Abstract
Background:
Ethanol causes hepatic cellular damage by alterations in biological functions. This study evaluated the hepatoprotective potential of the methanolic extract originating from Firmiana simplex (Sterculiaceae) stem bark against the ethanol-induced hepatotoxicity in rat primary hepatocytes.
Materials and Methods:
The extract of F. simplex stem bark was successively fractionated into n-hexane, chloroform, ethyl acetate (EtOAc), and n-butanol. Column chromatography with silica gel and sephadex LH-20 was used to isolate the EtOAc fraction. Rat primary hepatocytes were cultured to study the hepatoprotective activity of isolated substances against ethanol-induced toxicity. Intracellular reactive oxygen species (ROS) levels, the antioxidant activities of glutathione reductase (GR) and glutathione peroxidase (GSH-PX) enzymes, and the GSH content were measured to examine the antioxidative property of the isolated compounds.
Results:
Two flavonoid glycosides, quercitrin (1) and tamarixetin 3-O-rhamnopyranoside (2), were isolated from the active EtOAc fraction. Compound 1 significantly protected rat primary hepatocytes against ethanol-induced oxidative stress by reducing the intracellular ROS level and preserving antioxidative defense systems such as GR, GSH-PX, and total GSH.
Conclusion:
This is the first report on the hepatoprotective activities of the extract of F. simplex. The EtOAc fraction of F. simplex stem bark and its major constituent quercitrin (1) could function as hepatoprotective agents to attenuate the development of alcoholic liver disease.
Keywords: Ethanol, Firmiana simplex, hepatoprotective, primary rat hepatocytes, quercitrin
INTRODUCTION
Alcoholic liver disease (ALD) onset is mainly caused by excessive consumption of alcohol.[1] In the oxidative pathways of alcohol metabolism, ethanol is converted into acetaldehyde and then further metabolized to acetic acid. Intracellular reactive oxygen species (ROS) and adducts are additionally generated in this process, and they induce oxidative stress in hepatocyte cells and eventually cause hepatic damages.[2,3] Development of new substances that have hepatoprotective effects against ethanol-induced liver toxicity is a prevailing strategy for preventing the progression of alcoholic liver impairment.[4] Thus, we searched for hepatoprotective phytochemicals that could reduce hepatic damages caused by ethanol insult. We used primary cultured rat hepatocytes as an in vitro screening tool useful for studying the liver metabolism of xenobiotics.[5,6]
While searching for hepatoprotective agents in natural products, the methanolic extract of stem bark from Firmiana simplex was found to show protective activity against ethanol-treated primary rat hepatocytes. F. simplex (L.) W. Wight, commonly called the Chinese parasol tree, is a deciduous tree from the family of Sterculiaceae. It is native to the Asian region and grown as an ornamental in North America. This plant has been traditionally used as medicine in Korea for treatment of diarrhea and stomach disorders.[7,8] A study on the chemical composition of F. simplex revealed that it contains coumarins, lignans, and flavonoids.[9] However, biological study on the activities of F. simplex constituents has not been reported. In this study, we isolated bio-active compounds from the methanolic extract of F. simplex stem bark using column chromatography and investigated their hepatoprotective effects in primarily cultured ethanol-intoxicated rat hepatocytes.
MATERIALS AND METHODS
General experimental procedures
For the isolation of compounds from F. simplex, all organic solvents such as methanol (MeOH), n-hexane, chloroform (CHCl3), ethyl acetate (EtOAc), and n-butanol (n-BuOH) were obtained from Dae Jung Pure Chemicals and Metals (Korea). Thin layer chromatography was carried out on a pre-coated silica gel plate (Kieselgel, Merck, NJ, USA) and a Kieselgel 60 silica gel from Merck was used for column chromatography in this experiment. Bruker GP ×400 nuclear-magnetic resonance (NMR) spectrometer (400 MHz) was used to record NMR spectra. Sephadex LH-20 was used with bead size 25–100 μm (Pharmacia, Sweden). For the cell culture, ethylenediaminetetraacetic acid, pronase E, collagenase type IV, trypan blue, ethanol, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium (MTT), and dimethylsulfoxide were purchased from Sigma Chemical Co. (St. Louis, MO, USA), and Hank's balanced salt solution was purchased from Gibco Co. (Grand Island, NY, USA). Collagen type I for dish coating was purchased from BD (Becton, Dickinson and Company) (Franklin Lakes, NJ, USA).
