Abstract
Background:
The Antarctic moss Sanionia uncinata (Hedw.) Loeske has shown high ultraviolet (UV)-absorbers content after exposition to high levels of UV-B radiation and can be an important source of antioxidants.
Objective:
The aim was to investigate photoprotection and mutagenicity by the aqueous extract (AE) and hydroethanolic extract (HE) from the Antarctic moss S. uncinata.
Materials and Methods:
Photoprotective activities were determined through survival curves of Escherichia coli strains, after UV irradiation in an aqueous solution of thymine and in vitro sun protection factor (SPF). The Salmonella/microsome assays were applied to assess the mutagenicity.
Results:
Both extracts induced photoprotection against UV-C radiation. The AE showed a higher protection than the hydroethanolic one against UV-induced thymine dimerization. The SPFs were low in both extracts. In association to benzophenone-3 a significant increase in the SPF was detected for the AE, and a significant decrease was induced by the HE. No mutagenicity was found in the both extracts. Furthermore, it was observed absence of cytotoxicity.
Conclusion:
Water-extractable compounds seem to contribute on photoprotection of this Antarctic moss.
Keywords: Moss, mutagenicity, photoprotection, sun protection factor, thymine-dimer inhibition
INTRODUCTION
Bryophytes have simple structures, with leaves formed by thin-layer cells without protective structures such as cuticles or epidermal layers. Hence, they are especially susceptible to ultraviolet-B (UV-B) damage.[1] When exposed to intense UV radiation Antarctic mosses produce more of certain secondary metabolites, as antioxidants, UV-chromophore containing compounds and stimulators of DNA repair processes, which protect their biological systems against UV radiation.[2,3] In particular, Antarctic moss Sanionia uncinata (Hedw.) Loeske also showed increasing in level production of photoprotective pigments under ozone-depleted conditions.[4] Moreover, no increase in cyclobutane pyrimidine dimers was detected in its genome, suggesting good acclimatization to environmental stresses via DNA repair or by increasing synthesis of UV-chromophores.[5]
Responses to in vitro antioxidant activity have been correlated to the phenolic content in a hydroethanolic extract (HE) of S. uncinata collected from Antarctica, suggesting that this moss could be an important natural source of antioxidants for applications in medicine and cosmetics.[6]
The aqueous extract (AE) and HE of Antarctic moss S. uncinata have shown to protect plasmidial DNA against cleavage induced by reactive oxygen species. However, both extracts induced DNA cleavage by Fenton-like reactions, indicating that formulations involving metal ions must be strictly avoided in the direct use of these extracts.[7]
Photoprotective effects are an important subject of research for natural products that can be employed in cosmetic and pharmaceutical products against UV-induced DNA damage.[8] However, given the number of plants that contain mutagenic, carcinogenic and teratogenic compounds, it is important to evaluate the toxicological activities.[9] In the present study, we investigated photoprotective and mutagenic activities in the AE and HE of S. uncinata.
MATERIALS AND METHODS
Moss material
Sanionia uncinata specimen was collected from King George Island, Antarctic Peninsula in February 2004. The sampling site was described before.[7] The S. uncinata was identified by Dr. Denise Pinheiro da Costa of the Botanical Garden Research Institute of Rio de Janeiro (Brazil) and a voucher specimen was deposited (Herbarium of the State University of Rio de Janeiro, Brazil, H-RJ 11,811).
Preparation of extracts
Moss material was extracted by boiling for 15 min consecutively with 300 mL water and 300 mL 70% v/v ethanol. The extracts were filtered and centrifuged, and then the supernatant was sterilized by membrane filtration (0.22 μm Nylon membrane). The ethanol was evaporated under 300 mTorr pressures, and both extracts were further freeze-dried. Aliquots were freshly prepared and sterilized by membrane filtration immediately prior to assays, which were done in triplicate and repeated twice.
