Abstract
Background:
Lippia nodiflora (LN) Linn is a small herb distributed throughout the world. The plant extracts of LN is used traditionally as an analgesic, anti-inflammatory, antioxidant, antibacterial, antimicrobial, antipyretic, antitumor, antidiabetic, and possess hepatoprotective properties.
Materials and Methods:
To study the antibacterial and hepatoprotective effect of LN, we used methanolic extracts of leaves on HepG2 cells. Lipopolysaccharides (LPS) is a well-characterized hepatotoxin, so toxicity was induced on liver cells using LPS. Up-regulation of inflammation genes were quantified.
Results and Conclusions:
In our present study, we have showed that LN reduced reactive oxygen species (ROS) production against LPS induced toxicity on HepG2 cells, and ther by decreased the apoptotic gene expression and protect the liver cells against toxicity.
Keywords: Apoptosis, HepG2 cells, Lippia nodiflora, methanloic extraction, reactive oxygen species
INTRODUCTION
Sepsis is a systemic inflammatory response to bacterial infections, which are often associated with fatal ends. Antibiotics are generally used to protect and treat bacterial infections. However, in recent years, the heavy use of antimicrobial drugs increased new antibiotic-resistant bacterial populations, thus increasing the incidence of fatal cases of sepsis and inevitable nosocomial infections.[1] Every year sepsis affects 700,000 people and results in 200,000 deaths in the United States.[2] Both Gram-negative and Gram-positive bacterial infections are capable of causing sepsis especially lipopolysaccharides (LPS) from Gram-negative bacteria, and peptidoglycan/lipopeptides from Gram-positive bacteria.[3]
Liver is one of the largest organ in the human body plays an important role in sepsis. Cytokine released during sepsis results in profound physiological changes in the liver. These physiological changes injure liver cells and its functions are altered.[4] Besides sepsis, the liver is constantly flushed with toxins from environment, food, alcohol, drugs, diseases such as hepatitis, fatty liver, and cirrhosis. These varied disorders damage and weaken the liver. Every year about 20,000 deaths are found due to liver disorders, and approximately 25,000,000 Americans have been affected with liver diseases and are more susceptible to the development of sepsis, thus liver ailments remain a serious health problem.[5] Moreover, the readily available conventional hepatoprotective synthetic drugs used in the treatment of liver diseases are sometimes inefficient and causes severe side effects.[6] Hence, there is a need to search alternative drugs, and one such alternative way is using new compounds, not based on existing synthetic molecules.[7]
since ancient times, in India, different parts of plants are used to cure various diseases. Recent research on traditional medicines showed they are effective because of naturally occuring polyphenolic products as their active ingredients. Moreover, they have rich sources of antioxidants and secondary metabolites which are found to have antimicrobial, anti-infective, analgesic, anti-inflammatory, antioxidant, antipyretic, antitumor, antidiabetic, and hepatoprotective properties and are highly effective, cost-effective with fewer side effects.[8] Thus, these phytochemical molecules make ideal alternative for drugs.
Lippia nodiflora Linn (LN) is distributed throughout the world [Figure 1]. Its entire parts have been used as a traditional medicinal cure in Asian and African, systems of medicine. LN had a number of pharmacological properties such as analgesic, antimalaric, anti-inflammatory, hepatoprotective activity, antispasmodic, antipyretic, antibacterial activity, and anti-fungal. prior work show that LN had some promising hepatoprotective activity against paracetamol-induced liver injury on rats.[9]
Figure 1.

Lippia nodiflora Linn habitat
As there was no evidence carried on hepatoprotective activity of LN against LPS induced liver damages in HepG2 cells, to support the claim of natural practitioners as hepatoprotective, it was, therefore, our interest to characterize the antibacterial activity as well as hepatoprotective nature of LN in HepG2 cells against LPS toxicity.
MATERIALS AND METHODS
Chemicals
Cell culture media such as Dulbecco's Modified Eagle's Medium (DMEM), fetal calf serums (FCS), trypsin-ethylenediaminetetraacetic acid solution, penicillin, streptomycin minimal medium were purchased from Mediatech, USA. Acridine orange and LPS (Escherichia coli 0127:E8) were purchased from Sigma Chemicals Co, USA. Silymarin was a kind gift from Dr. Nicholas Oberlies, Washington University, USA. RNA isolation kit was purchased from Qiagen, USA. cDNA was synthesis using iScript kit from Bio-Rad, USA. Cell viability was measured using cell titer-glo luminescent cell viability kit from Promega, USA. Quantitative polymerase chain reaction (qPCR) was validated using Tagman probes from Life Technologies, USA.
Cell culture
HepG2 cells were grown in DMEM containing 10% FCS, supplemented with penicillin (100 units/mL), streptomycin (100 mg/L), minimal media in 5% CO2 at 37°C, and were sub-cultured regularly.
Cell treatment
Stock solutions of LPS, LN extracts, and silymarin were prepared fresh to avoid oxidation. For LPS toxicity experiments, crude extract of LN and silymarin were added to the cell cultures 2 h prior to LPS treatment for 24 h. Cells were then collected to test for various parameters.
