Abstract
Background:
Yuanhu Zhitong prescription (YZP) is a famous traditional Chinese medicine formula, which is officially recorded in Chinese Pharmacopoeia for the treatment of stomach pain, hypochondriac pain, headache and dysmenorrhea caused by qi-stagnancy and blood stasis. It is the first report for the simultaneous determination of 12 active components in YZP.
Objective:
A newly, simple, accurate and reliable method for the separation and determination of 12 active components (protopine, α-allocryptopine, coptisine, xanthotol, palmatine, dehydrocorydaline, glaucine, tetrahydropalmatine, tetrahydroberberine, imperatorin, corydaline, isoimperatorin) in YZP was developed and validated using HPLC-PAD.
Materials and Methods:
The analytes were performed on a Phenomenex Luna-C18 (2) column (250×4.6 mm, 5.0 μm) with a gradient elution program using a mixture of acetonitrile and 0.1% phosphoric acid water solution (adjusted with triethylamine to pH 5.6) as mobile phase. Analytes were performed at 30°C with a flow rate of 1.0 mL/min.
Results:
The validated method was applied to analyze four major dosage forms of YZP coming from different manufacturers with good linearity (r2, 0.9981~0.9999), precision (RSD, 0.24~2.89%), repeatability (RSD, 0.15~3.34%), stability (RSD, 0.14~3.35%), recovery (91.13~110.81%) of the 12 components.
Conclusion:
The proposed method enables the separation and determination of 12 active components in a single run for the quality control of YZP.
Keywords: Angelica dahurica, Corydalis yanhusuo, HPLC, quality control, Yuanhu Zhitong
INTRODUCTION
Traditional Chinese medicines (TCMs) are playing an important role (accounts for 20%) in Chinese health care system, and will have prospect future all over the world due to their reliable therapeutic efficacy.[1,2] Modern researches have shown that TCMs and their formulas contained a plenty variety of active chemical components. It is believed that the characteristics of TCMs are their systematism, multi-target and multi-channel due to the joint contribution of their multiple active components.[3] It is the characteristics of TCMs that make quality control become the key problem for the development of TCMs. However, the present quality control mode of TCMs in China which only choose one or a few mark components cannot reveal the real quality of TCMs.[4] The lack of strict quality control contributes to the marketing of TCMs of questionable quality. Therefore, a strict quality control method for TCMs is badly needed. Nowadays, quantitative analysis of multiple active components is taken for the most direct and important method for quality control of TCMs,[5] and will be the developing trends of quality control of TCMs.
Yuanhu Zhitong prescription (YZP), a famous traditional Chinese medicine formula, composed of Corydalis yanhusuo (Y.H.Chou and Chun C.Hsu) W.T.Wang ex Z.Y.Su and C.Y.Wu and Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav have been officially recorded in Chinese Pharmacopoeia for the treatment of stomach pain, hypochondriac pain, headache and dysmenorrhea caused by qi-stagnancy and blood stasis.[6] Up to now, YZP have been widely used and made into many dosage forms including tablets, capsules, soft capsules, oral liquids, granules, dropping pills, etc., Tetrahydropalmatine is the only marker component for the quality control of all dosage forms of YZP in Chinese Pharmacopoeia.[6] However, accumulating documentary records have indicated that alkaloids in Corydalis yanhusuo (Y.H.Chou and Chun C.Hsu) W.T.Wang ex Z.Y.Su and C.Y.Wu and coumarins in Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav were the active components of YZP.[7,8,9,10,11] The content of tetrahydropalmatine has already not revealed the real quality of YZP.
At the present time, a few analytical methods (HPLC, TLC, capillary electrophoresis (CE)) mainly focusing on tetrahydropalmatine (THP), protopine (PTE), imperatorin (IMP) or isoimperatorin (ISO)[12,13,14,15,16,17,18,19] have been reported as quality assessment for YZP in China. One study[20] using rapid resolution liquid chromatography coupled with a triple quadrupole electrospray tandem mass spectrometry (RRLC-QQQ) method determined 17 components of YZP tablets. However, this study may not suitable for other dosage forms due to different impurities in different dosage forms. To date, the methods for the simultaneous separation and quantitative determination of multiple active components in a single running for other dosage forms of YZP are still not available. Therefore, a universal method for the quantitative determination of multiple active components in different dosage forms of YZP is necessary and convenient for their quality control.