Bacterial cells
Exponential growth cultures of Escherichia coli AB1157 (wild-type nucleotide excision repair [NER] proficient), AB1884 (uvrC), AB1885 (uvrB), and AB1886 (uvrA) until 2 × 108 cells/mL were obtained at 37°C in Luria-Bertani broth (LB, 0.45% yeast extract, 0.9% tryptone, 0.9% NaCl) containing 100 μg/mL streptomycin. Cell concentration was adjusted by colorimetric calibration curve and plating method after dilution. Stationary growth cultures of Salmonella typhimurium TA97, TA98, TA100, TA102, and TA104 with 2 × 109 cells/mL were obtained at 37°C in LB containing 8 μg/mL ampicillin and 2 μg/mL tetracycline (alone TA102). All strains used in the present work are from our laboratory collection.
Phototoxicity inhibition
The E. coli cells were centrifuged, washed twice and resuspended with the same volume of 0.9% NaCl. A 24-well microplate with 100 μL/well-bacterial suspension and 100 μL/well AE or HE, at 2 mg/mL in water, was irradiated under the G30T8UV germicidal lamp (254 nm) at 1.17 J/m2/s, which was measured with a Latarjet UV dosimeter (Institut Curie, Paris). To determine cell viability after irradiation, cells were successively diluted at 1:10 in 0.9% NaCl and 100 μL of each dilution were plated (0.8% Oxoid#2, 1.5% agar). Thereafter, the plates were incubated at 37°C for 24 h and colony forming units (CFU) were counted.
Inhibition of ultraviolet-C induced thymine dimerization
A 24-well microplate with 13, 72 or 173 μg/mL AE or HE in water (100 μL) and 100 μL of 1 μg/mL thymine (Sigma-Aldrich) in water were placed in a liquid nitrogen bath for freezing and irradiated (30 min, 1.17 J/m2/s) under a G30T8UV germicidal lamp (254 nm). After irradiation, the mixtures were thawed in the dark, and the UV spectra were recorded in a spectrophotometer. Inhibition efficiency (E %) was calculated considering absorbances at 250, 260, and 270 nm: AM, reaction mixture after irradiation; AD and ATHY, of the irradiated and nonirradiated thymine solution.
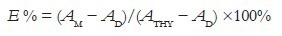
In the presence of the extracts, their contributions on absorption intensities were subtracted.
Sun protection factor
The sun protection factor (SPF) was determined by in vitro with isopropanol solutions.[10,11] In a 96-well microplate, the absorbance values of 200 μL of each extract (2 mg/mL) or benzophenone-3 (BP-3, Pharma Nostra, Brazil) at 0.1 μL/mL (positive control) were analyzed at290-320 nm at 5-nm intervals in a microplate reader (μ-Quant, Bio-tek, USA). In another test, 100 μL AE or HE and 100 μL BP-3 (0.05 μL/mL) were mixed in a 96-well microplate and absorbance values were determined. The concentration of BP-3 in the latter test was lower than in the first in order to detect synergistic effects. The SPF was calculated considering, for each wavelength λ:[10] Erythmogenic effect of radiation [E(λ)]; sunlight intensity [I(λ)]; absorbance [A(λ)]. Values of E(λ) multiplied by I(λ) are constants determined by Sayre et al.[12]
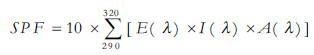
Salmonella/microsome assays
The extracts were screened for mutagenicity and cytotoxicity using a spot test procedure for the TA97, TA98, TA100, TA102, and TA104 strains without (−S9) and with (+S9) exogenous metabolization (Molecular Toxicology Inc., Boone).[13] From these results, it was performed the preincubation Salmonella/microsome assay procedure.[13] Salmonella typhimurium TA98, TA102, and TA104 strains (100 μL of culture) were then preincubated (20 min, 37°C, 150 rpm) with sample dilutions (100 μL) and 500 μL of sodium-phosphate buffer (0.2 M, pH 7.4) or of exogenous metabolizing fraction (S9 at 4% w/v).[13] About 2 mL top agar was included, and the mixture was poured onto plates containing minimal agar, following incubation (37°C, 72 h) and histidine revertant colonies (His+) were counted.[13] Aliquots (10 μL) from the Salmonella/microsome assay, after preincubation period, were diluted (1:105) in 0.9% NaCl and 100 μL of the final dilution was plated on nutrient agar. CFU per plate was counted after incubation at 37°C for 24 h.
