., Ltd. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and penicillin-streptomycin were obtained from HyClone. Bovine calf serum was obtained from Gibco. Paraformaldehyde, 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5 -di-phenytetrazoliumromide (MTT), Oil Red O, 3-isobutylmethylxanthine (IBMX), dexamethasone (DEX), dimethylsulfoxide (DMSO) and insulin were obtained from Sigma-Aldrich (St Louis, MO). Trizol reagent was purchased from Invitrogen and reverse transcription system from Takara. SYBR Green was purchased from BIO-RAD. All other reagents were of analytical grade.
The triterpenoid compound (TC) from Agrimonia Pilosa Ledeb was prepared by ourself in lab. The collected procedure and the constituents analysis of TC have been described in detail in reference.[14] TC has a high level of total triterpenoids with a value of 415.97 ± 5.15 mg/g and is abundant of 1β, 2β, 3β, 19α-tetrahydroxy-12-en-28-oic acid (265.2 mg/g) and corosolic acid (100.9 mg/g).[14]
Cell culture and preadipocyte differentiation
3T3-L1 preadipocytes were cultured at 37°C, 5% CO2. According to the standard protocols, 3T3-L1 preadipocytes were differentiated into adipocytes for 8 days.[15] The preadipocytes were maintained in DMEM (10% bovine calf serum, 1% penicillin-streptomycin). For the differentiation protocols, after reaching confluency (defined as day 0), the preadipocytes were cultured in differentiation medium DMEM (0.5 mM IBMX, 1 μg/ml insulin, 0.25 μM DEX and 10% FBS) for 2 days. After 2 days, the differentiation medium was replaced to DMEM (1 μg/ml insulin and 10% FBS) for 2 days. In day 5 to 8 days, the differentiation medium was changed again with fresh DMEM (10% FBS). The pioglitazone as positive control group and serial dilutions of TC from Agrimonia Pilosa Ledeb were administered at the initiation of differentiation and with every medium change. At 8th day, the differentiated cells (3T3-L1 adipocytes) were collected for analysizing mRNA expression level and Oil Red O staining.
The effect of TC on cell viability assay (MTT assay)
The MTT assay was performed according to the method of Mosmann.[16] 3T3-L1 preadipocytes were digested, centrifuged and prepared into single cell suspension, then calculated cell numbers in 1 mL by cell counting chamber. Then 3T3-L1 preadipocytes were plated into each well of a flat 96-well plate at a density of 2000 cells/well. After 24 h, the culture medium was replaced by pioglitazone and serial dilutions of TC from Agrimonia Pilosa Ledeb, and the cell cultures were incubated for 24 h or 48 h. Culture solutions were then removed and replaced by 80 μL of culture medium containing DMEM with 10% bovine calf serum and 20 μL filtered MTT solution (5 mg/mL) in phosphate-buffered saline (PBS, pH 7.4). After 4 h, solutions were removed and replaced by DMSO (150 μL/well) and the microtiter plate was closed by silver paper, and then measured spectrophotometer on microplate reader at 492 nm.
Oil Red O staining
At 8th day, discarding the culture and washing with PBS for three times. Then cells were fixed with 10% paraformaldehyde for 50 min, washed with PBS, and dyed with Oil Red O working dye (sealed, avoid light) for 50 min. The differentiated cells were photographed under a microscope (100× magnification) after washing with PBS twice.
Triglyceride Mass
3T3-L1 preadipocytes were cultured in 24-well microtiter plates, then stained with oil red O on 8th day. The redundant dye was washed twice by 60% isopropanol alcohol and then tri-distilled water for three times, and replaced by 1 mL 100% isopropanol alcohol, then vibrated 20 min by electronic oscillator. The absorbance was measured at 490 nm using a spectrophotometer to calculate TG mass.[17]
RNA preparation and quantitative real-time PCR
By Trizol reagent, total RNA was isolated from 3T3-L1 differentiated cells in 6 well plates. Then 1 μg RNA of each sample was reverse-transcribed to cDNA. At last, RT-PCR was performed with iQTM SYBR Green supermix according to the protocol. The PCR conditions were 1 cycle of 95°C for 3 min, followed by 39 cycles of 95°C for 10 s and 60°C for 30 s. The primer sequences were as follows: PPAR-γ, 5’-GCC CAC CAA CTT CGG AAT C-3’ and 5’-TGC GAG TGG TCT TCC ATC AC -3’; ADD1/SREBP1, 5’-TGG CTT GGT GAT GCT ATG TTG -3’ and 5’-GAC CAT CAA GGC CCC TCA A-3’; C/EBP-α, 5’-AGC TGA GTT GTG AGT TAG CCA TGT-3’ and 5’-ACC CCA CAA AGC CCA GAA A -3’; GLUT4, 5’-CAT GGC TGT CGC TGG TTT C-3’ and 5’-AAA CCC ATG CCG ACA ATG A-3’; aP2, 5’-CAA CCT GTG TGA TGC CTT TGT G-3’ and 5’-CTC TTC CTT TGG CTC ATG CC-3’; adiponectin, 5’-GAC ACC AAA AGG GCT CAG GAT-3’ and 5’-TGG GCA GGA TTA AGA GGA ACA-3’; LPL, 5’-GGA CTG AGG ATG GCA AGC AA -3’ and 5’-GCC ACT GTG CCG TAC AGA GA-3’.