In the present study, a simple, accurate, and reliable analytical method for quantitative determination of 12 active components contained in YZP including protopine (PTE), α-allocryptopine (ATP), coptisine (CTE), xanthotol (XTL), palmatine (PME), dehydrocorydaline (DCE), glaucine (GCE), THP, tetrahydroberberine (TDE), IMP, corydaline (CDE), ISO (they were chose according to our previous research,[21,22] Figure 1) was firstly developed and validated using a HPLC coupled with a photodiode array (PDA) detection. The results have indicated that the validated HPLC-PDA method is very simple, suitable and universal for the routine analysis of four main dosage forms (tablets, capsules, soft capsules, dropping pills) of YZP and their quality control.
Figure 1.
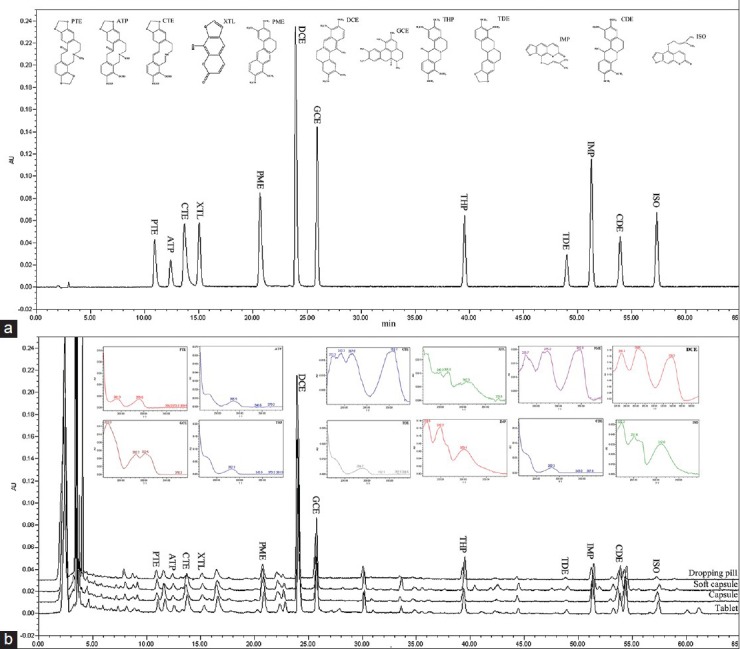
Chemical structures and chromatograms of 12 components (protopine, PTE; α-allocryptopine, ATP; coptisine, CTE; xanthotol, XTL; palmatine, PME; dehydrocorydaline, DCE; glaucine, GCE; tetrahydropalmatine, THP; tetrahydroberberine, TDE; imperatorin, IMP; corydaline, CDE; isoimperatorin, ISO) (a); representative chromatograms and ultraviolet spectrogram for determination of 12 components in four dosage forms of YZP (b).
MATERIALS AND METHODS
Chemicals and Reagents
HPLC-grade acetonitrile and methanol were obtained from Honeywell (Muskegon, MI, USA). HPLC-grade phosphoric acid and triethylamine were obtained from Tianjin Kermel Chemical Reagent Co., Ltd. The deionized water was prepared from Millipore water purification system (Milford, MA, USA) and filtered with a 0.22 μm membrane. Four dosage forms containing 17 batches of YZP coming from eight manufacturers were obtained from commercial sources [Table 1].
Table 1.
A summary of the tested YZP samples
PTE, CTE, XTL, PME, GCE, TDE (>98%, purity) were acquired from Shanghai Tauto Biotech Co., Ltd. ATP was obtained from Shenzhen Meihe Biotech Co., Ltd. CDE and DCE (>98%, purity) were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). THP, IMP and ISO were obtained from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Equipments and Chromatographic Conditions
The analysis was performed on a Waters 2695 Alliance HPLC system (Waters Corp., Milford, MA, USA), equipped with a quaternary solvent delivery system, an on-line degasser, an autosampler, a thermostated compartment and a 2996 photodiode array detection. Data collection and integration were accomplished by Empower™ Software (Waters Corp., Milford, MA, USA). All analytes were separated on a Luna-C18 (2) column (250×4.6 mm, 5.0 μm, Phenomenex) and a C18 guard column was used before the analytical column. The separation was carried out with gradient elution procedure and mobile phase A (acetonitrile) and B (0.1% phosphoric acid water solution, adjusted with triethylamine to pH 5.6) ratios linear changed as follows: 0~15 min, 23% A; 15~26 min, 23~40% A; 26~65 min, 40~65% A. The total run time was 65 min at a flow rate of 1 mL/min. The following HPLC parameters were used for the analysis: Column temperature, 30°C; injection volume, 20 μL, and the detection wavelength, 280 nm.