Plant material
The stem bark of F. simplex was obtained in October 2010 from Seoul National University Medicinal Plant Garden, belonging to SNU College of Pharmacy and locating in Goyang, Korea; the plant was air-dried. The voucher specimen (SNUPH-11203) was deposited at the Herbarium in the Medicinal Plant Garden, Seoul National University.
Extraction and isolation
The dried stem bark (2.0 kg) of F. simplex was extracted with MeOH (with 12 L for 90 min, 3 times) in an ultrasonic apparatus. Once the solvent was removed in vaccuo, this extract (874.0 g) was suspended in water and partitioned into n-hexane (55.8 g), CHCl3 (39.5 g), EtOAc (7.8 g), and with n-BuOH (37.1 g). Among these fractions, the EtOAc fraction was subjected to silica gel column chromatography with a mixture of (CHCl3–MeOH = 50:1, 1:1) to obtain ten fractions (E1–E10). Compound 1 (1.0 g) was isolated from E8 by recrystallization. Compound 2 (40.0 mg) was obtained from E5 with Sephadex LH-20 (MeOH).
Compound 1
1H NMR (CD3OD, 400 MHz); d 7.32 (1H, dd, J = 8.4 and 2.0 Hz, H-6’), 7.28 (1H, d, J = 2.0 Hz, H-2’), 6.88 (1H, d, J = 8.3 Hz, H-5’), 6.35 (1H, d, J = 2.0 Hz, H-8), 6.18 (1H, d, J = 2.0 Hz, H-6), 5.34 (1H, J = 1.7 Hz, rha H-1”), 3.29–3.45 (4H, m, rha H-2”,3”,4”,5”), 0.92 (3H, J = 6.0 Hz, rha CH3).
Compound 2
1H NMR (CD3OD, 400 MHz); d 7.32 (1H, dd, J = 8.4 and 2.0 Hz, H-6’), 7.28 (1H, d, J = 2.0 Hz, H-2’), 6.83 (1H, d, J = 8.3 Hz, H-5’), 6.26 (1H, d, J = 2.0 Hz, H-8), 6.09 (1H, d, J = 2.0 Hz, H-6), 5.28 (1H, J = 1.7 Hz, rha H-1”), 3.84 (3H, s, 3’-OCH3), 3.29–3.45 (4H, m, rha H-2”,3”,4”,5”), 0.82 (3H, J = 6.0 Hz, rha CH3).
High-performance liquid chromatography with diode array detector and electrospray ionization mass spectrometer analysis
The total extract and each fraction of F. simplex stem bark were resolved respectively in MeOH at a concentration of 1 mg/mL. The injection sample was prepared by filtering through a nylon membrane filter (0.2 μm, Millipore, USA). Chromatographic separation was achieved with an Ascentis Express C18 column (4.6 mm i.d. × 150 mm, 2.7 μm) at 30°C column temperature. The high-performance liquid chromatography-diode array detector-mass spectrometer (HPLC-DAD-MS) system consisted of a Finnigan Surveyor MS pump plus (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a Finnigan Surveyor photodiode array detector (Thermo Fisher Scientific, Waltham, MA, USA) and Finnigan LCQ advantage Max ion trap MS (Thermo Fisher Scientific, Waltham, MA, USA). The solvent system was a mixture of 0.1% formic acid (A) and acetonitrile (B) with a flow rate of 0.3 mL/min and an injection volume of 10 μL with a multistep gradient system (0 min 10% (v/v) B, 25 min 90% B, 25.1 min 10% B and 30 min 10% B).
