Abstract
Background:
Quercetin is universally distributed in the plant kingdom and is the most abundant flavonoid in the human diet. In a previous study, we have reported that quercetin stimulated glucose uptake in cultured C2C12 skeletal muscle through an insulin-independent mechanism involving adenosine monophosphate-activated protein kinase (AMPK). AMPK is a key regulator of the whole body-energy homeostasis. In skeletal muscle, activation of AMPK increases glucose uptake through the stimulation of the glucose transporter GLUT4 translocation to the plasma membrane. In liver, AMPK decreases glucose production mainly through the downregulation of the key gluconeogenesis enzymes such as phosphoenolpyruvate carboxylase (PEPCK) and Glucose -6-phosphate (G6Pase).
Objective:
To study the effect of quercetin on glucose homeostasis in muscle and liver.
Materials and Methods:
L6 skeletal muscle cells, murine H4IIE and human HepG2 hepatocytes were treated with quercetin (50 μM) for 18 h.
Results:
An 18 h treatment with quercetin (50 μM) stimulated AMPK and increased GLUT4 translocation and protein content in cultured rat L6 skeletal muscle cells. On the other hand, we report that quercetin induced hepatic AMPK activation and inhibited G6pase in H4IIE hepatocytes. Finally, we have observed that quercetin exhibited a mild tendency to increase the activity of glycogen synthase (GS), the rate-limiting enzyme of glycogen synthesis, in HepG2 hepatocytes.
Conclusions:
Overall, these data demonstrate that quercetin positively influences glucose metabolism in the liver and skeletal muscle, and therefore appear to be a promising therapeutic candidate for the treatment of in type 2 diabetes.
Keywords: Akt, adenosine monophosphate-activated protein kinase, glucose transporter, glucose-6-phosphatase, glycogen synthase, insulin resistance, Quercetin, type 2 diabetes mellitus
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by elevated blood glucose levels stemming from impaired insulin secretion and/or increased cellular insulin resistance. This form of diabetes accounts for 90-95% of diabetes cases and is usually associated with unhealthy body weight and sedentary lifestyle.[1] The current number of people affected by T2DM is approximately 382 million and is expected to rise to 471 million in the next 20 years.[2,3]
There is a growing trend among health care professionals to treat metabolic diseases including T2DM with food or isolated food ingredients. Nutraceuticals is now a widely used term, which describes medicinal products isolated or purified from food, and used to prevent or treat chronic diseases.[4]
Flavonoids constitute a large class of polyphenols that are common in plants and food of plant origin. They mostly exist in nature as glycosides (attached to sugar) and are subdivided into the following main structural subclasses: Flavonols, flavones, flavanols, flavanones, anthocyanidins and isoflavones.[5] Compounds belonging to this class have been reported to possess a wide range of biological activities including antioxidant, antibacterial, antiviral, anti-fungal, anti-inflammatory and cardioprotective effects.[6,7,8,9,10,11]
Quercetin (3,3′,4′,5,7 pentahydroxyflavone), also referred to as quercetin aglycone, has a flavonol backbone, and is one of the major dietary polyphenols found in vegetables, fruits, coffee and tea. In the United States, the daily consumption of quercetin is estimated to be 25 mg/day and mostly comes from food.[12] Like certain other flavonoids, quercetin has been reported to possess anti-cancer, anti-inflammatory, anti-coagulant and anti-hypertensive properties.[13,14,15,16,17] In addition, it has the most potent anti-oxidant activity within the flavonoid family.[18]
Growing evidence also indicates that quercetin influences glucose and lipid metabolism. Studies carried out in human colon adenocarcinoma (Caco-2) cells have shown that quercetin reduced the intestinal glucose absorption through the inhibition of the GLUT2 glucose transporter.[19] In the pancreas, the in vitro studies revealed that quercetin improved the glibenclamide-induced insulin secretion and protected the β-cells from oxidative damage by hydrogen peroxide.[20] In addition, quercetin exerted antidiabetic effects in db/db mice and streptozotocin (STZ)-induced rat models of diabetes.[21,22]
In a previous study, we have demonstrated that quercetin increased basal glucose uptake in cultured C2C12 skeletal muscle cells through an insulin-independent mechanism implicating the activation of AMPK.[23] Of note, AMPK is known to promote glucose uptake though the recruitment of GLUT4 transporters to the plasma membrane.[24] The present study was carried out firstly to determine the effect of quercetin on GLUT4 translocation in skeletal muscle. L6 myocytes were selected because they express more GLUT4 proteins than our previous C2C12 cellular model.