Standard stock solutions
The stock solution of the 12 components (PTE, ATP, CTE, XTL, PME, DCE, GCE, THP, TDE, IMP, CDE, ISO; each is accurately weighed) was prepared in 75% methanol water solution. A series of working standard solutions of the 12 components were prepared by further dilution of the stock solution with 75% methanol water solution. All stock and working standard solutions were stored in brown bottles at 4°C until used for analysis.
Preparation of sample solutions
One gram of the content of YZP for each dosage form was accurately weighted and dissolved in 50 mL 75% methanol water solution. Then the solution was extracted with ultrasonic for 30 min, settled to the volume of 50 mL, and filtered with a 0.45 μm microporous membrane prior to analysis. 20 μL of the sample solution was injected into the HPLC system for analysis.
RESULTS AND DISCUSSION
Optimization of HPLC conditions
A Hypersil BDS C18 column (250 × 4.6 mm, 5.0 μm, elite) and a Phenomenex Luna-C18 (2) column (250 × 4.6 mm, 5.0 μm, Phenomenex) were tested to optimize the chromatographic condition. The resolution of Phenomenex Luna-C18 (2) column was proved better than that of Hypersil BDS C18 column. Because of the major components of YZP are alkaloids, different mobile phases with buffer were tried, such as methanol–water (0.1% phosphoric acid water solution, adjusted with triethylamine to pH 4, pH 4.5, pH 5, pH 5.5, pH 6), and acetonitrile–water (0.1% phosphoric acid water solution, adjusted with triethylamine to pH 4, pH 4.5, pH 5, pH 5.5, pH 5.6, pH 5.8, pH 6) with a step linear gradient procedure. When pH ≤ 5, the retention time of alkaloids was short, but they could not be separated no matter what conditions of the step linear gradient procedure we chose. When use methanol–water with buffer, the runtime of the test was long or the separation effect of the components of YZP was poor. Therefore, we did not choose methanol–water with buffer. The column temperature (20°C, 30°C, 35°C) were also tested. The detective components from YZP were identified by comparing both the retention times and ultraviolet spectrogram with those authentic standards. Finally, a Phenomenex Luna-C18 (2) column using acetonitrile-0.1% phosphoric acid solution (adjusted with triethylamine to pH 5.6) as a mobile phase system with a step linear gradient procedure was determined with a runtime of 65 min, the detection wavelength was set at 280 nm, and the column temperature were performed at 30°C. The validated chromatographic condition gave good resolution and acceptable peak parameters for PTE, ATP, CTE, XTL, PME, DCE, GCE, THP, TDE, IMP, CDE and ISO. Typical chromatograms of the authentic standards and four dosage forms of YZP (T1, C1, S1, D1) are shown in figure 1 (process by Photoshop CS5, Adobe Systems Incorporated, USA).
Method Validation
Calibration curves and the limit of detection
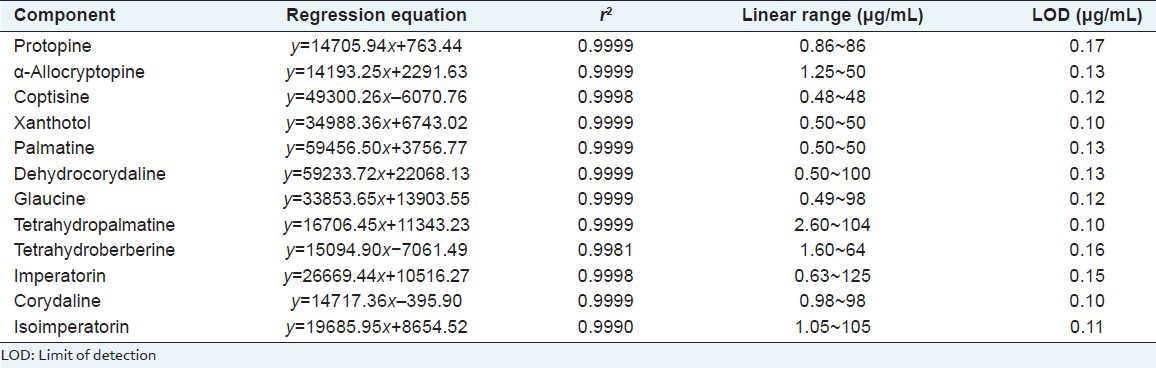
Through calculation of each standard peak area (y; the peak area value was the average values of three replicate injections) against its concentration (x, μg/mL), good linear calibration curves (r2 ≥.9981) were obtained over series of working standard solutions [Table 2]. Each curve was made at least 6 levels. The limit of detection (LOD) is defined as three-fold of the ratio of the signal-to-noise (S/N).