Animals
Male Sprague–Dawley rats were obtained from Koatech Co., Limited (Yongin, Korea) with body weights of 200–250 g, housed in conventional cages (two per cage) at room temperature under a 12 h light-dark cycle and fed ad libitum with free access to water. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Seoul National University and conducted with special care taken to avoid any undue animal pain or suffering.
Culturing of primary rat hepatocytes
Using the collagenase perfusion technique of Berry and Friend, primary rat hepatocytes were isolated from SD male rats.[10] The freshly isolated hepatocytes were suspended in Dulbecco's modified Eagle's Medium with high glucose (Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum (Hyclone, Logan, UT, USA), 1 μM dexamethasone (Sigma, St. Louis, MO, USA), 0.1 μM insulin (Sigma, St. Louis, MO, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma, St. Louis, MO, USA). Cells were inoculated on rat tail collagen-coated 35 mm × 10 mm style culture dishes (Corning, New York, NY, USA) at a density of 2 × 105 cells/mL and maintained in a humidified incubator containing 95% air and 5% CO2 gas at 37°C until cell attachment.
Measurement of hepatoprotective activity on cell viability
To investigate the possible protective activities from the fractions, an appropriate hepatocyte intoxicant model was made in preliminary experiments performed in our lab. Stabilizing the isolated primary rat hepatocytes, the culture medium was replaced 16 h after seeding. Then, the cells were pre-treated with vehicle or the substances of interest for 2 h.[11] Compared to the untreated hepatocytes, 200 mM ethanol exposure for a further 48 h caused a sufficient decrease (71.0% ±4.9%) to evaluate the hepatoprotective activities of the substances in the cultured hepatocytes. The culture dishes containing ethanol treated cells were sealed tightly with parafilm (Bemis, Neenah, WI, USA) to prevent evaporation of the ethanol. The hepatoprotective activity was assessed by MTT assay for the extract, fractions, and compounds. The measurements were represented in percentage of viability of the cells in respect to control cultures as the means from three independent experiments.
Cell viability (% of control) = (Abs. of sample-treated)/(Abs. of control) × 100
Measurement of cellular reactive oxygen species formation
Ethanol-treated primary rat hepatocytes were pre-treated for 2 h with compounds or vehicle alone and then maintained for 24 h. The relative intracellular level of ROS was measured by peroxide sensitive dye 2,7-dichlorofluoresein diacetate (DCF-DA) (Sigma, St. Louis, MO, USA).[12] Cells were incubated with 5 μM of DCF-DA for 40 min followed by two washes in phosphate buffered saline. DCF-DA fluorescence measurements in the cells were done with excitation and emission wavelengths at 485 nm and at 530 nm, respectively. The results were expressed as the mean ± standard deviation (SD) from three independent experiments, each with triplicate wells.
Measurement of antioxidant enzyme activities
Cells from three culture dishes were suspended in 2 mL of 0.1 M phosphate buffer (pH 7.4) and homogenized. This homogenate was centrifuged at 12,000 g for 15 min at 4°C, and its supernatant was collected to measure antioxidant enzyme activities and glutathione (GSH) contents. Total GSH in the supernatant was spectrophotometrically determined with the enzymatic cycling method.[13] The glutathione reductase (GR) activity was measured according to the method of Carlberg.[14] The activity of glutathione peroxidase (GSH-PX) was measured by quantifying the oxidation rate of GSH to oxidized GSH using cumene hydroperoxide.[15] Values shown were the mean ± SD of three independent experiments. Protein concentrations were determined with a bicinchoninic acid kit from Sigma and bovine serum albumin as a standard.[16]
Statistical analysis
ANOVA test was used to evaluate the statistical significance of the acquire data. They were considered significant when the probability was set as 0.05 or lower.