RESULTS
Protection against ultraviolet-induced toxicity
To evaluate the protective effects of the extracts against UV-induced toxicity, survival curves NER-deficient and proficient E. coli were used. All values obtained with or without the extracts were different from the non-irradiated control (P < 0.01, Dunnett) [Figure 1]. The extracts did not affect the survival rate of the wild-type NER-proficient when they were exposed to UV radiation until a dose of 23 J/m2 (data not shown). However, at higher doses, in which UV radiation significantly decreases viability, significant (P < 0.01, Bonferroni) photoprotective effects of the both extracts were observed [Figure 1a].
Figure 1.
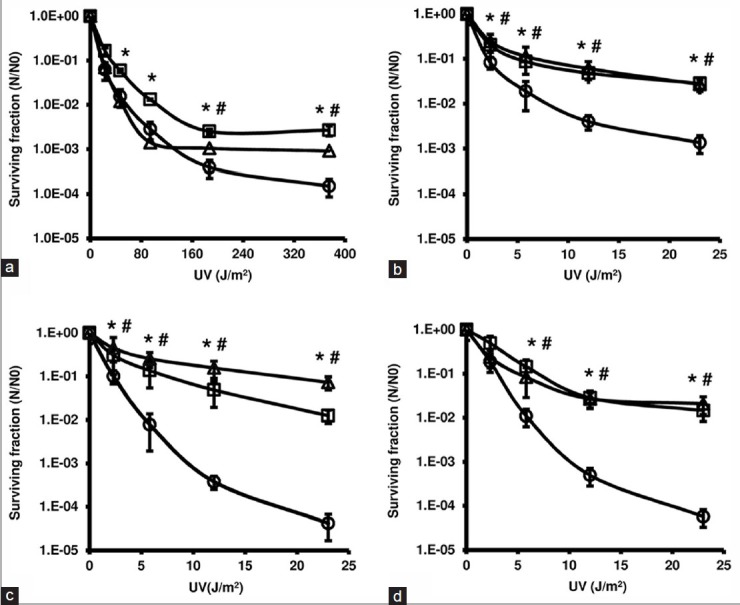
Logarithmic survival curves of the nucleotide excision repair (NER)-proficient (a: AB1157) and NER-deficient (b: AB1884; c: AB1885; d: AB1886) Escherichia coli strains at exponential growth phase after UV-C exposure, in the absence (○) or the presence of 1 mg/mL aqueous extract (AE) (□) and hydroethanolic extract (HE) (Δ). Significant differences (P < 0.01, ANOVA and Bonferroni) relative to the respective curves without extracts are indicated (*AE and #HE)
Except for the lowest dose of the AB1886 strain [Figure 1d], surviving fractions of NER-deficient strains were significantly (P < 0.01, Bonferroni) higher in the presence of the extracts, indicating significant photoprotective effects [Figure 1b-d].
Activity against ultraviolet-inducing pyrimidine dimerization
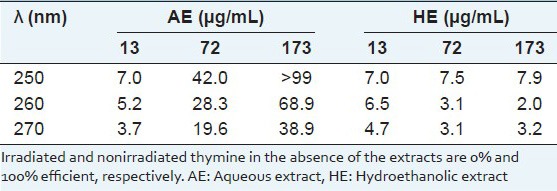
To evaluate the activities against UV-inducing pyrimidine dimerization, solutions of thymine after UV-C exposition over frozen state were used in the absence and presence of AE and HE. Both extracts significantly (P < 0.05, t-Student) prevented the cyclization (pyrimidine dimerization) of the thymine [Table 1]. The inhibition efficiency showed a linear dose–response relationship with the AE dose and intercepted the origin at correlation coefficient > 0.98. For 72 and 173 μg/mL the HE induced much lower photoprotection than the AE.