Table 2.
Calibration curves, linear ranges, LOD of 12 components (n=3)
Precision, repeatability and stability
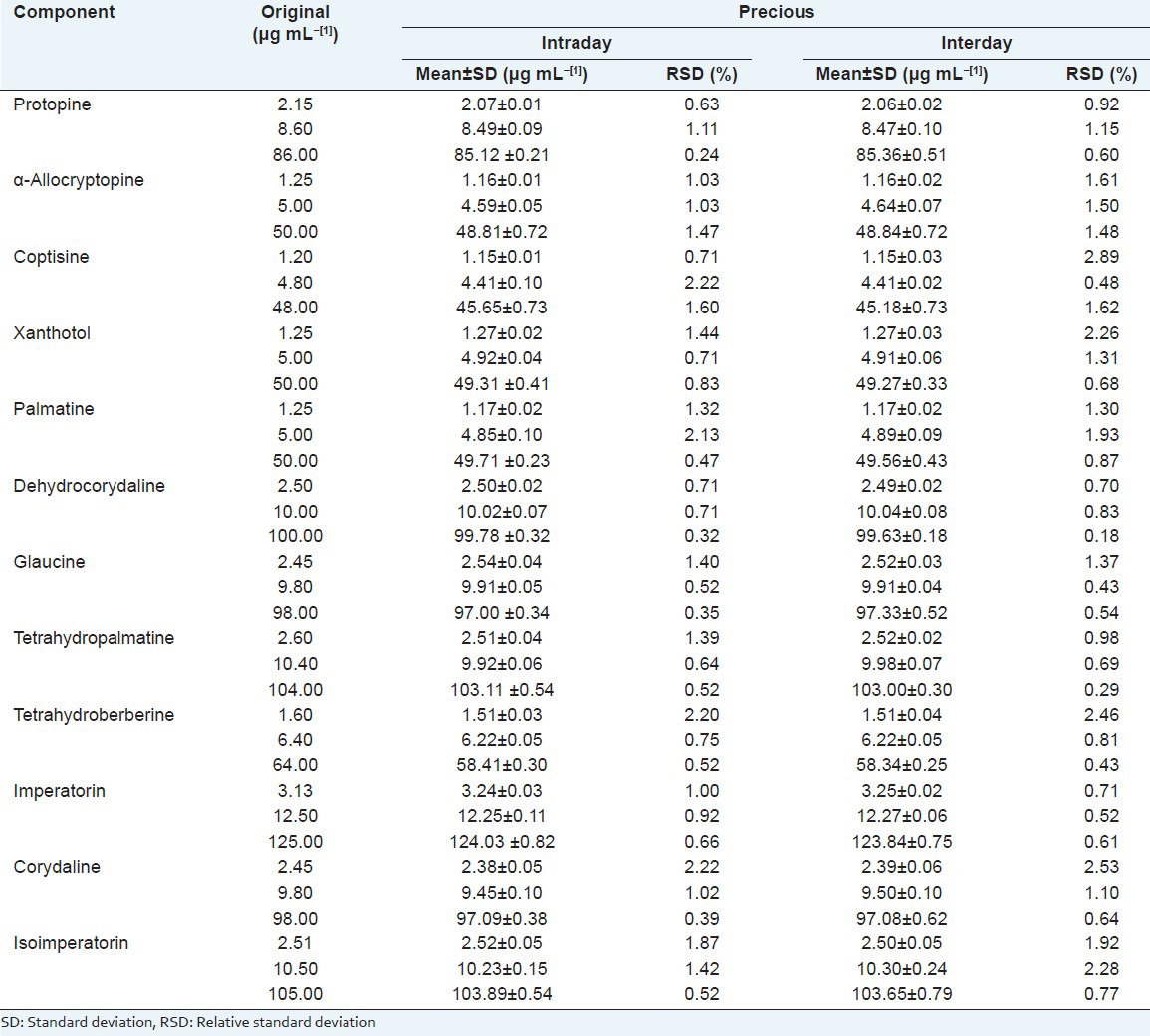
The precision of the method were studied by determination of interday and intraday variances. The interday and intraday precisions were evaluated by measuring a standard mixture solution composed of 12 components at three concentrations six times a day and twice a day over 3 consecutive days separately under the optimized conditions. The relative standard deviation (RSD) was used to estimate interday and intraday precisions. As the results shown in Table 3, RSD values were all ≤ 2.89%.