Cytotoxicity assays
Cytotoxicity is defined as the potential of a compound to induce cell death. Hence, cell viability is an index of cytotoxicity. To measure the viable, as well as dead cells, the cells were seeded onto 96-well plates, and after the corresponding treatment, 20 μL of the sample was mixed with 20 μL of AO/PI staining mix of cellometer viability dyes. Cell viability was further confirmed using MTT assay. Cells were seeded onto 96-well plates, and after the corresponding treatment, the medium was removed and cell viability was evaluated to measure the number of viable cells in culture based upon the quantitation of the ATP present, which signals the presence of metabolically active cells. The cell titer-glo assay is designed for cell proliferation and cytotoxicity assays and determined by ELISA reader at 565 nm (Multiskan Spectrum; Thermo Electron Corporation, USA).
Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) release assay was done to determine the effect of methanolic extract of LN on membrane permeability in HepG2 cells. The cells were seeded in a 96-well plate at a density of 104 cells/well. After corresponding treatments, 100 μl of reaction buffer from LDH cytotoxicity assay kit (Cayman Chemical, USA) was added to each well. The contents of each well were mixed well using a plate shaker at 300 rpm for 30 min at RT, and the presence of LDH enzyme in the cell culture medium was measured at 490-520 nm (Multiskan Spectrum; Thermo Electron Corporation, USA). The average values from treated wells were subtracted from the control wells, and the value was plotted on a graph.
The formula for LD activity was (U/I) = value of LDH activity from the graph/total volume of sample.
The amount of formazan produced is proportional to the amount of LDH released into the culture medium as a result of cellular cytotoxicity and cytolysis.
Caspase-3 enzyme activity to detect apoptosis
To measure the caspase-3 activity, cells were plated in 60 mm dishes at a density of ~ 3 × 105, after corresponding treatment, 100 μl of Caspase-Glo reagent (Caspase-Glo® 3/7 Assay kit from Promega, USA) was added to each well. The contents of each well were mixed well using a plate shaker at 500 rpm for 30 s and incubated at RT for 2.30 h. Caspase-3 enzyme activity was measured calorimetrically at 510-570 nm (Multiskan Spectrum; Thermo Electron Corporation, USA).
DNA laddering
HepG2 cells both treated and untreated were collected, centrifuged at 1500 × g for 5 min. Washed twice with ice cold PBS, and gDNA was isolated using phenol/chloroform extraction and eluted in TE buffer. gDNA concentration was measured using NanoDrop ND-1000 spectrophotometer. Nearly 1.5 μg of gDNA was stained with ethidium bromide and visualized by 2.0% TAE to analyze DNA laddering apoptotic fragmentation.[10]
RNA extraction, reverse transcription and real-time quantitative polymerase chain reaction
When cells showing ~85-90% confluence, crude extracts of LN, silymarin and DMSO4 were added to HepG2 cells in 5% FCS medium two h prior to LPS treatment for 24 h. After 24 h, cells were collected, and total RNA was isolated using RNeasy Qiagen column. Total RNA concentrations were measured using NanoDrop ND-1000 spectrophotometer. Reverse transcription was performed using 1 μg of total RNA using modified MMLV-derived reverse transcriptase in 20 μL total reaction mix. Real-time qPCR was performed using Roche Light Cycler 480 RT PCR Instrument (Roche, USA) using Taqman master mix (Life technologies). The 18S ribosomal RNA (rRNA) gene was used as a reference.
Collection and identification of test material
Fresh leaves of LN were collected from Houston, TX, USA and were authenticated by the Department of Botany, Morgan State University, Maryland, USA. The leaves were cleaned with sterile distilled water to remove dirt, dried under shadow, and powdered in a grinder. The powdered leaves were soaked separately for 24 h in different solvents like water, hexane, chloroform, petroleum ether and methanol, and extracted repeatedly. After 24 h, the extraction was filtered and concentrated further under a vacuum evaporator at 40°C, and stored in an air tight container until use at 4°C. Before use, each crude extract was re-suspended in their respective solvent.
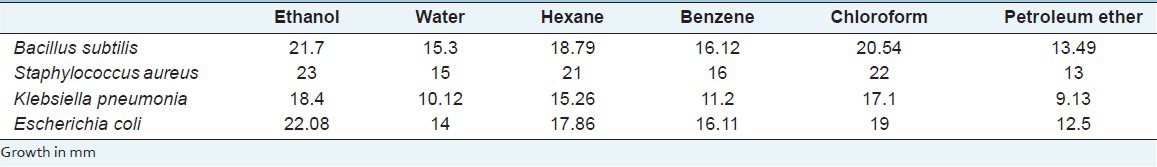
Screening for antibacterial activity of crude extracts
The crude extracts of leaves were screened initially for their antibacterial activity using agar disc-diffusion method.[11]
Bacterial strains
Two Gram-positive bacterial strains such as Bacillus subtilis and Staphylococcus aureus and two Gram-negative bacterial strains Klebsiella pneumonia and E. coli from Pathology Department, Methodist Hospital Research Institute, TX, USA were used. These strains were maintained on Nutrient Agar (NA) medium for further experiments.