Table 1.
Inhibition efficiency (E%) by Sanionia uncinata extracts on the UV-induced thymine dimerization
Sun protection factor
Sun protection factor was determined in vitro according to the spectrophotometric method in order to evaluate protection efficacy of the extracts.[10,11] The SPF values obtained were 1.10 ± 0.23 to AE and 1.11 ± 0.18 to HE and do not differ statistically among themselves (P > 0.05, t-Student). These SPF values are much lower than that for the positive control (18.00 ± 1.14). In association with BP-3 the AE significantly (P < 0.05, t-Student) increase in the SPF from 11.00 ± 0.46 (BP-3 alone) to 14.70 ± 0.10. However, the HE induced decrease in the SPF reaching 8.16 ± 0.06 for HE (P < 0.05, t-Student).
Salmonella/microsome
The mutagenic activity of the extracts was investigated through Salmonella/microsome assays, primarily by the spot-test and further after preincubation. Once in the spot test only TA98 and TA102 strains showed positive results for mutagenicity and cytotoxicity (data not shown), quantitative reversion induction was investigated further only with TA98, TA102, and TA104 using the preincubation procedure [Tables 2 and 3].
Table 2.
Number of Salmonella typhimurium revertant colonies per plate with the aqueous extract of Sanionia uncinata (AE) in the absence (–) and in the presence (+) of S9
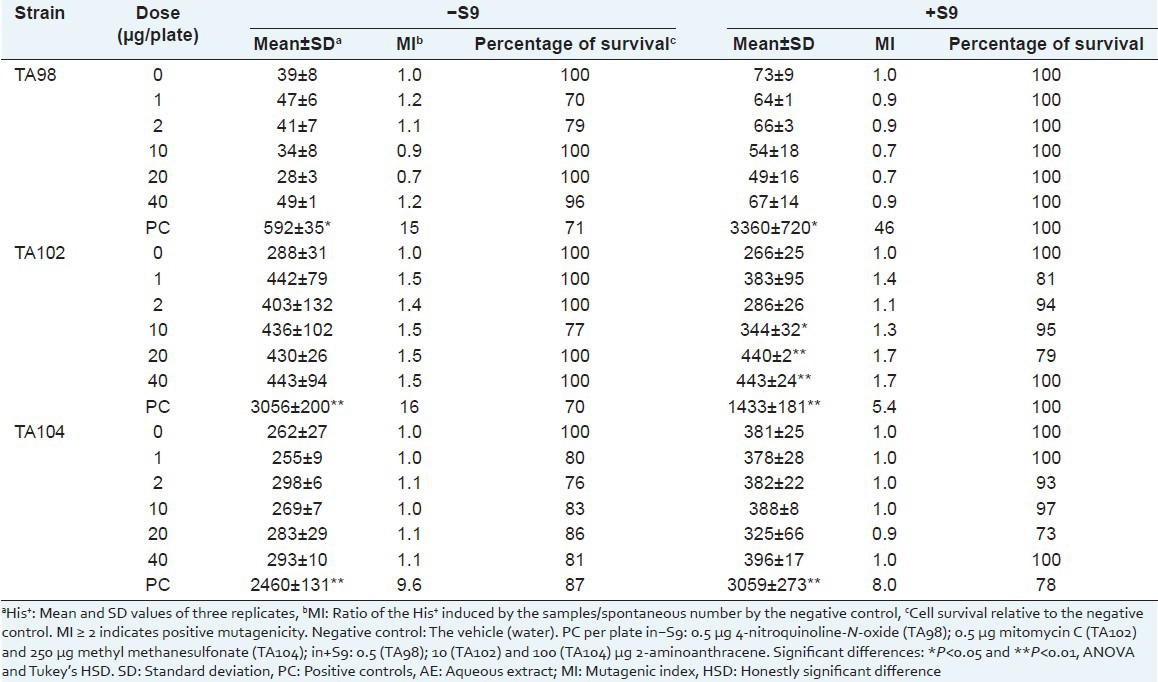
Table 3.