Statistical analysis
Data are presented as means ± SD. A paired-samples t-test was used for the difference analysis between groups by using SPSS 19.0 software. Difference with a value of P < 0.05 were considered statistically significant.
RESULTS
Effect of TC on the cell viability of 3T3-L1 preadipocytes
To assess cytotoxic effect of TC, cell viability in 3T3-L1 preadipocytes was evaluated using MTT assay [Figure 1]. At concentrations of 1-1000 μg/mL (1, 10, 50, 100, 500 and 1000 μg/mL) for 24 h and 1-500 μg/mL (1, 10, 50, 100 and 500 μg/mL) for 48 h, TC had no significant inhibitory effects on cell viability compared with the control group. At the concentration of 1000 μg/mL, TC showed inhibition activity on cell growth in 48 h with a value of 22.33%. The cell viability in pioglitazone group had no obvious change in 24 h, but decreased to 91.30% in 48 h. The results showed that TC had slight influence on cell viability in 48 h at 1-500 μg/mL.
Figure 1.

The effect of TC from Agrimonia Pilosa Leded on the cell viability of 3T3-L1 preadipocytes. The values are means ± SD of three independent experiments
TC promotes 3T3-L1 cells differentiation and triacylglycerol accumulation
Now, adipose tissue is recognized as not only an energy-storage tissue but also an endocrine tissue that secretes a variety of bioactive substances to participate in the occurrence and development of IR and obesity. The key link between these symptoms is the proliferation and differentiation of preadipocytes.[18,19] Under stimulation of specific hormones, the cell had fundamental changes in form and function through the expression of different genes and regulation of various kinds of cytokines.[20] As an azo dye, red oil O can combine with triacylglycerol (lipid droplet) to give out jacinth lipid droplets. When preadipocytes differentiate into adipocytes, large numbers of jacinth lipid droplets will be observed. In this paper, we used 3T3-L1 preadipocytes as cell model to investigate the effect of TC on the preadipocytes differentiation through the cellular morphology and triacylglycerol accumulation.
Preadipocytes were irregular form, but adipocytes turned round after induction [Figure 2]. Pioglitazone, as a PPARγ agonist, can promote the preadipocytes differentiation and triacylglycerol accumulation.[21] So we used pioglitazone as positive control. We found TC could induce differentiation obviously in dose-dependent manner. In addition, pioglitazone had similar effect [Figure 3a]. To further characterize the effects of TC on differentiation, we measured intracellular TG content. The results showed that TC groups could increase TG content in cells with a dose-dependent manner. Compared with control, TG content in cells treated with 1, 5, 25 and 125 μg/mL TC were about 1.25, 1.43, 1.76 and 2.01 fold greater, respectively [Figure 3b].
Figure 2.
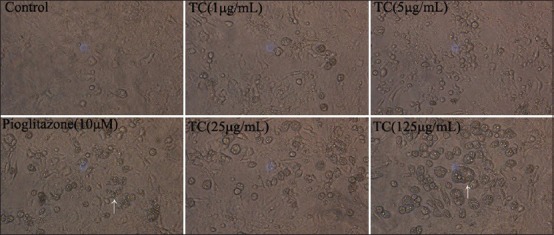
The morphology of 3T3-L1 cells treated without or with Pioglitazone (10 μM) or TC (1 μg/mL, 5 μg/mL, 25 μg/mL, or 125 μg/mL) for 7 days (×200)
Figure 3.
