Abstract
Background:
To extract, purify and identify the active constituents in ethanol extract of Radix Salviae Miltiorrhizae, and to analyze the protective effects of tanshinone IIA on cerebral ischemia-reperfusion injury in rats.
Materials and Methods:
Radix Salviae Miltiorrhizae was extracted by ultrasonic extraction, effective parts were extracted by extraction method, compounds were isolated by preparative TLC and preparative HPLC, and structures of compounds were identified by 1H NMR and 13C NMR; the effects of tanshinone IIA on cerebral ischemia-reperfusion injury in rats were determined by establishing rat model of middle cerebral artery occlusion (MCAO).
Results:
The experimental data show four compounds were isolated, namely tanshinone IIB, hydroxymethylene tanshinone, salvianolic acid B and 9”’-methyl lithospermate B. Tanshinone IIA could alleviate the symptoms of neurological deficit in rats, the neurological deficit alleviating effect became more obvious with the increase of dose; tanshinone IIA experimental groups could reduce the cerebral infarction size and brain water content in rats, different concentrations of tanshinone IIA could decrease the SOD content and increase the MDA content in the frontal and parietal cortices of ischemic hemisphere in the ischemia reperfusion group, the differences were statistically significant compared with the ischemia reperfusion group.
Conclusion:
Radix Salviae Miltiorrhizae has the protective effects on cerebral ischemia reperfusion injury in rats.
Keywords: 9”’-methyl lithospermate B, ischemia reperfusion, radix Salviae Miltiorrhizae, tanshinone IIB
INTRODUCTION
Radix Salviae Miltiorrhizae is the dried root and rhizome of Salvia miltiorrhiza bge in the family Lamiaceae, which was recorded as early as in the “Shen Nong's Herbal Classic” as “Radix Salviae Miltiorrhizae is bitter in taste, slightly cold and non-toxic. It mainly treats heart and abdominal pathogens, bowel rumble, cold and heat accumulation, and can eliminate abdominal mass, stop dysphoria and tonify qi. It is also named Quechancao, which grows in the mountain valleys.” and listed it as the top grade drug. Radix Salviae Miltiorrhizae is slightly cold, bitter in taste and enters the heart and liver meridians, which has the effects of dispersing blood stasis, promoting blood circulation and restoring menstrual flow, relieving restlessness and inducing tranquilization.[1] “Compendium of Materia Medica” recorded the functions of Radix Salviae Miltiorrhizae more comprehensively, which included irregular menstruation, prenatal fetal movement, postpartum lochiostasis, hot and cold fatigue, lumbar pain, vexing pain in the joints, pediatric hematochezia, cold hernia and abdominal pain, infantile fever, acute mastitis, hot oil burns, fire burns, etc.
Scholars at home and abroad have done a lot of studies on the extraction and purification processes of compounds from Radix Salviae Miltiorrhizae,[2,3,4,5] the present study focus on the isolation and identification of active constituents of Radix Salviae Miltiorrhizae, as well as the exploration of the effects of tanshinone IIA on repair of cerebral ischemia reperfusion injury in rats.
MATERIALS AND METHODS
Instruments and reagents
Advance 600 NMR spectrometer (Bruker Corporation, Switzerland); XHD micro melting point apparatus (shenyang Tech Instrument Co., Ltd.); reagents were all of analytical grade.
Drugs
Crude drug of Salvia miltiorrhiza bge was purchased from the pharmacy, which was identified as Salvia miltiorrhiza bge. Tanshinone IIA was prepared during the preliminary phase of the experiment, its purity was 82%. Nimodipine tablets (CFDA approval No. H20043915), Tianjin Central Pharmaceutical Co., Ltd.
Experimental animals
Wistar rats, male, 280-320 g, provided by the Liaocheng Brain Hospital. All experimental procedures were approved by the Animal Research Ethics Committee. The certificate number is GB-201232430B.
Extraction and isolation of compounds from Radix Salviae Miltiorrhizae
5 kg of crude Salvia miltiorrhiza bge was crushed, passed through a 40 mesh sieve, then ultrasonically extracted for 2 h twice with an 8-fold volume of 95% ethanol, the extracts were combined, and ethanol was evaporated to give pasty extract. The pasty extract was dissolved by addition of appropriate amount of water, and extracted with ethyl acetate, which was evaporated to give extract. The extract was loaded on a silica gel column, gradient-eluted with petroleum ether-ethyl acetate system (ascending polarity), then isolated by column chromatography with 250 ml as one fraction, the same fractions were combined. The resulting crude extract was purified by means of preparative TLC and preparative liquid chromatography, and four compounds were isolated there from.