Secondly, our objective was to study the effect of quercetin on hepatic glucose homeostasis. Notably, AMPK plays an important role in the liver and is an important target for oral anti-diabetic agents such as metformin and thiazoldinediones.[25,26] Indeed, AMPK activation leads to the suppression of hepatic gluconeogenesis and to the lowering of fasting blood glucose in diabetic patients. We hypothesized that quercetin also stimulates hepatic AMPK, thereby inhibiting glucose-6-phosphatase, the rate-limiting enzyme of gluconeogenesis in liver. Finally, T2DM is associated with altered glycogen metabolism in the form of a reduction of postprandial glycogen synthesis.[27] Therefore, we aimed to investigate the effect of quercetin on hepatic glycogenesis in an attempt to gain further insight into the cellular and molecular mechanisms underlying the antidiabetic effects of quercetin.
MATERIALS AND METHODS
Measurement of 3H-glucose uptake
L6 skeletal muscle cells stably transfected to overexpress GLUT4 harboring a myc epitope on the first exofacial loop of the transporter (L6 GLUT4myc) were provided by Dr. Amira Klip (The Hospital for Sick Children, Toronto, ON, Canada). Cell culture and the 3H-deoxyglucose uptake assay were performed as previously described.[28] Briefly, cells were cultured under standard condition in 12-well plates and were proliferated to 70% confluence in minimum essential medium alpha (α-MEM) supplemented with 10% (v/v) fetal bovine serum (FBS). Cells were then switched to a medium containing 2% FBS for 5-7 days to allow differentiation into multinucleated myotubes. Myotubes were serum-starved for 4 h before being treated with quercetin (50 μM, Sigma-Aldrich, St. Louis, MO, USA) or vehicle (DMSO, 0.1%) for 18 h in complete differentiation medium. Stimulation with 100 nM insulin for 15 min served as the positive control. Pilot dose–response study of quercetin indicated that glucose uptake following an 18 h treatment peaked at a dose of 50 μM. The 50 μM concentration was therefore selected for further testing. Moreover, this concentration had no effect on the viability of the cells (not shown). By the end of the treatment, myotubes were incubated in transport solution [140 mM NaCl, 20 mM HEPES-Na, 2.5 mM MgSO4, 1 mM CaCl2, 5 mM KCl, 10 μM 2-Deoxy-Glucose and 0.5 μCi/ml 2-deoxy-D-[3H] glucose, (pH 7.4)] for 5 min at room temperature. The cells were then lysed with 1 M KOH and the cell lysates were transferred to scintillation vials for 3H radioactivity counting and expressed as fold increase over control. Nonspecific uptake was measured in the presence of cytochalasin B (10 μM) and was subtracted from all values.
Determination of cell surface GLUT4 (OPD assay)
Cell surface GLUT4myc levels were assessed by an antibody-coupled colorimetric assay.[29] Briefly, L6-GLUT4myc myoblasts were cultured in 24-well plates until confluence and serum-starved for 4 h before incubation with either quercetin (50 μM) or vehicle (DMSO, 0.1%) for 18 h; insulin (100 nM) incubated for the last 15 min served as a positive control. At the end of incubation, cells were quickly washed with ice-cold PBS and put in the presence of an anti-c-myc antibody (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 60 min at 4°C. Cells were then washed and fixed in 3% paraformaldehyde for 3 min on ice. The fixative was neutralized by incubation in 10 mM glycine in ice-cold PBS for 10 min. Goat serum (5%) was used to block cells for 30 min before incubation for an additional 60 min with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG at 4°C (1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA). Cells were then washed five times with ice-cold PBS and incubated with O-phenylenediamine dihydrochloride (OPD) reagent (1 ml/well) (Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 30 min. Addition of 0.25 ml of 3 M HCl to each well stopped the reaction. The supernatant was collected and its absorbance was measured at 492 nm. Absorbance associated with nonspecific binding (primary antibody omitted) was used as a blank.