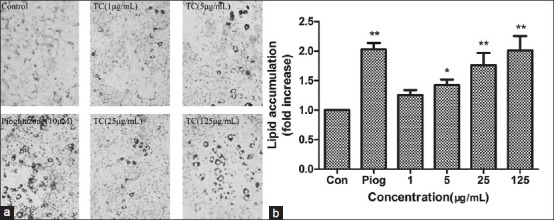
(a) The Red Oil staining of 3T3-L1 cells treated with various concentrations of TC for 7 days (×100). (b) The triglyceride content of 3T3-L1 cells treated with various concentrations of TC for 7 days. Data are expressed as means ± SD (n= 6). *P < 0.05, **P< 0.01, TC or Piog vs Control
TC activates the expressions of genes related to preadipocytes differentiation
To explicit whether TC had effect on expression of target genes related to differentiation, we performed RT-PCR to analysis target genes related to differentiation, including C/EBP-α, PPARγ and ADD1/SREBP1.[22,23] C/EBPs can regulate differentiation of preadipocytes through multiple paths, mainly by aP2 and GLUT4.[24] PPARγ plays a critical role in regulation of glucose, lipid homeostasis and downstream genes including aP2, GLUT4 and adiponectin.[25,26,27] Overexpression of PPARγ can induce and accelerate adipocyte differentiation.[28] As shown in Figure 4, TC could promote the expressions of PPARγ, C/EBP-α and ADD1/SREBP-1 in a dose-dependent manner. The expressions of PPARγ and ADD1/SREBP-1 after treatment with TC at 125 μg/mL were comparable to that of pioglitazone and significant higher than that of the control group (P < 0.05), suggesting that TC might have ideal insulin sensitizer effect as pioglitazone. As for C/EBP-α, TC at 125 μg/mL promoted its expression significantly compared with the control group (P < 0.05), but the promotion was not as strong as the pioglitazone group.
Figure 4.
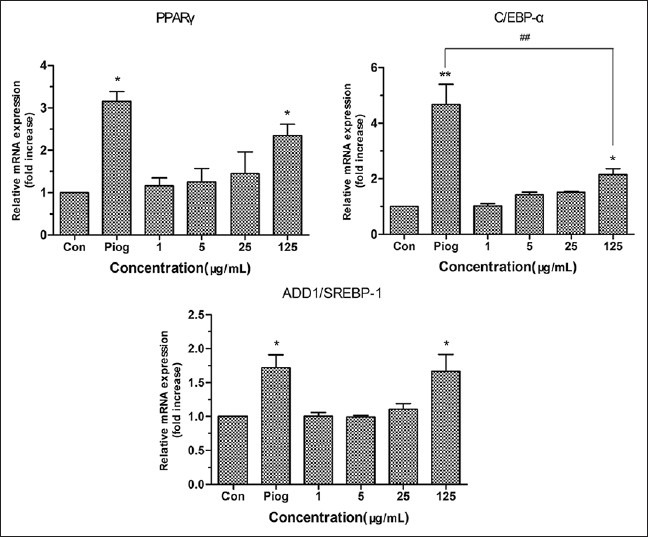
Effects of TC on the expression of main genes related to differentiation in 3T3-L1 adipocytes. The values are expressed as means ± SD of three independent tests. *P<0.05 and **P<0.01 as compared with 1% DMSO-treated control group; ##P <0.01 TC vs Piog
The gene aP2 plays an important role in adiposity and other side effects after activating PPARγ.[29,30,31] We found the expression of aP2 increased significantly in cells treated with TC at 25 and 125 μg/mL (P < 0.05), but was far lower than that in the pioglitazone group [Figure 5]. Compared with the control group, the expression of aP2 of 25 μg/mL TC group, 125 μg/mL TC group and pioglitazone group was about 2.15, 2.41 and 5.62 fold greater, respectively. The expression of GLUT4 was also significantly up-regulated by TC with concentrations of 25 μg/mL and 125 μg/mL (P < 0.05). What's more, the activation of PPARγ can trigger the expression of LPL which can produce free fatty acid (FFA), and then accumulate TG in cells and promote differentiation further. In this research, TC could increase the expression of LPL about 1.34 fold greater and pioglitazone can increase about 1.89 fold greater than the control group [Figure 5]. Adiponectin, a crucial adipokine secreted by adipocyte, can regulate metabolism of lipid and glucose,[32] which associates with anti-diabetic, anti-inflammatory and anti-atherogenic effects of PPARγ.[33] In this paper, adiponectin [Figure 6] was in agreement with other genes expression. Treating with TC at 25 μg/mL and 125 μg/mL, the expression of adiponectin in cells was significantly increased by 1.86 and 1.98 fold greater than that of the control group.