Number of Salmonella typhimurium revertant colonies per plate with the hydroethanolic extract of Sanionia uncinata (HE) in the absence (–) and in the presence (+) of S9
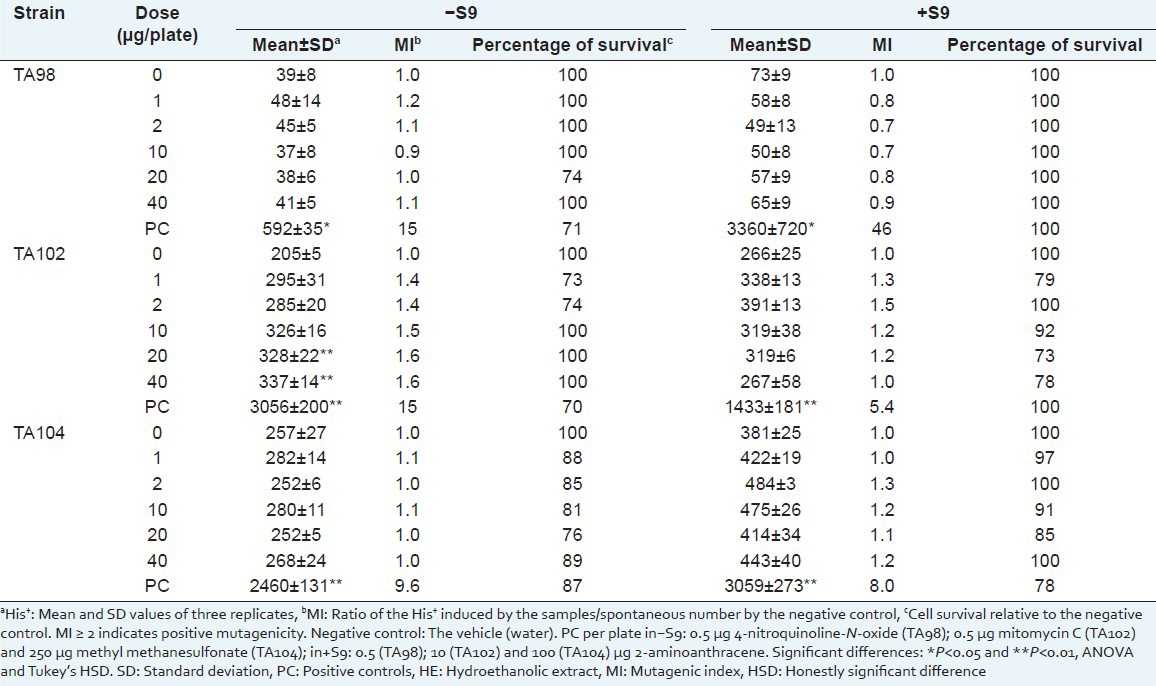
To evaluate the cytotoxic effects of the AE and HE, cell viability was evaluated by survival fraction determination [Tables 2 and 3]. No mutagenic activity (mutagenic index <2) was observed for the extracts.
DISCUSSION
Antarctic mosses have acclimated through the development of photoprotective mechanisms avoiding and repairing UV-B damage in order to survive under stratospheric ozone depletion.[1] In fact, S. uncinata moss has been particularly important for studies in photoprotection aiming cosmetic and pharmaceutical applications.[4,5,6]
In general, compounds could lead to UV-protection while also inducing photocytotoxicity. In this case, this effect would not be identified on survival curves when the NER system is active. NER-deficient E. coli strains (AB1184, AB1885, and AB1886) are more sensitive by UV-induced damage and NER pathway is able to repair about 20 types of DNA damage. The proteins associated with NER act to repair DNA damage.[14] However, both extracts also induced photoprotection against UV-C radiation in the NER-deficient E. coli strains, suggesting that the extracts do not present photocytotoxicity, and protect the bacterial system under UV-C radiation.