Effect of crude extract on the growth of bacteria by agar disc diffusion method
To see the antibacterial activity, about 400 mg/mL of crude extract of leaves in different solvents were loaded on a sterile whatman filter paper disc of 6 mm diameter. The petriplates with agar medium were spread with 100 μL of actively growing culture of the test bacteria and allowed to dry for 10 min. Then the impregnated discs were placed on the surface of inoculated agar medium and incubated for 37°C for 24 h. Discs impregmated with small volume of DMSO4 was served as control. The development of inhibition zone around the active extract loaded disc was recorded. Each experiment was repeated for three times, and the mean value was taken for each sample.
Phytochemical screenings
Various preliminary phytochemical screening was performed as per standardized procedures[12] in water, benzene, hexane, petroleum ether, chloroform, and ethanol.
Statistical analysis
Results are expressed as mean ± SD. Comparisons were made between control and treated groups unless otherwise indicated using unpaired Student's t-test and P < 0.01 were considered statistically significant.
RESULTS
Throughout the world, herbal medicines are claimed to provide relief against many of the liver diseases. But such claimed herbal medicines should be validated scientifically manner. In our present work, an effort was taken to prove the hepatoprotective activity of LN in HepG2 cells.
Different solvents such as water, hexane, benzene, chloroform, methanol and petroleum ether, are used to measure cell viability activity on HepG2 cells. Among which, methanol was found to be more effective followed by chloroform, hexane, benzene, water and petroleum ether [Table 1]. Dose-response experiments were studied using different concentrations of crude extract of LN leaves and silymarin. The crude extract of LN above 800 mg/L was found to be toxic, while 400 mg/L was found to have a protective effect [Figure 2]. For silymarin, a concentration above 50 mg/L showed loss of cell viability [Figure 3].
Table 1.
Phytochemical analysis of L. nodiflora in different solvents
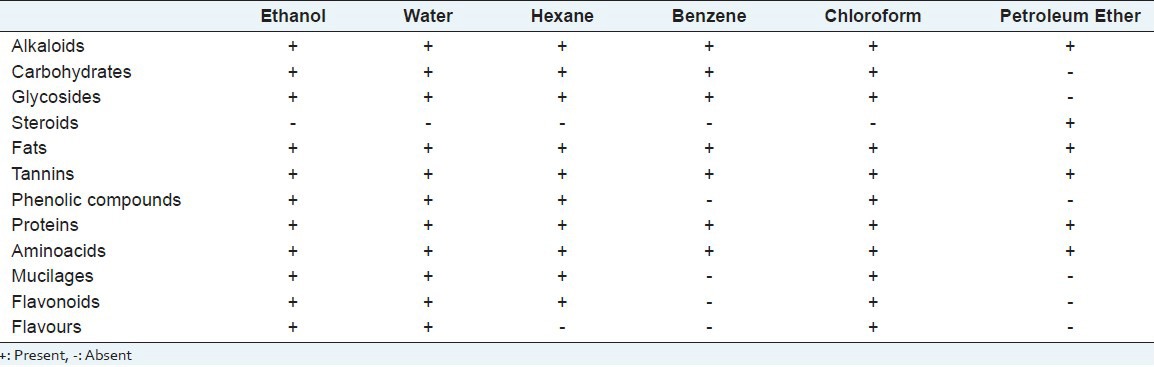
Figure 2.
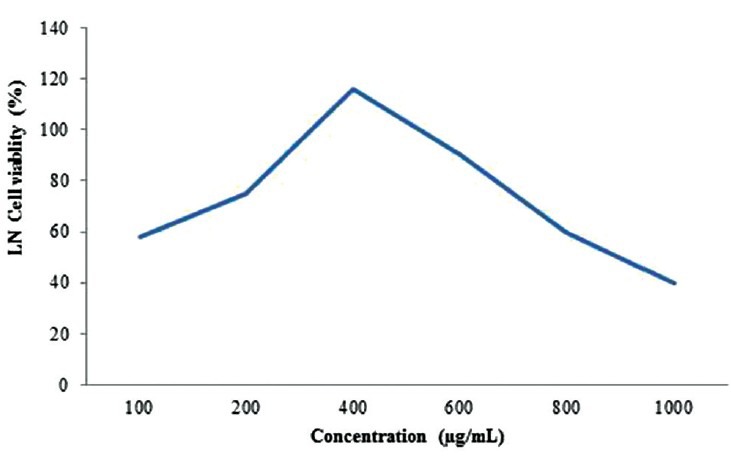
Cytoprotective effect of Lippia nodiflora on lipopolysaccharide-induced cytotoxicity
Figure 3.