Determination of G6Pase activity in H4IIE hepatocyte
H4IIE murine hepatocytes (American Type Culture Collection, Rockville, MD, USA) were cultured in monolayer in 10% FBS containing Dulbecco's modified Eagle's medium (DMEM; 4.5 g/l glucose). Cells were grown to 90% confluence in 12-well plates and treated with quercetin (50 μM), vehicle control (DMSO) or insulin (100 nM) for 16 h in serum-free medium. At the end of treatment, cells were washed in HEPES-buffered saline (10 mM HEPES, pH 7.4, 150 mM NaCl) at 37°C. The rate of glucose production in the presence of a non-limiting amount of glucose-6-phosphate (G6P) served to assess G6Pase activity. Total glucose production was measured using a commercial glucose assay kit (AutoKit Glucose; Wako Diagnostics, Richmond, VA, USA) with modifications, as previously described.[30,31] Briefly, 200 μL of AutoKit glucose buffer solution was diluted in water (1:4) and added to each well. Cells were then lysed using 50 μl of 0.05% Triton X-100 in similarly diluted AutoKit Glucose buffer solution. Twenty micromoles (final concentration) of G6P were then added to each well for a final volume of 275 μl, and the plates were incubated for 40 min at 37°C. Subsequently, 500 μl of AutoKit Glucose color reagent were added and incubation was continued for 5 min. Samples were rapidly transferred to microcentrifuge tubes and centrifuged at 3000 × g for 5 min at room temperature. Absorbance of the supernatant at 505 nm was measured at room temperature. A standard curve was run in parallel and served to calculate glucose concentrations. Control wells without exogenous G6P were included on each plate for each treatment condition and activity measured from these wells was subtracted from activity measured in the presence of exogenous G6P. The bicinchoninic acid (BCA) assay (Thermo Scientific Pierce Protein Research, Rockford, IL, USA) was used to assess cell protein content.[32] G6Pase activity was then expressed in relation to protein content on a well-by-well basis. Three independent experiments in cells of different passages were performed for each of the selected test compounds, with four replicates per condition per experiment.
Measurement of GS activity in HepG2 hepatocytes
To assess GS activity, [14C]-glucose incorporation into glycogen was measured with modifications of the previously described method.[33] Human hepatoma HepG2 cell lines were obtained from American Type Culture Collection (Rockville, MD, USA). Cells were grown in DMEM/nutrient mixture F12 medium 50:50 (Fisher Scientific, Tustin, CA, USA) supplemented with 10% FBS. Cells were cultured to confluence in 6-well plates then treated overnight (16-18 h) with quercetin (50 μM) or vehicle (DMSO, 0.1%); cells stimulated for 15 min with 100 nM of insulin served as positive controls. After treatment, cells were washed with PBS and were scraped into 500 μl of GS assay buffer (50 mM glycylglycine, 100 mM NaF, 20 mM EDTA, 0.5% glycogen, pH 7.4), to which complete protease inhibitor cocktail was added just before the assay. After centrifugation at 1000 × g for 20 min at 4°C, supernatants were separated into aliquots with GS assay buffer to equal protein content (Bio-Rad Laboratories, CA, USA). Thirty microlitre of supernatant were added to 100 μL of active GS buffer (25 mM glycylglycine, 0.275 mM UDP-glucose, 0.12 μCi/mL U-14C UDP-glucose, 1% glycogen, 1 mM EDTA, 10 mM sodium sulfate, pH 7.5). Another 30 L of supernatant were added to 100 uL of buffer solution for total GS (25 mM Tris, 5 mM UDP-glucose, 0.12 μ Ci/mL U-14C UDP-glucose, 1% glycogen, 3 mM EDTA, 5 mM G6P, pH 7.9). Assay tubes were incubated at 30°C for 120 min. After incubation, 90 μL of contents were spotted onto Whatman 31ET Chr filter papers, which were immediately immersed in 70% ethanol at 4°C, mixed for 30 min, then washed twice in 60% ethanol for 30 min to remove unincorporated substrate from precipitated glycogen. Filters were covered with acetone for 2-3 min, air-dried, and radioactivity was counted with 6 ml of liquid scintillation fluid. Activity of GS was expressed as the activity ratio of active form to total GS (fractional activity).