The DNA molecule directly absorbs the UV light energy due to the nature of the chemical bonds and most photoproducts are expected to be produced, mainly cyclobutane pyrimidine dimers.[15] It is known that aqueous solutions of thymine at room temperature present absorption near 260 nm and that significant dimerization by UV irradiation does not occur. However in a frozen state, thymine molecules are spatially oriented, allowing pyrimidine dimerization under UV-C by [2 + 2] cyclization. It is known that the thymine base absorbs UV-C radiation with high efficiency, promoting its dimerization.[16] Both extracts allowed similar UV light intensity to pass through them, such as 47% or 50% transmittance at 260 nm when AE and HE, respectively were used at 173 μg/mL.
The extracts prevented dimer formation by UV-irradiation on a frozen solution of thymine and the AE showed greater inhibition efficiency than the HE [Table 1]. Thus, the photoprotection observed for the AE seems to follow a pathway beyond the physical barrier by light absorption. Therefore, it appears that water-soluble components of this Antarctic moss contribute strongly to photoprotection in UV-induced cyclobutane pyrimidine dimerization, protecting the genome.
The SPF designs the sunscreen efficacy that corresponds to the ratio between the UV energies necessary to induce a minimal erythema on treated and on untreated skin.[17] The spectrophotometric method to determine in vitro SPF is proposed for the control of sun preparations and can provide preliminary information to develop formulations.[11] It has been found application for this method in sunscreen predictive before performing in vivo tests on humans thus reducing the risks related to UV exposure and is useful as an in vivo measure technique.[10,18]
Our results showed that both extracts presented very low SPF values (<1.1 at 2 mg/mL) when comparing to BF-3, a known sunscreen (16-fold higher levels at a 20-fold lower concentration). However, a significant and positive synergistic effect between AE and BP-3 was observed. The HE induced a decrease in the SPF in the association with the BP-3 probably antagonizing the photoprotective effect of this sunscreen. The SPF value obtained from AE/BF-3 differs statistically (P < 0.05, t-Student) from SPF value of HE/BF-3. Synergism has been widely reported as with topical vitamin C/vitamin E against photoaging.[19] Studies about in vitro SPF in the Antarctic moss S. uncinata have not been reported so far, even though they can be found in medicinal plants.[20]
In the preliminary evaluation of induction of bacterial reversion and toxicity, using the spot test, the S. uncinata extracts induced changing in the His+ of TA98 and TA102. Based on these results, Salmonella typhimurium TA98 and TA102 were selected to a bacterial reverse mutation assay applying the preincubation procedure. TA104 was included because it is genetically similar to TA102.[13] No mutagenic activity was found for a frameshift mutation (TA98 strain) and transition/transversion mutation (TA102 and TA104 strains) in the both extracts. Furthermore, it was observed absence of cytotoxicity.
CONCLUSION
The extracts from the Antarctic moss S. uncinata show promise for future applications once they presented photoprotective properties without inducing point mutation. The water-extractable compounds contributed better than the compounds extracted by 70% ethanol, mainly against the pyrimidine dimerization and for a favorable synergism in a sunscreen efficacy.