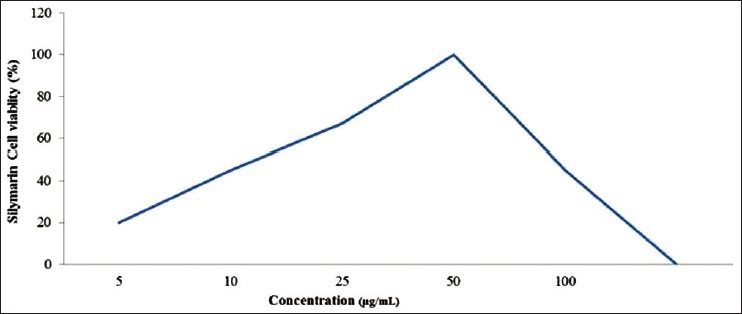
Cytoprotective effect of Silymarin on lipopolysaccharide-induced cytotoxicity
To see the antibacterial activity using agar disc method, among the four different bacterial strains, we noticed S. aureus was inhibited, followed by E. coli, B. subtilis and K. pneumonia using methanolic extract of LN leaves and there was no inhibition zone around the control DMSO4 [Table 2].
Table 2.
Antibacterial activity of L. nodiflora in different solvents
Cytotoxicity test by the cell titer-glo assay helped to identify the viable cells after treatment with LPS in HepG2 cells. Among the different concentrations of LPS checked for cytotoxicity, 200 mg/L was found to be effective in generating oxidative stress [Figure 4]. Acridine orange staining under fluorescence microscopy helps to identify the dead cells. Control cells treated with DMSO4 showed round-shaped nuclei [Figure 5a], while LPS treatment cells were swelled, distorted, detached, showed membrane blebbing and cytoplasmic shrinkage [Figure 5b]. These structural changes were prevented to the maximum extent by treating with silymarin at 50 mg/L and crude extracts of LN (400 mg/L) [Figure 5c and d]. Even the LDH assay confirmed the above acridine orange staining conclusions. Therefore, we can assume that stronger apoptosis is associated with a higher concentration of VN methanolic extract against the LPS induced apoptotis changes [Figure 6].
Figure 4.
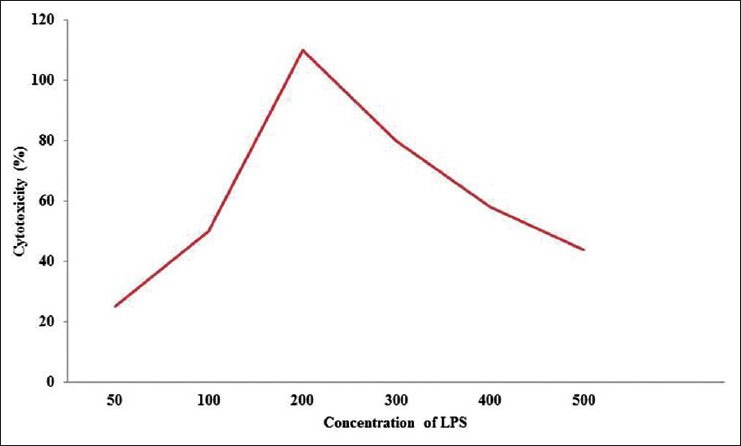
Effect of lipopolysaccharide on cell viability
Figure 5.
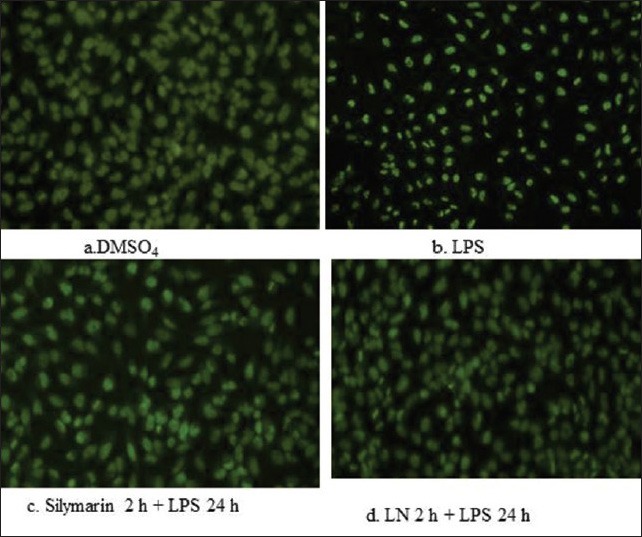
Effect of lipopolysaccharide on Lippia nodiflora and silymarin
Figure 6.