Inhibition of cerebral ischemia-reperfusion injury by tanshinone IIA.
Grouping
Rats were divided into five groups, namely the ischemia-reperfusion group, Tanshinone IIA groups (low-dose, medium-dose and high-dose groups, concentrations of 10, 20 and 40 mg/kg) and nimodipine group (0.5 mg/kg).
Model establishment and experimental procedure
Rat model of MCAO was established. The modified Zea Longa suture method was used to prepare the rat left MCAO model. 90 min after the ischemia, nylon suture was removed under anesthesia to achieve reperfusion. Each dose of drug was intravenously injected after ischemia and after reperfusion, respectively. Each group was scored separately 24 h after reperfusion.
Neurological scoring
Scoring was performed referring to the Longa's method, which had five grades. 0 point: No symptoms of neurological deficit; 1 point: Occurrence of signs of Horner, failure to fully extend the right forepaw; 2 points: Circling to the right when walking, circling; 3 points: Falling to the right during autonomic movement, and even cannot stand; 4 points: Cannot walk spontaneously, presence of a disturbance of consciousness. Model would be successful if the neurological deficit score was between 1 and 3 points.
Determination of cerebral infarction size and brain water content
Cerebral ischemia-reperfusion, rats were decapitated and their brains were removed after 24 h, white tissue (infarction area) was carefully peeled, and the cerebral infarction size and brain water content were calculated. Method of calculation of cerebral infarction size: Mass of infarcted brain/total mass of brain tissue × 100%; water content (%) = (1-brain tissue dry weight/brain tissue wet weight) ×100%.
Effects of tanshinone IIA on SOD and MDA in rats
24 h after cerebral ischemia-reperfusion, rats were decapitated and their brains were removed, under ice bath, frontal and parietal cortices 7-11 mm from the tip of left olfactory bulb were removed, and MDA and SOD contents were assayed strictly in kit instructions.
Statistical analysis
SPSS 13.0 statistical software was used, the experimental data were analyzed by the t-test, and comparison among groups were performed using analysis of variance, the difference was considered statistically significant when P < 0.05.
RESULTS
Structural identification of compounds
Compound 1, red needle crystals. 1H-NMR (600 MHz, CDCl3), d: 1.28 (3H, s, H-18), 1.37 (4H, m, H-2,3), 2.12 (3H, s, H-17), 3.14 (2H, m, H-1), 3.36 (1H, d, J = 10.2Hz, H-19b), 3.49 (1H, d, J = 10.6Hz, H-19a), 7.58 (1H, d, J = 8.2Hz, H-7), 7.63 (1H, d, J = 7.8Hz, H-6), 7.74 (1H, s, H-16). 13C-NMR (500 MHz, DMSO-d6) d: 9.6 (C-17), 27.2 (C-18), 18.3 (C-2), 29.3 (C-1), 32.2 (C-3), 40.7 (C-4), 69.6 (C-19), 120.2 (C-7), 120.4 (C-1S), 120.5 (C-13), 126.7 (C-9), 127.4 (C-8), 134.2 (C-6), 142.3 (C-16), 144.8 (C-10), 147.9 (C-5), 160.4 (C-14), 175.6 (C-12), 183.3 (C-11). The above data were basically consistent with the literature,[6] so the structure of compound 1 was tanshinone IIB. Its chemical structure as follows [Figure 1].
Figure 1.