Western immunoblotting
Effects of quercetin on insulin and AMPK signaling pathways in L6 and H4IIE cells, as well as on GLUT4 expression in L6 cells were assessed by western immunoblot. Cells were cultured in 6-well plates and quercetin (50 μM) or vehicle alone (DMSO) were applied for 18 h to 5-7 day differentiated L6 cells. Thirty minutes prior to the end of the treatment, insulin (100 nM) or aminoimidazole carboxamide ribonucleotide (AICAR; 1 mM) were added to some vehicle-treated wells as positive controls. Cells were lysed in 250 ml of lysis buffer (25 mM Tris–HCl, 25 mM NaCl, 0.5 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, pH 7.4). This lysis buffer contained protease inhibitors (Complete Mini; Roche, Mannheim, Germany), 1 mM phenylmethanesulfonyl fluoride, as well as phosphatase inhibitors (1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium fluoride). Cells were lysed on ice for 15 min, Cell lysates were transferred into microcentrifuge tubes, periodically vortexed, and centrifuged at 600 g for 10 min at 4°C. Supernatants were decanted and stored at −80°C until further analysis. Protein content was again assayed using the bicinchoninic acid method and bovine serum albumin as a standard. Lysates were diluted to a concentration of 1.25 mg total protein per ml and boiled for 5 min in reducing sample buffer (62.5 mM Tris–HCl, 2% SDS, 10% glycerol, 5% b-mercaptoethanol and 0.01% bromophenol blue, pH 6.8). For each sample, 20 μg of protein were separated on 10% polyacrylamide mini-gels and electrotransferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Skim milk (5%) in Tris-buffered saline (20 mM Tris–HCl, 137 mM NaCl, pH 7.6) containing 0.1% Tween 20 (TBST) was used to block membranes for 2 h at room temperature. Membranes were then incubated overnight with phospho-AMPK, phospho-Akt, GLUT4 or β-actin antibodies (1:1000 dilution, Cell Signalling Technology, Danvers, MA, USA). Thereafter, membranes were washed four times in wash buffer for 15 min each at room temperature before being incubated with HRP-coupled secondary antibody (1:10 000) for 1 h. Finally, membranes were washed five times for 10 min in wash buffer. Immunoblotted proteins were visualized by enhanced chemiluminescence and quantified by the Scion Image program (Scion Corporation, Frederick, MD, USA). Experiments were repeated on three different passages of cells, each passage containing all conditions in parallel.
Statistical analysis
Data are presented as the mean ± SEM for the indicated number of replicates and of independent experiments. The StatView software (SAS Institute Inc, Cary, NC, USA) served to analyse results by one-way analysis of variance (ANOVA) with a Fisher post-hoc test. A P ≤ 0.05 was taken to represent statistical significance.
RESULTS
Quercetin increases glucose uptake, GLUT4 translocation and GLUT4 protein content in L6 myotubes
After 18 h treatment, quercetin significantly stimulated basal glucose uptake in L6 cells by 70 ± 20%, [P < 0.05, Figure 1a]. Insulin treated cells (100 nM insulin, 15 min) served as positive control and exhibited 75 ± 13% increase in glucose uptake over vehicle control [P < 0.05, Figure 1a].
Figure 1.