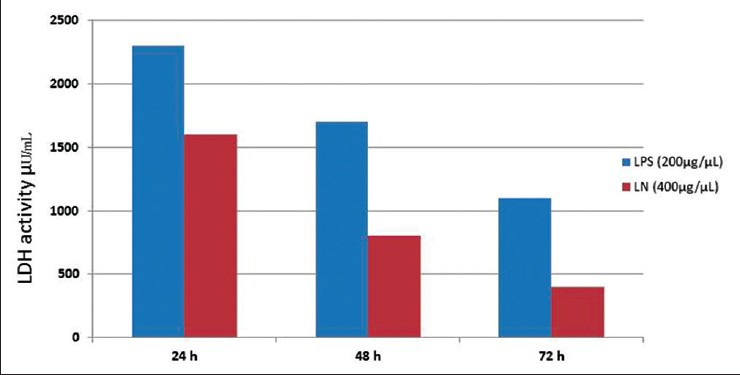
Effect of lipopolysaccharide and Lippia nodiflora treatment on lactate dehydrogenase release
After LPS treatment for 24 h, we observed an increase in mRNA gene expression of few inflammatory genes such as nuclear factor kappa β (NFκβ), interleukin 1β (IL1β), tumor necrosis factor α (TNF α), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX2) in HepG2 cells. The mRNA expression of proinflammatory factor IL-1β was increased almost 25 fold in LPS treated cells. Pretreatment with silymarin and LN followed by LPS treatment decreased the mRNA expression to 18.0 and 12.0 times fold [Figure 7]. The mRNA expression of NFκβ was increased to 17.5 fold in LPS treated cells, while in silymarin and LN treated cells significantly reduced to 14.5 and 12.0 fold respectively [Figure 8]. The mRNA expression of TNF α was almost 18.5 fold higher in LPS treated cells, while cells treated with silymarin and LN was reduced to 9.0 and 4.0 fold, respectively [Figure 9]. The mRNA expression of iNOS was 25.5 fold higher in LPS treated cells. But the mRNA expression of treated cells with silymarin and LN were reduced to 12.0 and 5.5-fold, respectively [Figure 10]. The mRNA expression of proinflammatory factor COX2 was increased almost 69 fold in LPS treated cells. Pretreatment with silymarin and LN followed by LPS treatment decreased the mRNA expression to 55.0 and 30.0 fold [Figure 11]. All these results confirmed the rescuing and hepatoprotective properties of LN.
Figure 7.
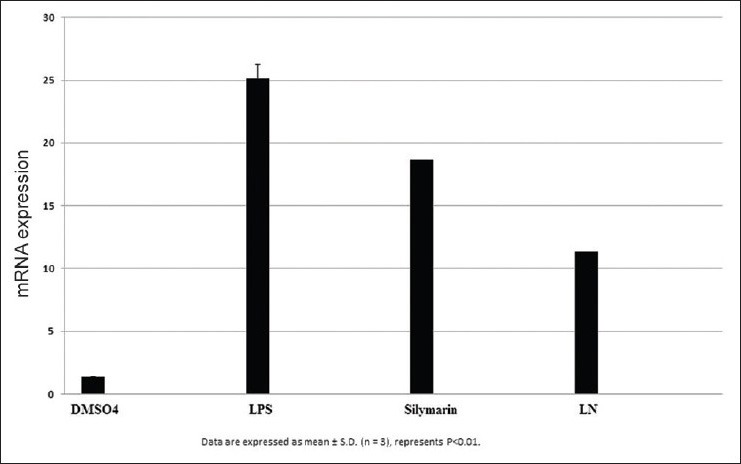
Effect of interleukin 1β on HepG2 cells
Figure 8.
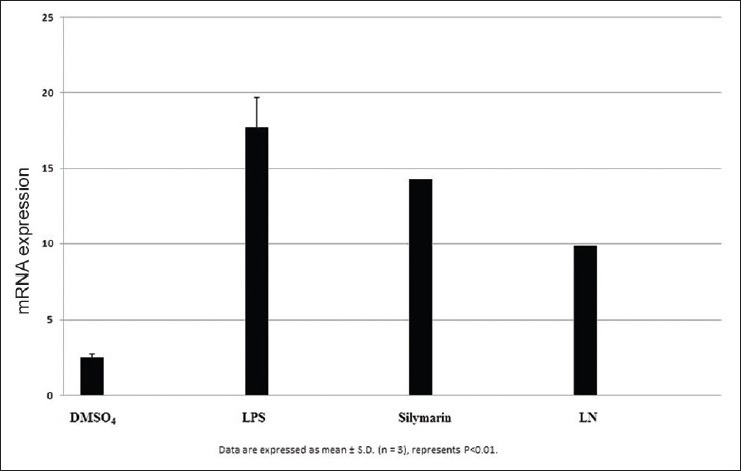
Effect of NFkβ on Hepg2 cells
Figure 9.
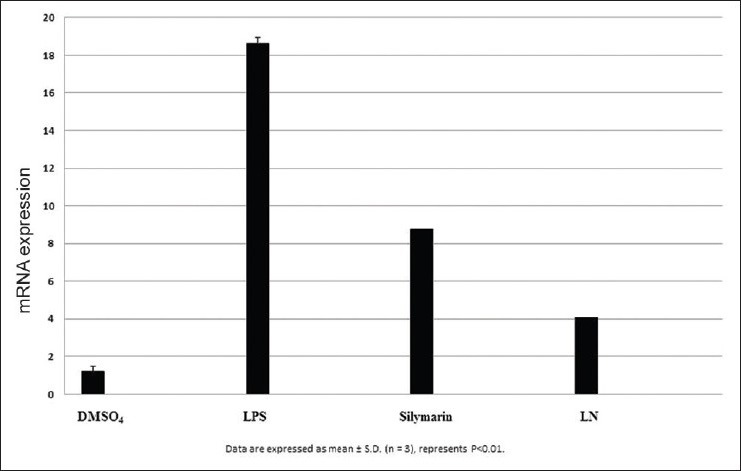
Effect of Tumor necrosis factorα on HepG2 cells
Figure 10.