Figure 5.
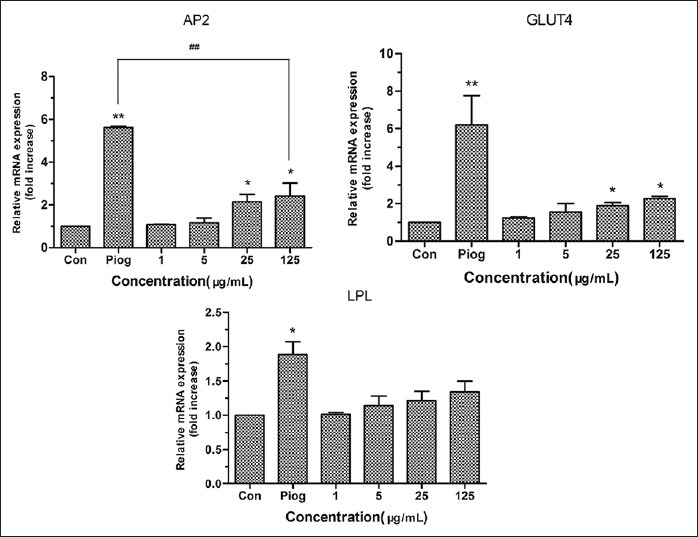
Effects of TC on the expression of main downstream genes related to differentiation in 3T3-L1 adipocytes. The values are expressed as means ± SD (n= 3). *P<0.05 and **P<0.01 TC vs 1% DMSO-treated control group; ##P <0.01 TC vs Piog
Figure 6.
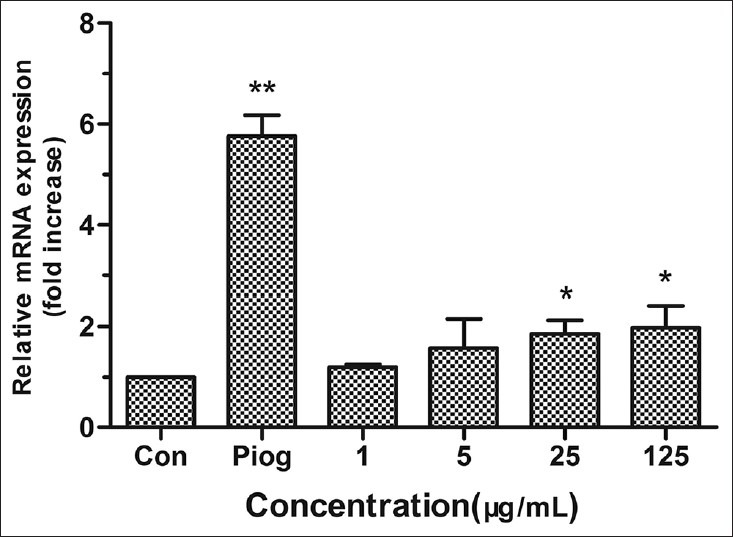
Effects of TC on the mRNA expression of adiponectin in 3T3-L1 adipocytes. The values are expressed as means ± SD (n= 3). *P<0.05 and **P<0.01 as compared with control group
DISCUSSION
T2DM is characterized by persistent hyperglycemia, IR and β-cell dysfunction, leading to macro- and micro-vascular diseases, such as diabetic retinopathy, nephropathy, neuropathy and sexual dysfunction.[34] In the clinic treatment of T2DM, TZDs, as PPARγ agonists, have an outstanding effect. But on the other hand, TZDs are associated with numerous safety issues.[35,36] So the research on exploring some new alternatives is urgent. In Chinese folk medicine, Agrimonia Pilosa Ledeb has showed good therapeutic effect on treating T2DM. However, the bioactive components and therapeutic mechanism are still obscure. Understanding these problems could be of value in developing natural drugs to fight IR and T2DM. Considering TC is one of the most abundant components in Agrimonia Pilosa Ledeb, we collected TC from Agrimonia Pilosa Ledeb and studied its effect on the differentiation of 3T3-L1 preadipocytes to develop new insulin sensitizing agent. Our results indicate that TC can promote 3T3-L1 preadipocytes differentiation and TG accumulation in the differentiated protocol of 3T3-L1 cells. Further studies show that TC can up-regulate the mRNA expression of genes related to insulin sensitization significantly, including PPARγ, SREBP-1 and C/EBP-α. As for downstream genes controlled by PPARγ, TC did not have drastic promoting effects on aP2 and LPL comparing with pioglitazone. But TC could significantly activate the mRNA expression of adiponectin and GLUT4.