RESULTS AND DISCUSSION
Isolation of fractions from Firmiana simplex stem bark
When compared to other fractions, the EtOAc fraction-from the bioassay-guided fractionation of the methanolic extract of F. simplex stem bark-demonstrated the highest protective activity against ethanol insult to primary rat hepatocytes (79.4 ± 5.8% at 10 μM, 99.0 ± 6.3% at 50 μM, and 101.7 ± 6.1% at 100 μM) [Table 1a]. Further separation of the EtOAc fraction with activity-guided isolation resulted to two flavonoid compounds. The structures of the isolated compounds were identified as the quercitrin (1) and tamarixetin 3-O-rhamnopyranoside (2) by spectroscopic data comparison [Figure 1b].[9]
Table 1.
The protective activities of (a) the total extract, each fraction and (b) the two isolated compounds from F. simplex stem bark against ethanol-induced hepatotoxicity in primary rat hepatocytes
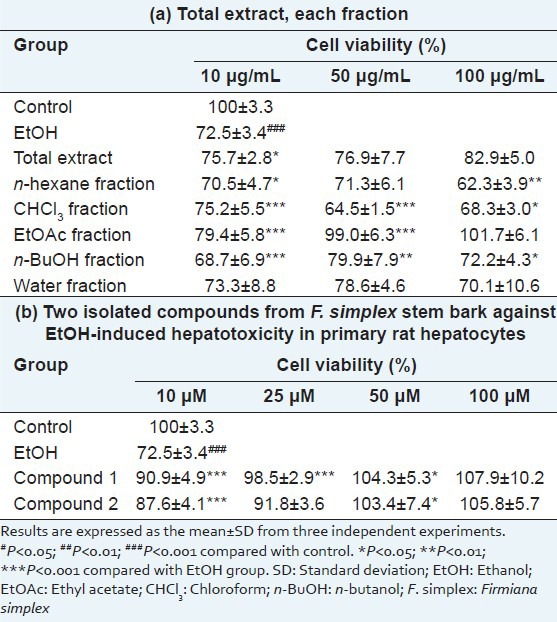
Figure 1.
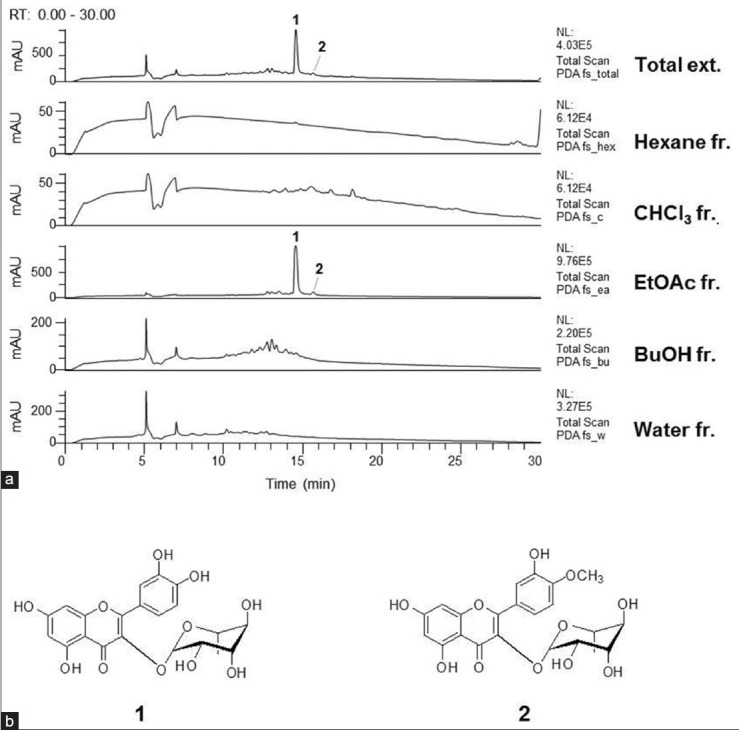
(a) The high-performance liquid chromatography with diode array detector chromatograms (total scan) of the total extract and each fraction of Firmiana simplex stem bark. (b) Structures of compounds 1 and 2 isolated from the ethyl acetate fraction
High-performance liquid chromatography with diode array detector and electrospray ionization mass analysis
The HPLC-DAD-MS analysis showed the presence of compounds 1 and 2 in the total extract of F. simplex stem bark. The corresponding peaks were numbered by comparing the retention times and spectral data of the chromatographic peaks with those of each of the isolated compounds. (Peak 1; Quercitrin (1); [M-H]− =447; [M + H]+ =449, Peak 2; Tamarixetin 3-O-glucopyranoside (2); [M-H]− =461; [M + H]+ =463) [Figure 1a]. The peaks of the two compounds were also observed in the chromatogram of the EtOAc fraction but not in those of the other fractions. In addition, compound 1 was a major constituent of the EtOAc fraction. Therefore, it is suggested that compounds 1 and 2 contribute to the significant hepatoprotective activity of the EtOAc fraction against the ethanol-induced hepatotoxicity in primary rat hepatocytes.