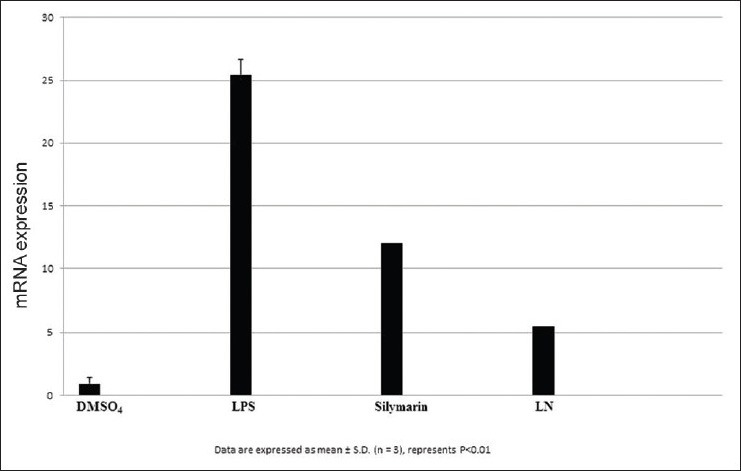
Effect of inducible nitric oxide synthase on HepG2 cells
Figure 11.
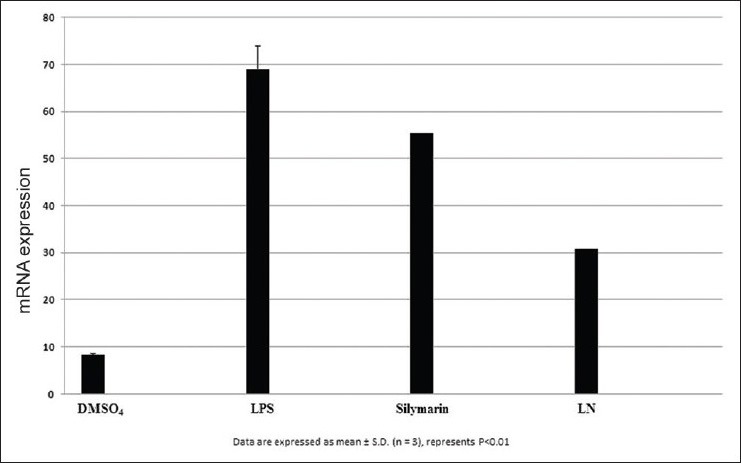
Effect of cyclooxygenase-2 on HepG2
Caspase-3 activation is an important element in the apoptotic signaling cascade pathway. Caspase-3 colorimetric assay confirmed the induction of apoptosis by LPS for 2 h in HepG2 cells. Treatment of LN for 24 h reduced the caspase 3 activation [Figure 12]. Cells treated with LPS for 24 h showed a DNA ladder kind of formation confirmed the apoptotic nature. DNA fragmentation is programmed cell death which is a key feature of apoptosis. This programmed cell death is characterized by the degradation of nuclear DNA into nucleosomal units. Treatment with crude extracts of LN and silymarin for 2 h protected cells against LPS induced DNA fragmentation [Figure 13]. Caspase 3 activation, as well as DNA laddering, strongly showed the apoptotic nature of LPS on HepG2 cells. LPS treated cells for 24 h showed increase in nuclei condensation. Apoptotic bodies increased the apoptotic cells significantly to 49%. Nearly 91% of cells treated with LPS for 24 h showed cell death. But crude extract of LN and silymarin had anti-apoptosis effect as LN reduced the percentage of apoptotic cells to 16%, while silymarin to 28%, respectively, during cell cycle analysis using cellometer automatic cell counters.
Figure 12.
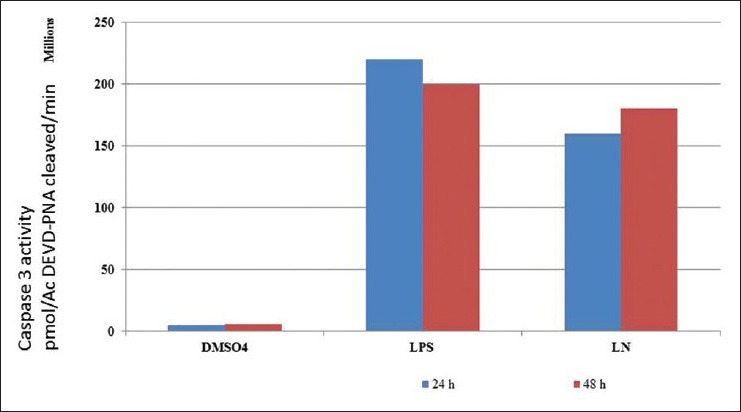
Caspase 3 activity of HepG2 cells treated with lipopolysaccharide and Lippia nodiflora
Figure 13.