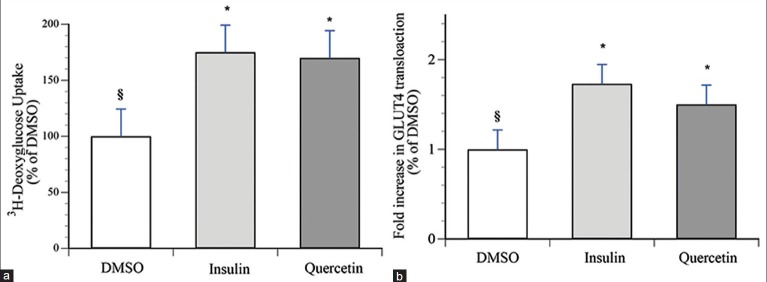
Quercetin increases glucose uptake and GLUT4 translocation in L6 GLUT4myc myotubes. Cells were treated with either 50 μM of quercetin, or with vehicle (0.1% DMSO) for 18 h. 100 nM insulin was applied for the last 15 min of the treatment in vehicle-treated cells. (a) Glucose uptake was assessed by the incorporation of 3H-deoxyglucose as described in Materials and Methods. Data are expressed relative to basal uptake observed in vehicle control treated cells (100%). Data represent the mean ± SEM of 3 experiments, each experiment composed of 3-4 replicates per condition. (b) GLUT4 translocation was assessed by measuring cell surface GLUT4myc using an enzyme-linked colorimetric assay, as described in Materials and Methods. Data are expressed relative to basal translocation observed in vehicle control treated cells (1.0). Data represent the mean ± SEM of 3 experiments, each experiment composed of 3-4 replicates per condition. * Indicates a significant (P ≤ 0.05) difference from the vehicle control group and § indicates a significant (P ≤ 0.05) difference from insulin group as assessed by ANOVA
Translocation of glucose transporter GLUT4 to the skeletal muscle cell plasma membrane underlies glucose uptake in this tissue. Therefore, we examined the effect of quercetin on GLUT4 translocation in L6-GLUT4myc cells. Our results show that quercetin significantly stimulated GLUT4 translocation in L6 muscle cells by 1.5-fold, compared to 1.7-fold, the maximal effect of insulin [P < 0.05, Figure 1b]. Furthermore, quercetin increased GLUT4 content by approximately 2 fold compared to DMSO vehicle control [P < 0.05, Figure 2].
Figure 2.
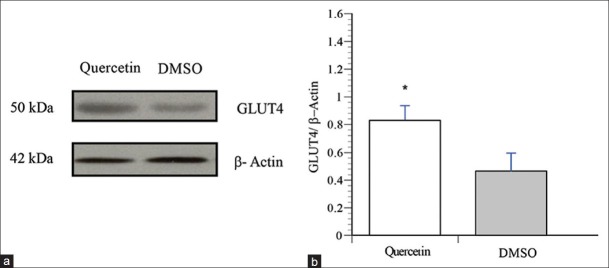
Quercetin increases GLUT4 content in L6 myotubes. Cells were treated with vehicle (0.1% DMSO, 18 h), quercetin (50 μM, 18 h), or AICAR (1mM, 30 min). Immunoblots were probed with an anti-GLUT4 antibody. (a) Representative blots are shown. (b) Data are expressed as GLUT4/β-actin, and are given as mean ± SEM from 3 experiments
Quercetin increases the phosphorylation of AMPK in L6 myotubes
To investigate the signaling pathways involved in quercetin's actions, L6 myotubes were treated with quercetin (50 μM) for 18 h. AICAR (2 mM), a well-known activator of AMPK, served as a positive control and was applied to the cells for 30 min. The phosphorylation of AMPK was significantly increased by quercetin [Figure 3, P < 0.05] indicating an elevation in AMPK activity. As previously reported in C2C12 cells[23] and in contrast to insulin, quercetin had no effect on Akt, a downstream target of PI3K in insulin-stimulated glucose uptake (results not shown).
Figure 3.