Table 3.
Intraday and interday precisions of 12 components (n=6, (χ̄)±SD)
Evaluation of repeatability was used to evaluate the repeatability of the present method by the injection of six different samples of four dosage forms of YZP (T1, C1, S1, D1 was selected) prepared by the same sample preparation procedure. The RSD values of 12 components were 0.15~3.34%, which are listed in Table 4.
Table 4.
Repeatability and stability of 12 components in four dosage forms of YZP (n=3)
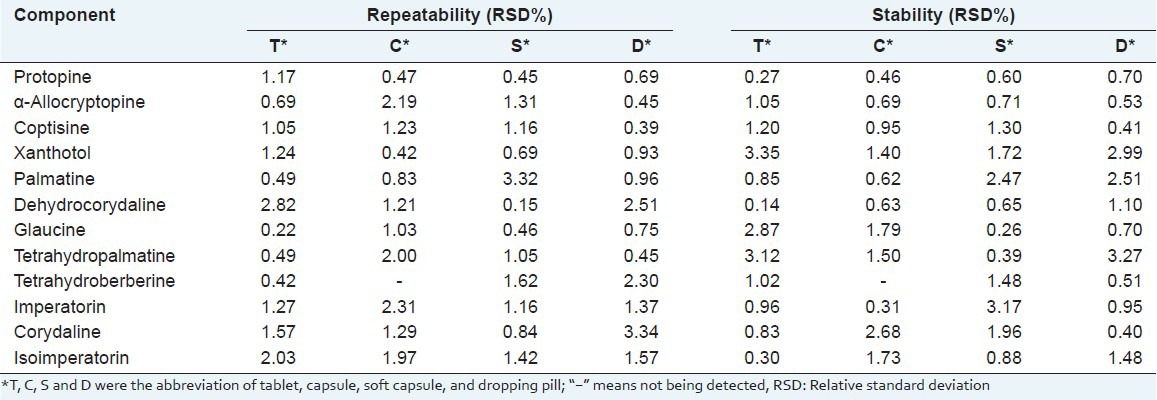
For the stability test (T1, C1, S1, D1 was selected), sample solutions were analyzed after being set in vial racks for 48 hours, and the sample solutions were found to be rather stable within 48 hours (RSD, 0.14~3.35%, see Table 4). The results demonstrated that the solutions were stable within 48 hours.