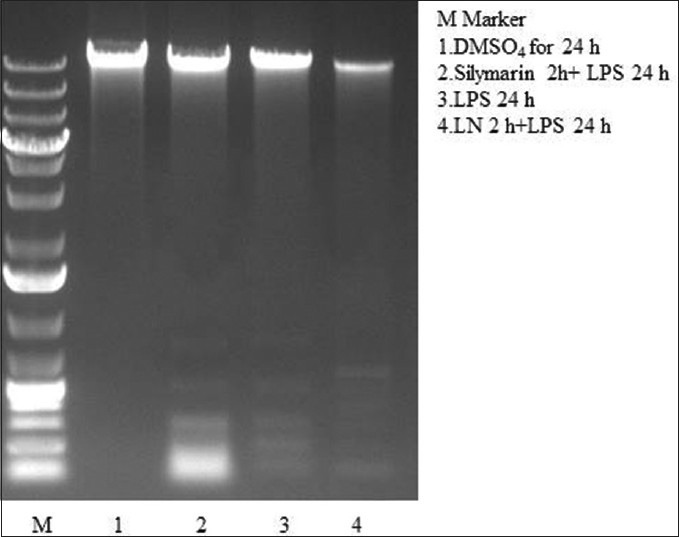
Effect of Lippia nodiflora and silymarin against lipopolysaccharide induced DNA fragmentation
DISCUSSION
The present study showed that the methanolic extract of LN suppressed the LPS-induced inflammatory responses in the cell line HepG2. Among the different solvents, tested, methanolic extracts were found to be effective in our studies. Results of our studies showed that the crude extract of LN above 800 mg/L was found to be toxic, while 400 mg/L was found to be a protective effect. Our result correlated with Durairaj et al., 2008[9] showed that 400 mg/kg of methanolic extracts of LN leaves showed the maximum percentage of viability against paracetamol-induced liver toxicity in rats. We noticed in our study in agar disc diffusion method, S. aureus was highly inhibited, followed by E. coli, B. subtilis and K. pneumonia using methanolic extract of LN leaves and there was no inhibition zone around the control DMSO4. Zare et al.,[13] also reported that S. aureus was inhibited by the methanolic extract of leaf followed by B. subtilis. These two bacteria are principle among other bacterial populations causing infections in cirrhosis related death.[4] Thus, these results confirmed that LN can be used as a source to improve the treatment of infection caused by these organisms. Phytochemical analysis of LN methanolic extract revealed the presence of sterols, saponins, coumarins, quinones, tannins, flavonoids, and reducing sugars[14] besides lippiacin, a new triterpenoid was also isolated from the methanolic extract of the LN.[15] Research have shown that bioactive natural products produced potential physiological activities, due to their polyphenols which act as cytoprotective, anti-inflammatory and hepatoprotective agents, forms an important source for human health research. Hence, these polyphenols present in LN can act as an antioxidant decreasing the production of reactive oxygen species, thus protect the liver against LPS-induced liver toxicity and oxidative stress.[16]
Among different cell lines, HepG2 cells were used, since it retains many of the phenotypic, and genotypic characteristics of liver cells, so many drugs, chemicals or compounds, metabolism and enzyme activities, phytochemical properties of plant extracts have been studied.[17] LPS-induced liver damage is similar to that of acute viral hepatitis.[18] During sepsis, liver responds to LPS with the intense production of inflammatory cytokines, such as TNF α, IL1β, and reactive oxygen intermediates through the nuclear factor-kappa β (NF-kB)/COX2 pathway.[19] Overexpression of iNOS has been implicated in the pathogenesis of septic shock, inflammation, and carcinogenesis. LPS is a potent stimulator of nitric oxide (NO) and large amounts of NO to be generated in the liver during sepsis, which could impair hepatic function by direct injury to hepatocytes.[20] COX2 is overexpressed during inflammation, and malignant condition.[21] Prostaglandin E2 (PGE2) synthesized from arachidonic acid by COX2 as a response to inflammation. During the sepsis pathogenesis, COX2 is generally expressed only in cells where prostaglandins are up-regulated by pro-inflammatory mediators.[22] More than any other cytokine family, the IL-1 family is closely linked to innate immune response. IL-1 β comprise a major pro-inflammatory family of cytokines play an important role in both acute and chronic inflammation especially in the auto inflammatory response.[23] TNF α is one of a potent pro-inflammatory cytokine which stimulates the secretion of other inflammatory cytokines and also found to be a growth factor for most tumor cells. Induction of proinflammatory genes by TNF α has been linked to most diseases due to its activation through NF-kβ. When exposed to TNF α, NF-kβ up-regulates the expression of many inflammatory genes such as COX-2, lipoxygenase-2 (LOX-2), cell-adhesion molecules, inflammatory cytokines, chemokines, and iNOS.[24] NFκβ is an inducible transcription factor which alters gene expression and activates up-regulation of many cytokines, chemokines, adhesion molecules, survival genes, thus regulates the expression of numerous genes involved in immune and inflammatory responses.[25] The regulation of iNOS, COX-2, and TNF α via the NFκβ pathway plays an important role in inflammation.[26] Thus, the anti-inflammatory agents inhibiting them have a potential therapeutic effect on the treatment of inflammatory and infectious diseases. Hence, many attempts have been made to develop a new generation of anti-inflammatory agents from natural compounds due to their fewer side effects[27] and LN is one among them.