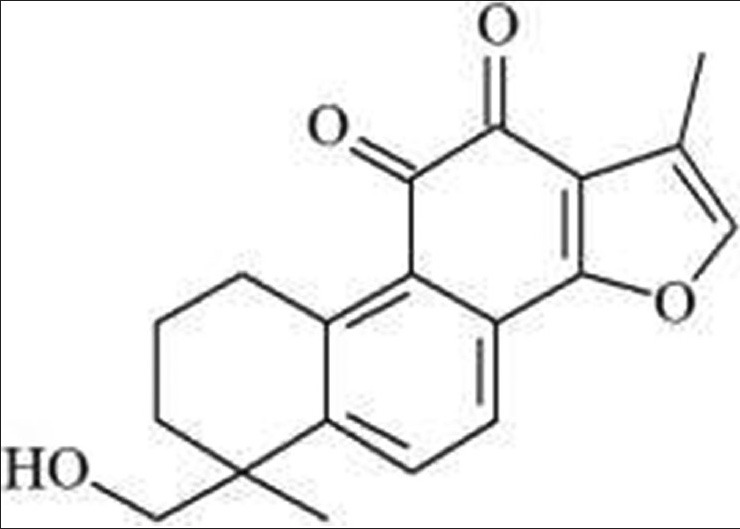
Tanshinone IIB
Compound 2, purplish red needle crystals. 1H-NMR (600 MHz, CDCl3): 3.37-3.56 (2H, m, H-1), 1.99-2.16 (2H, m, H-2) 4.57 (1H, d, J = 7.2Hz, H-3), 1.77 (1H, brs, OH-3), 7.92 (1H, d, J = 7.8Hz, H-6), 7.65 (1H, d, J = 7.8Hz, H-7), 7.29 (1H, s, H-16), 2.31 (3H, s, CH3-17), 5.08 (1H, s, H-18a), 5.50 (1H, s, H-18b). 13C-NMR (500 MHz, DMSO-d6) d: 25.8 (C-1), 31.1 (C-2), 69.7 (C-3), 88.8 (C-17), 110.3 (C-18), 120.4 (C-15), 120.8 (C-7), 121.4 (C-13), 131.7 (C-6), 126.1 (C-9), 129.3 (C-8), 136.8 (C-10), 141.7 (C-16), 143.3 (C-4), 145.9 (C-5), 161.1 (C-14), 175.3 (C-12), 183.2 (C-11). The above data were basically consistent with the literature,[7] so the structure of compound 2 was identified as hydroxymethylene tanshinone. Its chemical structure as follows [Figure 2].
Figure 2.
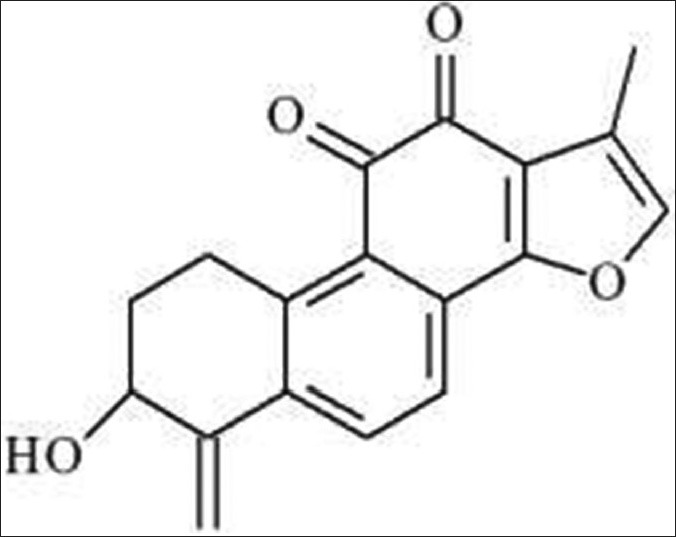
Hydroxymethylene tanshinone
Compound 3, molecular formula C36H30O16, yellow powder (methanol). 1H-NMR (600 MHz, CDC13) d: 7.53 (1H, d, H-2, J = 15.6Hz, H-A7), 6.37 (1H, d, J = 16.8Hz, H-A8), 6.38 (1H, s, H-B2), 7.35 (1H, d, J = 7.5Hz, H-SA), 6.82 (1H, brs, H-6A), 6.50-6.78 (8H, m, H-B5, B6, C2, C-5, C6, D2, D5, D6), 2.85-2.95 (4H, m, H-B7, D7), 5.12 (2H, s, H-B8, D8), 5.75 (1H, s, H-C7), 4.48 (1H, s, H-C8). 13C-NMR (500 MHz, DMSO-d6) d: 36.3 (C-D7), 36.4 (C-B7), 55.3 (C-C8), 73.4 (C-B8), 74.6 (C-D8), 86.7 (C-C7), 112.6 (C-C6), 115.4 (C-B2), 115.5 (C-BS), 117.4 (C-C3), 117.5 (C-A8), 117.8 (C-C2), 120.2 (C-A6), 120.3 (C-AS), 116.5 (C-C2), 117.4 (C-DS), 121.6 (C-A1), 123.5 (C-D6), 123.6 (C-B6), 125.7 (C-A2), 127.5 (C-B1), 127.6 (C-Dl), 131.4 (C-C1), 142.3 (C-A7), 144.7 (C-C4), 144.8 (C-CS), 144.8 (C-B4), 145.4 (C-D4), 145.7 (C-B3), 145.7 (C-D3), 146.4 (C-A3), 147.3 (C-A4), 166.6 (C-A9), 170.4 (C-C9), 170.4 (C-D9), 171.6 (C-B9). The above data were generally consistent with the data of salvianolic acid B in the literature,[8] so compound 3 was salvianolic acid B. Its chemical structure as follows [Figure 3].