Recovery
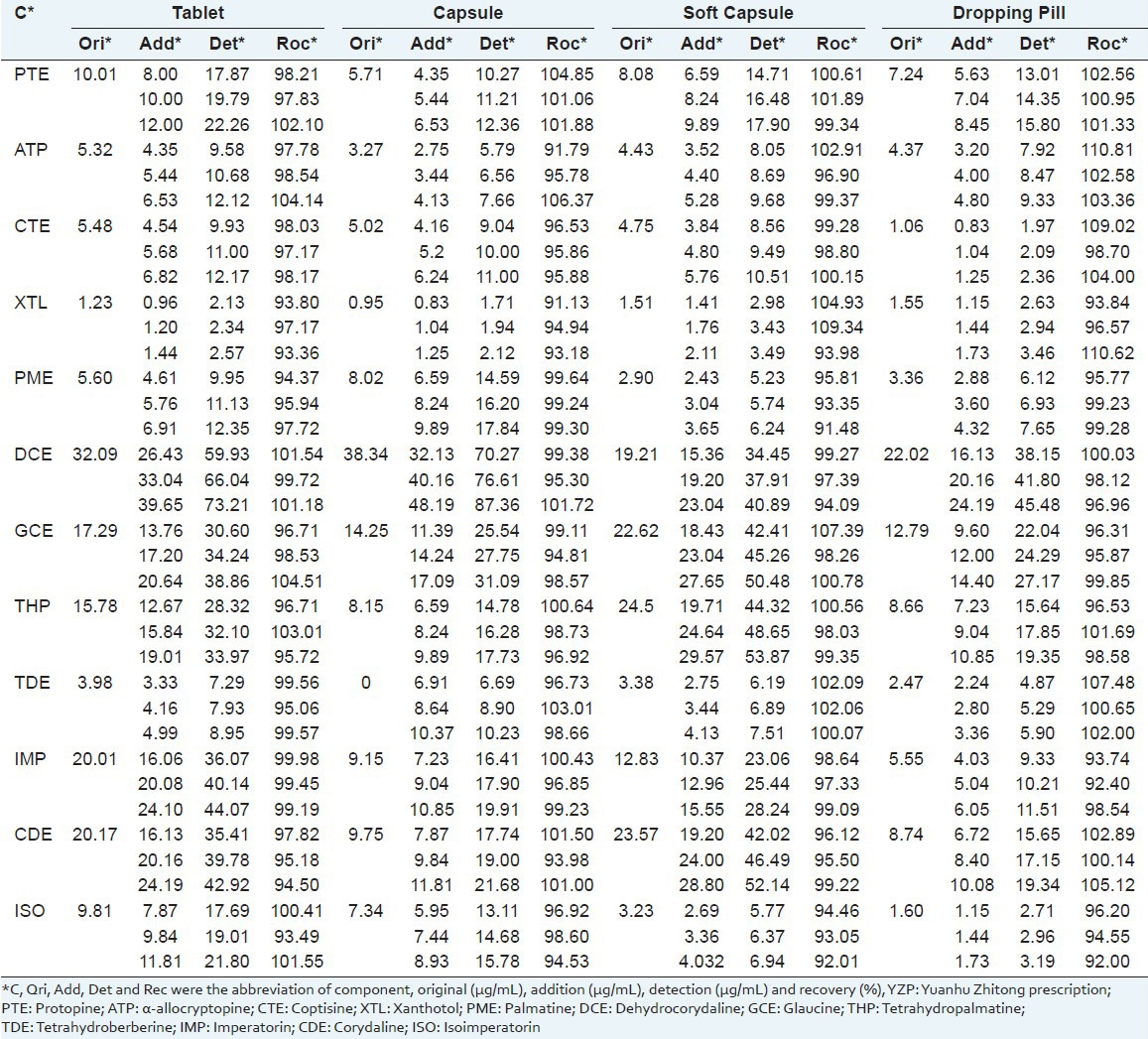
To determine the recovery, three different quantities (low, medium, and high) of the 12 standards were added to a previously analyzed real sample of YZP (T1, C1, S1, D1 was selected) for which the concentrations of the compounds of interest were known. Then the samples were extracted and analyzed by the established method. Each sample was determined in three times. The average recoveries were estimated by the formula (1):
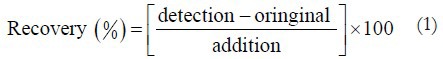
The mean recovery rates of 12 components ranged from 91.13% to 110.81% [Table 5].
Table 5.