ACKNOWLEDGMENTS
Thanks to Dr. Denise Pinheiro da Costa for the identification of the moss. Thanks are due to PROANTAR (CNPq 556971/2009-4) and INCT-Criosfera for allowing sampling in Antarctica. This research was funded by the CNPq and by the FAPERJ (E26/111-158/2011).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Robinson SA, Wasley J, Tobin AK. Living on the edge: Plants and global change in continental and maritime Antarctica. Glob Change Biol. 2003;9:1681–717. [Google Scholar]
- 2.Lovelock CE, Robinson SA. Surface reflectance properties of Antarctic moss and their relationship to plant species, pigment composition and photosynthetic function. Plant Cell Environ. 2002;25:1239–50. [Google Scholar]
- 3.Pereira BK, Rosa RM, da Silva J, Guecheva TN, Oliveira IM, Ianistcki M, et al. Protective effects of three extracts from Antarctic plants against ultraviolet radiation in several biological models. J Photochem Photobiol B. 2009;96:117–29. doi: 10.1016/j.jphotobiol.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Newsham KK, Hodgson DA, Murray WA, Peat HJ, Lewis Smith RI. Response of two Antarctic bryophytes to stratospheric ozone depletion. Glob Change Biol. 2002;8:972–83. [Google Scholar]
- 5.Lud D, Moerdijk TC, van de Poll WH, Bruma AG, Huiskes AH. DNA damage and photosynthesis in Antarctic and Arctic Sanionia uncinata (Hedw.) Loeske under ambient and enhanced levels of UV-B radiation. Plant Cell Environ. 2002;25:1579–89. [Google Scholar]
- 6.Bhattarai HD, Paudel B, Lee HS, Lee YK, Yim JH. Antioxidant activity of Sanionia uncinata, a polar moss species from King George Island, Antarctica. Phytother Res. 2008;22:1635–9. doi: 10.1002/ptr.2538. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes AS, Mazzei JL, De Alencar AS, Evangelista H, Felzenszwalb I. Effects of Sanionia uncinata extracts in protecting against and inducing DNA cleavage by reactive oxygen species. Redox Rep. 2011;16:201–7. doi: 10.1179/1351000211Y.0000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alluis B, Dangles O. Acylated flavone glucosides: Synthesis, conformational investigation and complexation properties. Helv Chim Acta. 1999;82:2201–12. [Google Scholar]
- 9.Horn RC, Vargas VM. Mutagenicity and antimutagenicity of teas used in popular medicine in the Salmonella/microsome assay. Toxicol In Vitro. 2008;22:1043–9. doi: 10.1016/j.tiv.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Mansur JS, Breder MN, Mansur MC, Azulay RD. Determination of Sun protection factor by spectrophotometric methods. An Bras Dermatol. 1986;61:121–4. [Google Scholar]
- 11.Kaur CD, Saraf S. Photochemoprotective activity of alcoholic extract of Camellia sinensis. Int J Pharm. 2011;7:400–4. [Google Scholar]
- 12.Sayre RM, Agin PP, LeVee GJ, Marlowe E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem Photobiol. 1979;29:559–66. doi: 10.1111/j.1751-1097.1979.tb07090.x. [DOI] [PubMed] [Google Scholar]
- 13.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455:29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 14.Lage C, Gonçalves SR, Souza LL, De Pádula M, Leitão AC. Differential survival of Escherichia coli uvrA, uvrB, and uvrC mutants to psoralen plus UV-A (PUVA): Evidence for uncoupled action of nucleotide excision repair to process DNA adducts. J Photochem Photobiol B. 2010;98:40–7. doi: 10.1016/j.jphotobiol.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Füchtbauer W, Mazur P. Kinetics of the ultraviolet-induced dimerization of thymine in frozen solutions. Photochem Photobiol. 1966;5:323–35. [Google Scholar]
- 17.Kaur CD, Saraf S. in vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacognosy Res. 2010;2:22–5. doi: 10.4103/0974-8490.60586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos EP, Freitas ZM, Souza KR, Garcia S, Vergnanini A. in vitro and in vivo determinations of sun protection factors of sunscreen lotions with octylmethoxycinnamate. Int J Cosmet Sci. 1999;21:1–5. doi: 10.1046/j.1467-2494.1999.181658.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin JY, Selim MA, Shea CR, Grichnik JM, Omar MM, Monteiro-Riviere NA, et al. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol. 2003;48:866–74. doi: 10.1067/mjd.2003.425. [DOI] [PubMed] [Google Scholar]
- 20.Khazaeli P, Mehrabani M. Screening of sun protective activity of the ethyl acetate extracts of some medicinal plants. Iran J Pharm Res. 2008;7:5–9. [Google Scholar]