Assessment of hepatoprotective activity
Several flavonoids such as apigenin, quercetin, and rutin have been found to possess hepatoprotective activities.[17] Furthermore, there are several researches about the hepatoprotective effect of quercitrin (1).[18,19,20] However, previous investigations had reported about protective activity of quercitrin against tert-butyl hydroperoxide or CCl4 induced hepatotoxicity, but not against ethanol-induced toxicity. Therefore, we focused on investigating the hepatoprotective activities of compounds 1 and 2 against ethanol-induced toxicity in primary cultured rat hepatocytes. These two flavonoids exhibited the significant hepatoprotective activities in a dose dependent manner [Table 1b]. These results were in accordance with the previous studies that one of the required criteria for cytoprotective effects of flavonoids was the 2,3-double bond conjugation with a 4-oxo-fuction in the C-ring.[6,11,21,22,23] However, the overall increase in cell viability by compound 2 was of a less extent when compared to the effect of compound 1, even with their structural similarity. It seemed that the difference in the hepatoprotective activity of these two compounds could be explained by the presence of the methoxy group at C-4’ in the B-ring.
Antioxidative property
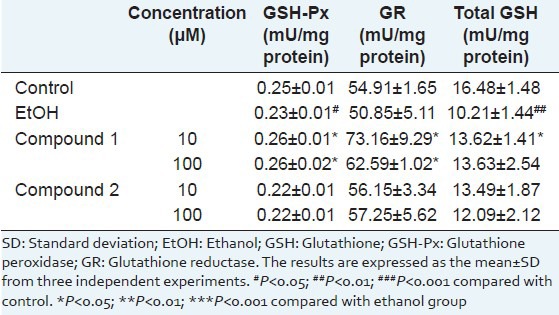
Since ethanol-induced hepatotoxicity is mediated by oxidative stress,[24] effects of compounds 1 and 2 were further studied; the results showed potent hepatoprotective effects on the antioxidative defense system in the ethanol-treated primary rat hepatocytes. Here, the effects of compounds 1 and 2 on cellular peroxide contents were first measured with DCF-DA fluorescence assay [Figure 2]. Compared with the control, cellular peroxide content was significantly increased (P < 0.001) in the ethanol group. The increased intracellular ROS by ethanol insult was decreased by the 2-h pre-treatment of compound 1. In contrast, when compound 2 was used for the 2-h pre-treatment, the increased peroxide level was not reduced. Second, we examined the effects of compounds 1 and 2 