Recoveries of 12 components in four dosage forms of YZP (n=3)
Sample analysis
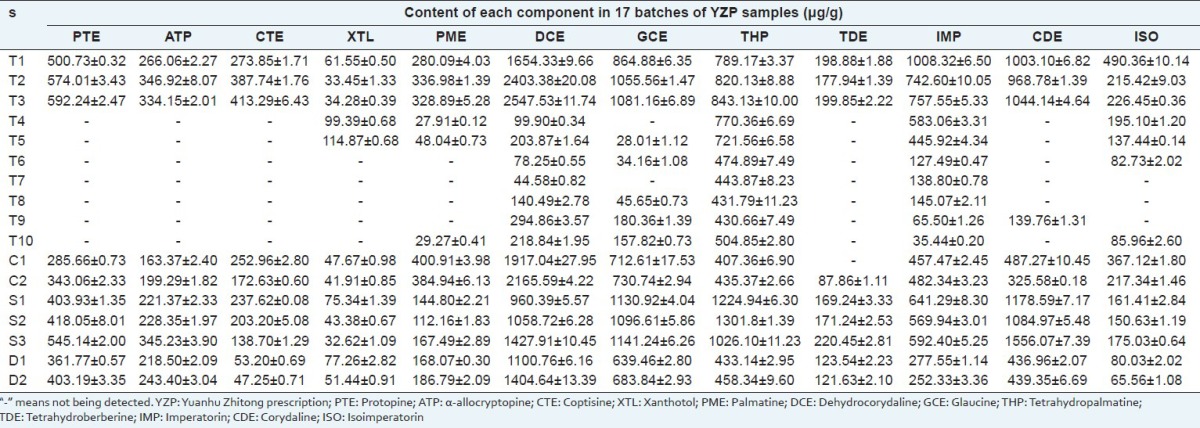
The validated analytical method was successfully applied to the simultaneous determination of PTE, ATP, CTE, XTL, PME, DCE, GCE, THP, TDE, IMP, CDE, ISO in four dosage forms of YZP containing 17 batches from the same manufacturer or different manufacturers. Each sample was determined in three times. As shown in Table 6, the results demonstrated that all batches of YZP were up to standard according to China Pharmacopoeia. However, the content and quality of each analyte obviously varied among the different dosage forms, different manufacturers or even different batches from the same manufacturer. Among the 12 components, DCE, GCE, THP, IMP were the main ones, whose content varied from 44.58 to 2547.53, 28.01 to 1130.92, 407.36 to 1301.8 μg/g, respectively. As we all know, geographic location, type of climate, environment and time of harvest, post-harvest handling, processing, storage and pharmaceutical technology can affect the quality of TCM and their formulas.[23] This may be the reason of variety. However, the content of each main active component in YZP should reach to a limited extent in order to attain good and stable efficacy, which needs a good quality control method. The results showed that the present method is suitable for the routine analysis and can contribute to quality control of commercial YZP.
Table 6.
Contents of 12 components in four dosage forms of YZP (n=3, (χ̄)±SD)
CONCLUSIONS
The change of the contents has an important influence on the quality of YZP. In the present paper, an accurate and reliable HPLC method to simultaneously determine 12 active components in YZP was validated. The method was applied to evaluate four commercial dosage forms of YZP containing 17 batches. The results showed that the content of each analyte obviously varied among the different dosage forms, different manufacturers or even different batches from the same manufacturer. This is the first report for the determination of 12 major active components in four dosage forms of YZP using HPLC coupled with PAD, which is helpful for the quality control for commercial YZP.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wang G, Mao B, Xiong ZY, Fan T, Chen XD, Wang L, et al. The quality of reporting of randomized controlled trials of traditional Chinese medicine: A survey of 13 randomly selected journals from mainland China. Clin Ther. 2007;29:1456–67. doi: 10.1016/j.clinthera.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Wang ZW, Li ZY. Strategy for market expansion: Medical services of Traditional Chinese Medicine. Tradit Chin Med. 2013;33:280–2. doi: 10.1016/s0254-6272(13)60140-5. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, David B, Tu PF, Barbin Y. Recent analytical approaches in quality control of traditional Chinese medicines-A review. Anal Chim Acta. 2010;657:9–18. doi: 10.1016/j.aca.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Yang GM, Li WD, Pan Y, Tu X. Rapid simultaneous determination of four alkaloids in lotus plumule by CZE with ephedrine hydrochloride as an internal standard. Chromatographia. 2012;75:1295–300. [Google Scholar]
- 5.Liang XM, Jin Y, Wang YP, Jin GW, Fu Q, Xiao YS. Qualitative and quantitative analysis in quality control of traditional Chinese medicines. J Chromatogr A. 2009;1216:2033–44. doi: 10.1016/j.chroma.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 6.China Pharmacopoeia. Vol. 1. Beijing: Chemical Industry Publishing House; 2010. China Pharmacopoeia Committee. [Google Scholar]