Figure 3.
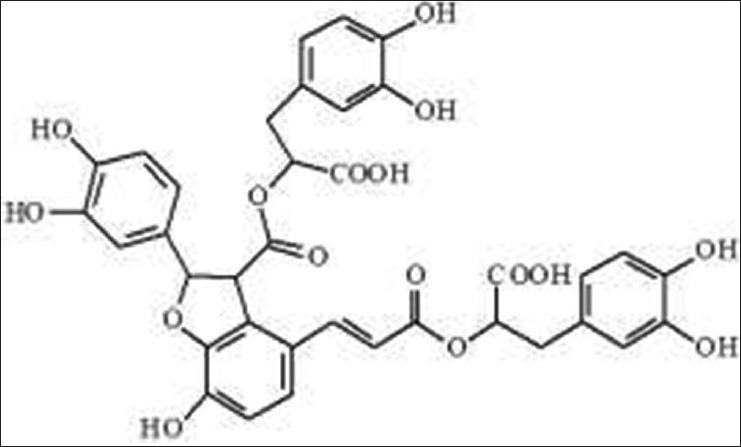
Salvianolic acid B
Compound 4, yellow amorphous powder (methanol), m.p.: 159-160°C, freely soluble in methanol. 1H-NMR (600 MHz, CDC13) d:6.83 (1H, d, J = 8.5Hz, H-5), 7.15 (1H, d, J = 8.5, 34H-6), 7.54 (1H, d, J = 16.0Hz, H-7), 6.30 (1H, d, J = 2.0Hz, H-8), 6.76 (1H, d, J = 2.0Hz, H-2′), 6.77 (1H, s, H-5′), 6.66 (1H, dd, J = 1.5, 8.0Hz, H-6′), 5.86 (1H, d, J = 4.5Hz, H-7′), 4.35 (1H, d, J = 5.0, H-8′), 6.78 (1H, d, J = 1.0Hz, H-2″), 6.69 (1H, d, J = 9.0Hz, H-5″), 6.64 (1H, dd, J = 2.0, 8.0Hz, H-6″), 3.00 (2H, m, H-7′), 5.16 (1H, m, H-8′), 6.54 (1H, d, J = 2.0Hz, H-2‴), 5.59 (1H, d, J = 8.0Hz, H-5‴), 6.32 (1H, dd, J = 2.0, 2.0Hz, H-6‴), 3.00 (1H, m, H-7‴), 4.69 (1H, d, J = 4.0Hz, H-8‴), 3.64 (3H, s, OCH3). 13C-NMR (500MHz, DMSO-d6) d: 124.58 (C-1), 126.39 (C-2), 149.14 (C-3), 145.12 (C-4), 118.34 (C-5), 121.88 (C-6), 143.85 (C-7), 116.58 (C-8), 167.98 (C-9), 133.54 (C-1’), 113.35 (C-2’), 146.72 (C-3’), 146.15 (C-4’), 116.39 (C-5’), 118.35 (C-6’), 88.12 (C-7’), 59.75 (C-8’), 172.32 (C-9’), 129.05 (C-1”), 117.53 (C-2”), 145.17 (C-3”), 146.47 (C-4”), 116.45 (C-5”), 122.27 (C-6”), 37.79 (C-7”), 74.72 (C-8”), 173.36 (C-9”), 128.66 (C-1”’), 117.53 (C-2”’), 145.26 (C-3”’), 145.92 (C-4”’), 116.36 (C-5”’), 121.73 (C-6”’), 37.48 (C-7”’), 75.26 (C-8”’), 52.67 (C-9”’). The above data were consistent with the reported literature,[9] so compound 4 was identified as 9”’-methyl lithospermate B. Its chemical structure as follows [Figure 4].
Figure 4.