on the activities of endogenous antioxidant enzymes GSH-PX and GR and the total GSH content in ethanol-treated primary rat hepatocytes. Under our experimental conditions, ethanol exposure to primary rat hepatocytes caused the depletion of GSH content and decreasing of GR and GSH-PX activities [Table 2]. The decreased activity of GSH-PX caused by ethanol insult was restored by treatment with compound 1 but not by treatment with compound 2. The decreased activity of GR by ethanol insult was recovered by treatment with compounds 1 and 2, respectively. The activities of antioxidant enzymes GR and GSH-PX were strengthened by compound 1 and thus effectively suppressing ethanol-induced GSH depletion. Compound 2 also restored the GSH content to a certain extent, but the degree of recovery was less than that of compound 1. These results show that compound 1 had a more potent antioxidant activity than that of compound 2 despite the structural similarity of the two compounds. It seems that the methylation at 4’-OH in the B-ring affected the decrease in the antioxidant property of compound 1, and this result corresponds to other studies on the structure-activity relationship of flavonoids. Actually, it is known that the 3’,4’-ortho-dihydroxy group in the B-ring and the 5-OH group in the A-ring with a 4-carbonyl group are required for the high antioxidant activity of flavonoids.[21,22] In addition, the presence of the o-catechol group (3’,4’-OH) in the flavonoid-B ring is a determinant for high antioxidant capacities in flavonoids.[25] Thus, it was revealed that compound 1, the major constituent of the EtOAc fraction of F. simplex stem bark, is a significant contributor to the hepatoprotective activity of this plant.
Figure 2.
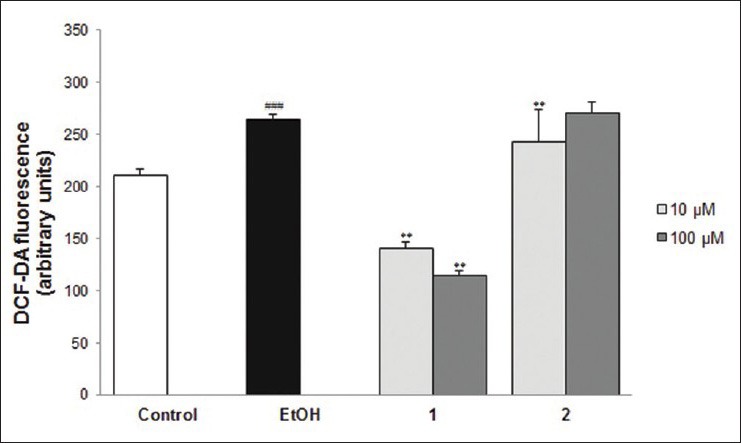
The effects of compounds 1 and 2 on intracellular reactive oxygen species formation in ethanol-treated primary rat hepatocytes. Values represent the mean ± standard deviation from three independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001 compared with control. *P < 0.05, **P < 0.01, ***P < 0.001 compared with ethanol group
Table 2.