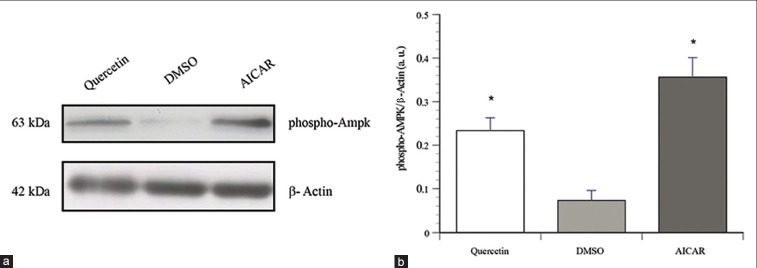
Quercetin increases AMPK phosphorylation in L6 myotubes. Shown are representative immunoblots of cells treated with vehicle (0.1% DMSO, 18 h), quercetin (50 μM, 18 h), or AICAR (1 mM, 30 min). Immunoblots were probed with phospho-specific antibodies against AMPK (Thr 172) as described in Materials and Methods section. Immunoblots were probed with β-actin as loading control. (a) Representative immunoblots. (b) Data are expressed as pAMPK/β-actin, and are given as mean ± SEM from 3 experiments
Quercetin promotes the phosphorylation of AMPK and suppresses G6Pase activity and in H4IIE hepatocytes
Inhibition of G6Pase activity was assessed following an 18 h treatment with quercetin. Results show that quercetin significantly reduced the activity of G6Pase per mg total protein by 39 ± 5%. As expected, insulin (100 nM, 18 h treatment) diminished G6Pase activity by 58.7 ± 1.6 [P < 0.05, Figure 4a]. On the other hand, after similar treatment period (18 h), quercetin induced a significant 3-fold increase in AMPK activation in H4IIE over the untreated control [P < 0.05, Figure 4b, c].
Figure 4.
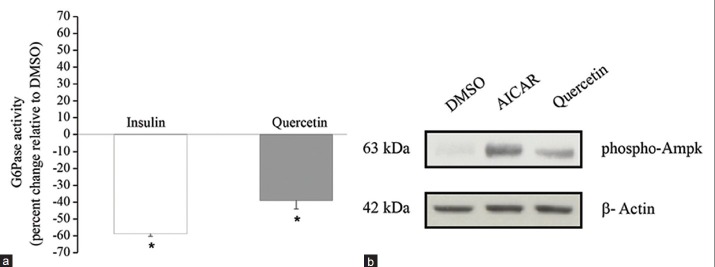
Quercetin treatment inhibits G6Pase activity and stimulated the phosphorylation of AMPK in H4IIE hepatocytes. H4IIE cells were treated with either vehicle (0.1% DMSO, 18 h) or quercetin (50 μM, 18 h). (a) G6Pase activity was assessed by measuring the rate of glucose formation in the presence of a non-limiting amount of G6P as described under “Materials and Methods.” Insulin (100 nM, 18 h) served as the positive control. Results are expressed as mean % change ± SEM, relative to a vehicle-treated control group for three independent experiments of four to six replicates per condition. G6Pase activity data were normalized to total protein content per well. * denotes a significant difference (P ≤ 0.05). (b) Phosphorylation of AMPK was measured by western immunoblot. AICAR (2 mM) applied for 30 min served as the positive control. The upper immunoblot was probed with anti-phospho-AMPK and the lower blot was probed with β-actin as loading control. Blots shown are representative from 3 experiments
Quercetin tends to increase GS activity in HepG2 cells
To study the effect of quercetin on GS activity, UDP-[14C] glucose incorporation into glycogen was determined in HepG2 cells. The data shown in Figure 5 demonstrate that quercetin shows only a mild tendency to increase fractional GS activity in HepG2 cells (30% increase over vehicle control, NS, P = 0.18). This contrasted the results obtained with insulin, which significantly increased fractional GS activity by two fold over vehicle control [Figure 5, P < 0.05].
Figure 5.