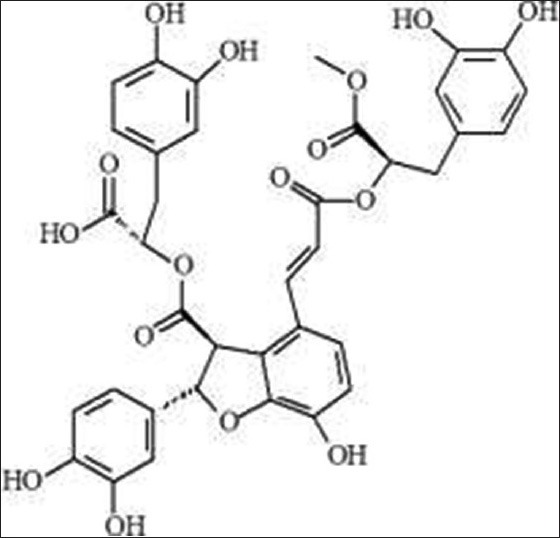
9”’-methyl lithospermate B
Effects of tanshinone IIA on neurological function in rats
Compared with the model group, each tanshinone IIA treatment group could apparently reduce the neurological score, indicating that tanshinone IIA could alleviate symptoms of neurological deficit in rats, the neurological deficit alleviating effect became more obvious with the increase of dose, and the neurological score of tanshinone IIA high-dose group reached 1.61 [Figure 5].
Figure 5.
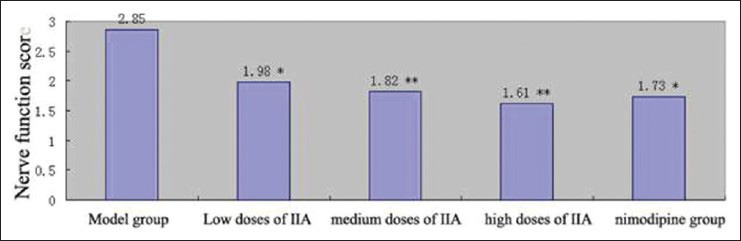
Effects of tanshinone IIA on neurological function in rats
Results for the effects of tanshinone IIA on cerebral infarction size and brain water content in rats
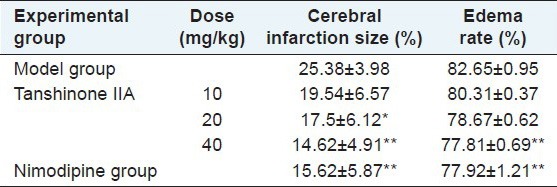
Compared with the model group, cerebral infarction size apparently decreased (P < 0.05) in the low-dose group; cerebral infarction size and brain water content significantly decreased (P < 0.01) in the high-dose group and the nimodipine group [Table 1].
Table 1.
Effects of tanshinone IIA on cerebral infarction size and brain water content in rats (x̄±s, n=8)
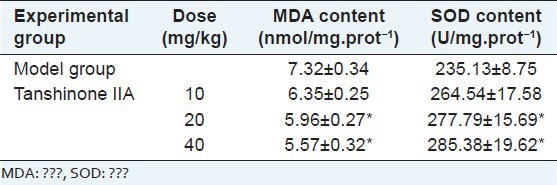
Result of the effects of tanshinone IIA on brain MDA, SOD contents in rats.
The results were shown in Table 2. Different concentrations of tanshinone IIA could reduce the SOD content and increase the MDA content in frontal and parietal cortices of the ischemic hemisphere in the ischemia reperfusion group, the differences were statistically significant compared with the ischemia reperfusion group.
Table 2.
Effects of tanshinone IIA on brain MDA, SOD contents in rats (x̄±s, n=6)
DISCUSSION
Cerebral ischemia-reperfusion injury (CIRI) is a common clinical pathological process, within a short time after cerebral ischemia, ATP, CP, glucose and glycogen all reduce, and cyclic adenosine monophosphate (cAMP) increases and activates phospholipase, leading to degradation of phospholipids, and an increase in free fatty acids.[10] During the blood reperfusion after ischemia, production of free radicals increases, which attacks proteins, nucleic acids and unsaturated fatty acids on biofilm, and reacts with free fatty acids to increase the production of lipid peroxide, thus causing cellular and tissue damage.[11]
Radix Salviae Miltiorrhizae has the effects of promoting blood circulation and removing meridian obstruction, eliminating blood stasis and relieving pain, studies showed that tanshinone IIA, its main active constituent, can apparently reduce the release of inflammatory mediators after cerebral ischemia-reperfusion, enhance superoxide dismutase (SOD) activity, and decrease malondialdehyde (MDA) content, thereby reducing the symptoms of cerebral edema, and effectively alleviating focal cerebral ischemia-reperfusion injury in rats.[12,13] The level of SOD activity indirectly reflects the body's ability to scavenge oxygen free radicals, while the level of MDA indirectly reflects the severity of free radical attack to body's cells.