The protective effects of compounds 1 and 2 on the activities of the antioxidant enzymes and on the GSH content in EtOH-treated primary rat hepatocytes
CONCLUSIONS
The data in the present study suggest that the EtOAc fraction of F. simplex stem bark exhibits a significant protective activity against the ethanol-induced hepatotoxicity in primary rat hepatocytes. Quercitrin (1), a major bioactive constituent of the EtOAc fraction, dose-dependently showed a potent protective effect against the ethanol-induced hepatotoxicity by alleviating oxidative stress. These results show that the EtOAc fraction of F. simplex and its major constituent quercitrin (1) can be used as a source of natural antioxidant or herbal supplement to prevent the progression of ALD. Further studies on additional biological responses and their molecular pathways are needed to discover the potential benefits of this plant.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Stewart S, Jones D, Day CP. Alcoholic liver disease: New insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408–13. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- 2.Tilg H, Day CP. Management strategies in alcoholic liver disease. Nat Clin Pract Gastroenterol Hepatol. 2007;4:24–34. doi: 10.1038/ncpgasthep0683. [DOI] [PubMed] [Google Scholar]
- 3.Zakhari S. Overview: How is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Park JH, Kim Y, Kim SH. Green tea extract (Camellia sinensis) fermented by Lactobacillus fermentum attenuates alcohol-induced liver damage. Biosci Biotechnol Biochem. 2012;76:2294–300. doi: 10.1271/bbb.120598. [DOI] [PubMed] [Google Scholar]
- 5.Jones CA, Moore BP, Cohen GM, Fry JR, Bridges JW. Studies on the metabolism and excretion of benzo (a) pyrene in isolated adult rat hepatocytes. Biochem Pharmacol. 1978;27:693–702. doi: 10.1016/0006-2952(78)90506-3. [DOI] [PubMed] [Google Scholar]
- 6.Kondeva-Burdina M, Zheleva-Dimitrova D, Nedialkov P, Girreser U, Mitcheva M. Cytoprotective and antioxidant effects of phenolic compounds from Haberlea rhodopensis friv.(Gesneriaceae) Pharmacogn Mag. 2013;9:294–301. doi: 10.4103/0973-1296.117822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae KH. Seoul: KyoHak Publishing Co., Ltd; 2000. Medicinal Plants of Korea. [Google Scholar]
- 8.Hotta M, Ogata K, Nitta A, Hosikawa K, Yanagi M, Yamazaki K. Tokyo: Heibonsha; 1989. Useful Plant of the World. [Google Scholar]
- 9.Son YK, Lee MH, Han YN. A new antipsychotic effective neolignan from Firmiana simplex. Arch Pharm Res. 2005;28:34–8. doi: 10.1007/BF02975132. [DOI] [PubMed] [Google Scholar]
- 10.Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J Cell Biol. 1969;43:506–20. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Hou W, Yao P, Zhang B, Sun S, Nüssler AK, et al. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicol In Vitro. 2010;24:516–22. doi: 10.1016/j.tiv.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Aranda A, Sequedo L, Tolosa L, Quintas G, Burello E, Castell JV, et al. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol In Vitro. 2013;27:954–63. doi: 10.1016/j.tiv.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 14.Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975;250:5475–80. [PubMed] [Google Scholar]
- 15.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–21. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 16.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: An overview 2013. 2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong CO, Rhee CH, Won NH, Choi HD, Lee KW. Protective effect of 70% ethanolic extract of Lindera obtusiloba Blume on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem Toxicol. 2013;53:214–20. doi: 10.1016/j.fct.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Hubert DJ, Dawe A, Florence NT, Gilbert KD, Angele TN, Buonocore D, et al. in vitro hepatoprotective and antioxidant activities of crude extract and isolated compounds from Ficus gnaphalocarpa. Inflammopharmacology. 2011;19:35–43. doi: 10.1007/s10787-010-0070-4. [DOI] [PubMed] [Google Scholar]
- 20.Tian L, Shi X, Yu L, Zhu J, Ma R, Yang X. Chemical composition and hepatoprotective effects of polyphenol-rich extract from Houttuynia cordata tea. J Agric Food Chem. 2012;60:4641–8. doi: 10.1021/jf3008376. [DOI] [PubMed] [Google Scholar]
- 21.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–84. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 22.Seyoum A, Asres K, El-Fiky FK. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry. 2006;67:2058–70. doi: 10.1016/j.phytochem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Cho N, Choi JH, Yang H, Jeong EJ, Lee KY, Kim YC, et al. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem Toxicol. 2012;50:1940–5. doi: 10.1016/j.fct.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:11–6. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- 25.Mendes AP, Borges RS, Neto AM, de Macedo LG, da Silva AB. The basic antioxidant structure for flavonoid derivatives. J Mol Model. 2012;18:4073–80. doi: 10.1007/s00894-012-1397-0. [DOI] [PubMed] [Google Scholar]