PPARγ has an outstanding function in the process of cell differentiation and lipid metabolism. Its agonists significantly ameliorate insulin sensitivity in patients with T2DM.[37] aP2 plays an important role in adiposity, is a key controller for peripheral insulin sensibility and glucose and lipid metabolism.[38] Overexpression of aP2 causes accumulation of lipid and metabolism disorder, so it has a very close relationship with lots of metabolic disorders and has been recognized as a potential marker of undesirable effects.[29,30,31,39] In our study, induction of aP2 expression by TC was only about 30% compared with pioglitazone. These data suggest that TC can promote expression of PPARγ without activating aP2 sharply.
LPL plays an important role in providing energy and metabolizing lipoprotein. When PPARγ is activated, LPL will express and produce FFA. In turn, FFA can promote PPARγ expression. This process can promote cell differentiation, but also cause accumulation of triacylglycerol in the meantime. In this study, we find that TC can promote LPL gene expression, but less than pioglitazone. The results illustrated TC might reduce the main side effects such as weight gain. Adiponectin, an adipokine, is produced when 3T3-L1 preadipocytes differentiate to be mature adipocytes. In vivo, the level of circulating adiponectin has positive relationship with IR generally and higher adiponectin in fatter people. In normal physiological condition, it improves absorbption of glucose, oxidation of fatty acid and sensibility of peripheral insulin,[40,41] acting as anti-diabetic, anti-atherogenic and anti-inflammatory.[42,43] Our results show that TC plays an obvious role in promoting its expression.
CONCLUSIONS
In conclusion, TC from Agrimonia Pilosa Ledeb can promote preadipocytes differentiation through activating the mRNA expression of PPARγ to improve IR. Excitingly, though TC could activate the expression of PPARγ sharply, which was comparable to the effect of pioglitazone, the promotion of TC on the expression of aP2 and LPL did not have drastic effects compared with that of pioglitazone. It implied that TC cannot induce over-production of FFA or worsen IR. In addition, mRNA expression of GLUT4 and adiponectin can also be up-regulated significantly. So TC from Agrimonia Pilosa Ledeb has a good prospect as a natural drug for IR and T2DM.
ACKNOWLEDGMENTS
We thank Changhua Wang (Chongqing Academy of Chinese Materia Medica, China) for the verification of the plant material. This work was supported by the Natural Science Foundation Project of CQ CSTC (No. 2014jcyjA10097), Program for Innovation Team Building at Institutions of Higher Education in Chongqing (KJTD201325), and Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (CQKLBST-2012-007).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Muoio DM, Newgard CB. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 2.Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. J Clin Invest. 2000;106:467–72. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, et al. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: A randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–10. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 4.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 5.Kumar BD, Krishnakumar K, Jaganathan SK, Mandal M. Effect of mangiferin and mahanimbine on glucose utilization in 3T3-L1 cells. Pharmacogn Mag. 2013;9:72–5. doi: 10.4103/0973-1296.108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009–13. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 7.Kouno I, Baba N, Ohni Y, Kawano N. Triterpenoids from Agrimonia pilosa. Phytochemistry. 1988;27:297–9. [Google Scholar]
- 8.Bilia A, Palme E, Marsili A, Pistelli L, Morelli I. A flavonol glycoside from Agrimonia eupatoria. Phytochemistry. 1993;32:1078–9. [Google Scholar]
- 9.Tomlinson C, Nahar L, Copland A, Kumarasamy Y, Mir-Babayev NF, Middleton M, et al. Flavonol glycosides from the seeds of Agrimonia eupatoria (Rosaceae) Biochem Syst Ecol. 2003;31:439–41. [Google Scholar]
- 10.Xu X, Qi X, Wang W, Chen G. Separation and determination of flavonoids in Agrimonia pilosa Ledeb by capillary electrophoresis with electrochemical detection. J Sep Sci. 2005;28:647–52. doi: 10.1002/jssc.200400095. [DOI] [PubMed] [Google Scholar]
- 11.Jung S, Ha Y, Shim E, Choi S, Jin J, Yun-Choi H, et al. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem J. 2007;403:243–50. doi: 10.1042/BJ20061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan MJ, Ye JM, Turner N, Hohnen-Behrens C, Ke CQ, Tang CP, et al. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem Biol. 2008;15:263–73. doi: 10.1016/j.chembiol.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Alqahtani A, Hamid K, Kam A, Wong K, Abdelhak Z, Razmovski-Naumovski V, et al. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr Med Chem. 2013;20:908–31. [PubMed] [Google Scholar]