- 7.Ding B, Zhou TT, Fan GR, Hong ZY, Wu YT. Qualitative and quantitative determination of ten alkaloids in traditional Chinese medicine Corydalis yanhusuo W.T. Wang by LC-MS/MS and LC-DAD. J Pharm Biomed Anal. 2007;45:219–26. doi: 10.1016/j.jpba.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Jin Y, Dong J, Xiao YS, Feng JT, Xue XY, et al. Systematic screening and characterization of tertiary and quaternary alkaloids from corydalis yanhusuo W.T. Wang using ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. Talanta. 2009;78:513–22. doi: 10.1016/j.talanta.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Wang SW, Fan GR, Zou HF. Screening of antinociceptive components in Corydalis yanhusuo W.T. Wang by comprehensive two-dimensional liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem. 2010;396:1731–40. doi: 10.1007/s00216-009-3409-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Fan GR, Chen B, Xie Y, Wu HL, Wu YT, et al. Separation and quantitative analysis of coumarin compounds from Angelica dahurica (Fisch. ex Hoffm) Benth. et Hook. f by pressurized capillary electrochromatography. J Pharm Biomed Anal. 2006;41:105–16. doi: 10.1016/j.jpba.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Zheng HC, Cai SQ. Beijing: People's Medical Publishing House; 2004. Pharmaceutical botany and Pharmacognosy. [Google Scholar]
- 12.Wei HZ, Xie F, Rao Y, Li XN, Zhong HH, Jin Hx. Determination of tetrahydropalmatine in Yuanhu Zhitong Capsule by HPLC and evaluation of its measurement uncertainty. Chin Tradit Herbal Drugs. 2012;43:299–302. [Google Scholar]
- 13.Yu YY, Zhang HY, Lu L, Teng HY, Wu SQ. Determination of Tetrahydropalmatine in Yuanhuzhitong Granule by HPLC. J Fujian Univ TCM. 2012;22:34–6. [Google Scholar]
- 14.Jiang YP. Determination of imperatorin in Yuanhu Zhitong Soft capsules by HPLC. Qilu Pharm Aceutical Aff. 2011;30:206–8. [Google Scholar]
- 15.Deng YT, Zhang XM, Ren YJ, Jia JX, Lu Y, Wu XQ. Study on quality standard for Yuanhu Zhitong dropping pills. Chin J Exp Tradit Med Formulae. 2012;18:127–9. [Google Scholar]
- 16.Liu RX, Wang QX. Determination of tetrahydropalmatine in yuanhuzhitong oral liquor by HPLC. Chin J Exp Tradit Med Formulae. 2010;16:64–6. [Google Scholar]
- 17.Wei HZ, Rao Y, Wang YM, Luo GA. Quantitative analysis of tetrahydropalmatine in two dosage forms of Yuanhu Prescriptions by High Performance Capillary Electrophoresis. Chin J Pharm Anal. 2002;22:272–4. [Google Scholar]
- 18.Yang F, Wan L, Xu SC, Ye N. Content Determination of imperatorin isoimperatorin and tetrahydropalmatione in Yuanhu Zhitong capsules by QAMS. Chin Pharm. 2012;23:3046–9. [Google Scholar]
- 19.Liao ZG, Wang GF, Liang XL, Zhao GW, Jiang QY. Optimization of microwave-assisted extraction of active components from Yuanhu Zhitong prescription. Sep Purif Technol. 2008;63:424–33. [Google Scholar]
- 20.Zhang YC, Xu HY, Chen XP, Chen C, Wang HJ, Meng FY, et al. Simultaneous quantification of 17 constituents from Yuanhu Zhitong tablet using rapid resolution liquid chromatography coupled with a triple quadrupole electrospray tandem mass spectrometry. J Pharm Biomed Anal. 2011;56:497–504. doi: 10.1016/j.jpba.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Chen YT, Cao Y, Xie YH, Zhang XK, Yang Q, Li XQ, et al. Traditional Chinese medicine for the treatment of primary dysmenorrhea: How do Yuanhu painkillers effectively treat dysmenorrhea? Phytomedicine. 2013;20:1095–104. doi: 10.1016/j.phymed.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XK, Xie YH, Cao W, Yang Q, Miao S, Wang SW. Brain distribution study of imperatorin in rats after oral administration assessed by HPLC. Chromatographia. 2011;74:259–65. [Google Scholar]
- 23.Xu S, Yang L, Tian R, Wang Z, Liu Z, Xie P, et al. Species differentiation and quality assessment of Radix Paeoniae Rubra (Chi-shao) by means of high-performance liquid chromatographic fingerprint. J Chromatogra A. 2009;1216:2163–8. doi: 10.1016/j.chroma.2008.04.064. [DOI] [PubMed] [Google Scholar]