CONCLUSIONS
HepG2 cells when treated with LPS for 24 h increased IL-1β, TNF α, NFκβ, iNOS, and COX2 mRNA expressions. But when HepG2 cells treated 2 h prior with LN followed by LPS for 24 h, decreased the mRNA expression of these genes, confirmed the inhibiting activity of LN. Thus, LN can be useful as a nontoxic, pharmacological active compound especially as antibacterial, hepatoprotective, and anticancer agent.
Further studies is required on the polyphenolic compounds and their mechanisms is involved in the regulation of inflammatory genes.
ACKNOWLEDGEMENT
We would like to thank Dr. Nicholas Oberlies, University of Washington for providing Silymarin as a kind gift.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–14. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Wang JE, Jørgensen PF, Almlöf M, Thiemermann C, Foster SJ, Aasen AO, et al. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect Immun. 2000;68:3965–70. doi: 10.1128/iai.68.7.3965-3970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, et al. Sepsis in cirrhosis: Report on the 7 th meeting of the International Ascites Club. Gut. 2005;54:718–25. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: Analysis of the National Hospital Discharge Survey. Chest. 2003;124:1016–20. doi: 10.1378/chest.124.3.1016. [DOI] [PubMed] [Google Scholar]
- 6.Day CP. Alcohol and the liver. Medicine. 2007;35:22–5. [Google Scholar]
- 7.Shah PM. The need for new therapeutic agents: What is the pipeline? Clin Microbiol Infect. 2005;11(Suppl 3):36–42. doi: 10.1111/j.1469-0691.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 8.Remington S. 17th ed. Eastan, PA: Mack Publishing Co; Pharmaceutical Sciences. [Google Scholar]
- 9.Durairaj A, Vaiyapuri TS, Mazumder UK, Gupta M. Protective activity and antioxidant potential of Lippia nodiflora extract in paracetamol induced hepatotoxicity in rats. Iran J Pharmacol Ther. 2008;7:83–9. [Google Scholar]
- 10.Anithachristy SA, Arunmani M, Evelyn K. Hepato-protective activity of Vitex negundo Linn against CCL4. Int J Appl Biol. 2014;4:61–6. [Google Scholar]
- 11.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 12.Kokate CK. 4th ed. New Delhi: Vallabh Prakasan; 2005. Practical Pharmacognosy; pp. 107–9. [Google Scholar]
- 13.Zare Z, Majd A, Sattari TN, Iranbakhsh A, Mehrabian S. Antimicrobial activity of leaf and flower extracts of Lippia nodiflora L.(Verbenaceae) J Plant Prot Res. 2012;4:401–3. [Google Scholar]
- 14.Terblanché FC, Kornelius G. Essential oil constituents of the genus Lippia (Verbenaceae)-A literature review. J Essent Oil Res. 1996;8:471–85. [Google Scholar]
- 15.Siddiqui BS, Ahmad F, Sattar FA, Begum S. Chemical constituents from the aerial parts of Lippia nodiflora Linn. Arch Pharm Res. 2007;30:1507–10. doi: 10.1007/BF02977318. [DOI] [PubMed] [Google Scholar]
- 16.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 17.Sassa S, Sugita O, Galbraith RA, Kappas A. Drug metabolism by the human hepatoma cell, Hep G2. Biochem Biophys Res Commun. 1987;143:52–7. doi: 10.1016/0006-291x(87)90628-0. [DOI] [PubMed] [Google Scholar]
- 18.Stacey NH, Klaassen CD. Comparison of the effects of metals on cellular injury and lipid peroxidation in isolated rat hepatocytes. J Toxicol Environ Health. 1981;7:139–47. doi: 10.1080/15287398109529965. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Kim DH, Baek SH, Lee HJ, Kim MR, Kwon HJ, et al. Rengyolone inhibits inducible nitric oxide synthase expression and nitric oxide production by down-regulation of NF-kappaB and p38 MAP kinase activity in LPS-stimulated RAW 264.7 cells. Biochem Pharmacol. 2006;71:1198–205. doi: 10.1016/j.bcp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Roland CR, Naziruddin B, Mohanakumar T, Flye MW. Gadolinium chloride inhibits Kupffer cell nitric oxide synthase (iNOS) induction. J Leukoc Biol. 1996;60:487–92. doi: 10.1002/jlb.60.4.487. [DOI] [PubMed] [Google Scholar]
- 21.Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: Independence from COX-2 protein expression. Clin Cancer Res. 1997;3:1679–83. [PubMed] [Google Scholar]
- 22.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–61. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Guadagni F, Ferroni P, Palmirotta R, Portarena I, Formica V, Roselli M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: An early target in anticancer therapeutic strategy. In Vivo. 2007;21:147–61. [PubMed] [Google Scholar]
- 25.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 26.Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935–50. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