- 14.Liu X, Zhu LC, Tan T, Zhou XM, Xiao L, Yang X, et al. Glucosidase inhibitory activity and antioxidant activity of flavonoid compound and triterpenoid compound from Agrimonia Pilosa Ledeb. BMC Complement Altern Med. 2014;14:12–21. doi: 10.1186/1472-6882-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nugent C, Prins JB, Whitehead JP, Savage D, Wentworth JM, Chatterjee VK, et al. Potentiation of glucose uptake in 3T3-L1 adipocytes by PPAR gamma agonists is maintained in cells expressing a PPARγ dominant-negative mutant: Evidence for selectivity in the downstream responses to PPAR gamma activation. Mol Endocrinol. 2001;15:1729–38. doi: 10.1210/mend.15.10.0715. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Goto T, Nagai H, Egawa K, Kim YI, Kato S, Taimatsu A, et al. Farnesyl pyrophosphate regulates adipocyte functions as an endogenous PPARγ agonist. Biochem J. 2011;438:111–9. doi: 10.1042/BJ20101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: Peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–78. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 19.Saito T, Abe D, Sekiya K. Nobiletin enhances differentiation and lipolysis of 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2007;357:371–6. doi: 10.1016/j.bbrc.2007.03.169. [DOI] [PubMed] [Google Scholar]
- 20.Smas CM, Sul HS. Control of adipocyte differentiation. Biochem J. 1995;309:697–710. doi: 10.1042/bj3090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallakou S, Doare L, Foufelle F, Kergoat M, Guerre-Millo M, Berthault MF, et al. Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46:1393–9. doi: 10.2337/diab.46.9.1393. [DOI] [PubMed] [Google Scholar]
- 22.Johmura Y. Characterization of novel genes regulating adipocyte differentiation. Yakugaku Zasshi. 2007;127:135–42. doi: 10.1248/yakushi.127.135. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBP beta, C/EBP delta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–36. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adelmant G, Gilbert JD, Freytag SO. Human translocation liposarcoma-CCAAT/enhancer binding protein (C/EBP) homologous protein (TLS-CHOP) oncoprotein prevents adipocyte differentiation by directly interfering with C/EBP beta function. J Biol Chem. 1998;273:15574–81. doi: 10.1074/jbc.273.25.15574. [DOI] [PubMed] [Google Scholar]
- 25.Rosen ED, Spiegelman BM. PPARγ: A nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–4. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 26.Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue--understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92:386–95. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- 27.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 28.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–71. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Bhalla K, Hwang BJ, Choi JH, Dewi R, Ou L, McLenithan J, et al. N-Acetylfarnesylcysteine is a novel class of peroxisome proliferator-activated receptor gamma ligand with partial and full agonist activity in vitro and in vivo. J Biol Chem. 2011;286:41626–35. doi: 10.1074/jbc.M111.257915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rival Y, Stennevin A, Puech L, Rouquette A, Cathala C, Lestienne F, et al. Human adipocyte fatty acid-binding protein (aP2) gene promoter-driven reporter assay discriminates nonlipogenic peroxisome proliferator-activated receptor gamma ligands. J Pharmacol Exp Ther. 2004;311:467–75. doi: 10.1124/jpet.104.068254. [DOI] [PubMed] [Google Scholar]
- 31.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–34. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 32.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: A key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110:267–78. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 34.Munana KR. Long-term complications of diabetes mellitus, Part I: Retinopathy, nephropathy, neuropathy. Vet Clin North Am Small Anim Pract. 1995;25:715–30. doi: 10.1016/s0195-5616(95)50064-6. [DOI] [PubMed] [Google Scholar]
- 35.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA. 2007;298:1180–8. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 36.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108:2941–8. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki Y, Mahankali A, Wajcberg E, Bajaj M, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:4312–9. doi: 10.1210/jc.2004-0190. [DOI] [PubMed] [Google Scholar]
- 38.Fu Y, Luo N, Lopes-Virella MF. Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res. 2000;41:2017–23. [PubMed] [Google Scholar]
- 39.Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta. 2000;1486:28–44. doi: 10.1016/s1388-1981(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 40.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: Mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263–70. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 41.Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2007;9:282–9. doi: 10.1111/j.1463-1326.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–91. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 43.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]