In this paper, the ethanol extract of Radix Salviae Miltiorrhizae was extracted, and the resulting crude extract was purified by means of preparative TLC and preparative liquid chromatography, four compounds were isolated there from, which were tanshinone IIB, hydroxymethylene tanshinone, salvianolic acid B and 9”’-methyl lithospermate B, respectively. To further investigate the effects of Radix Salviae Miltiorrhizae on recovery of cerebral ischemia reperfusion injury in rats, this paper established the rat model of MCAO, and evaluated the symptoms of neurological deficit in each experimental group using the modified Zea Longa suture method and by intravenous injection of different concentrations of tanshinone IIA and nimodipine. In the meanwhile, cerebral infarction size, brain water content, as well as the effects of tanshinone IIA on SOD and MDA in rats were determined.
CONCLUSIONS
Tanshinone IIA could alleviate symptoms of neurological deficit in rats, and the neurological deficit alleviating effect became more obvious with the increase of dose; tanshinone IIA experimental group could reduce the cerebral infarction size and brain water content in rats, different concentrations of tanshinone IIA could reduce the SOD content and increase the MDA content in the frontal and parietal cortices of the ischemic hemisphere in the ischemia reperfusion group, the differences were statistically significant compared with the ischemia reperfusion group. According to the research, Radix Salviae Miltiorrhizae has the protective effects on cerebral ischemia reperfusion injury in rats objectively.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Writing Group. 2nd ed. Beijing: People's Medical Publishing House; 1996. Compilation of National Chinese Herbal Medicine; pp. 221–2. [Google Scholar]
- 2.Tan JK. Doctoral thesis of Sun Yat-sen University. Guangzhou: Sun Yat-sen University; 2005. Studies on the separation of tanshinones and their chemical transformations; pp. 43–5. [Google Scholar]
- 3.Tian GL, Zhang YB, Zhang TY, Yang FQ, Ito Y. Separation of tanshinones from Salvia miltiorrhiza Bunge by high-speed counte-current chromate graphy using step wise elution. J Chromatogr A. 2000;904:107–11. doi: 10.1016/s0021-9673(00)00916-x. [DOI] [PubMed] [Google Scholar]
- 4.Pan X, Niu G, Liu H. Micro wave-assisted extraction of tanshinones from Salvia miltiorrhiza Bunge with analysis by high-performance liquid chromatography. J Chromatogr A. 2001;922:317–75. doi: 10.1016/s0021-9673(01)00949-9. [DOI] [PubMed] [Google Scholar]
- 5.Jiao SL, Zhang ZY, Chu ZD, Ma YT, Meng XL, Zhu XW, et al. Microwave extracting technique of Radix Salviae Miltiorrhizae. Chin Tradit Herb Drugs. 2005;36:1640–2. [Google Scholar]
- 6.Li AR, Wu WL, Channg WL. Isolation and bioactivity of new tanshinones. J Nat Prod. 1987;50:157–60. doi: 10.1021/np50050a004. [DOI] [PubMed] [Google Scholar]
- 7.Feng BS, Li SR. Study on the chemical constituents of Radix Salviae Miltiorrhizae. Acta Pharm Sin. 1980;15:489–93. [PubMed] [Google Scholar]
- 8.Jia NA. Study on the chemical constituents of Radix Salviae Miltiorrhizae. Acta Pharm Sin. 1980;15:489–93. [Google Scholar]
- 9.Wu ZJ, Ouyang MA, Yang CR. Polyphenolic Constituents of Salvia przewalskii. Acta Bot Yunnan. 2007;21:512–6. [Google Scholar]
- 10.He LN, He SB, Yang J, Wang J, Liu C. Protective effect of tanshinones against ischemia injury in cultured primary cortex neurons. Chin Pharmacol Bull. 2001;17:214–6. [Google Scholar]
- 11.Hu XM, Zhou MM, Hu XM, Wang J. Protective effects of Tan IIA on brain injury induced by cerebral ischemia-reperfusion in rats and its effects on energy metabolism. Chin J Clin Pharm. 2006;15:176–9. [Google Scholar]
- 12.Li H, Liu KX. Effects of Tanshinone IIA on expressions of Bcl-2 and caspase-3 following cerebral ischemic reperfusion injury in rats. Chin J Rehabil Med. 2008;23:691–3. [Google Scholar]
- 13.Ye LB, Xi T, Chen F, Wang Y. Protective effect of tanshinone IIA against focal cerebral ischemia reperfusion injury in rats. J Chin Pharm Univ. 2004;35:267–70. [Google Scholar]