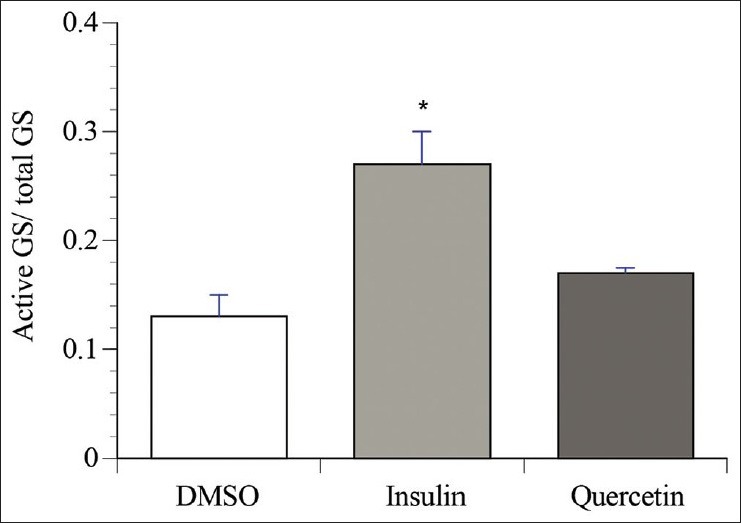
Effect of quercetin on glycogen synthase in HepG2. HepG2 cells were incubated for 18 h with either 0.1% DMSO (vehicle), or quercetin (50 μM). Insulin (100 nM) applied for 15 min and served as positive control. GS activity was assayed in the supernatants of cell lysates as described under “Materials and Methods”. Results were expressed as fractional activities (active/total). Values shown are means ± S.E.M. for three different experiments. * denotes a significant difference (P ≤ 0.05) from the vehicle control group
DISCUSSION
Skeletal muscle and liver are the main regulators of peripheral glucose homeostasis. Around 70-80% of postprandial glucose uptake is carried out by skeletal muscle. On the other hand, the liver is responsible for more than 80% of glucose release during the fasting state. In T2DM, increased hepatic glucose output and decreased glucose uptake by skeletal muscle cells are the principal contributors to the associated hyperglycemic state.[34,35]
In the fed state, insulin stimulates glucose uptake by peripheral tissues including skeletal muscle. GLUT4 is the main insulin-responsive glucose transporter and is located primarily in skeletal muscle cells, cardiac muscle cells and adipocytes. In the basal state, about 95% of GLUT4 are present in small intracellular vesicles; the rest reside in the plasma membrane. Upon insulin stimulation, GLUT4 undergoes a rapid translocation to the plasma membrane and glucose uptake is increased. Insulin-stimulated GLUT4 translocation is mainly mediated through a PI3K-dependent pathway.[36] AMPK is a metabolic stress-sensing protein kinase that is also known to stimulate GLUT4 translocation. Under stress conditions such as hypoxia, physical exercise and inhibition of mitochondrial respiration, AMPK responds to the depletion of ATP content and to an increase in the cellular AMP/ATP ratio with an increase in its phosphorylated active form.[37] We have previously shown that quercetin is responsible, at least in part, for the biological activity of certain antidiabetic Boreal forest plants stemming from Canadian Aboriginal traditional medicine.[23] We notably demonstrated that this flavonoid increases glucose transport and leads to the phosphorylation of AMPK in C2C12 myocytes. The present studies confirm that quercetin acts similarly in L6 myotubes, a cell line expressing a higher level of GLUT4. Moreover, the flavonoid exerted an effect that was comparable to an optimal concentration of insulin. In the present studies, we were also able to further evaluate the mechanism of action of quercetin by measuring the cell surface content of GLUT4, thereby directly assessing GLUT4 translocation to the plasma membrane. The results clearly demonstrate that quercetin significantly increases the appearance of GLUT4 transporters on the surface of L6 GLUT4myc cells. Interestingly, quercetin also increased the total content in GLUT4 protein in L6 cells. Together with the aforementioned increases in AMPK and in glucose transport, our results are consistent with the interpretation that quercetin activates AMPK-dependent and insulin-independent pathways to enhance GLUT-4 content and translocation in skeletal muscle cells.
We also examined the effects of quercetin in cultured hepatocytes in order to expand the understanding of the antidiabetic potential of the flavonoid. Indeed, the liver regulates glucose homeostasis by maintaining equilibrium between glucose storage in the form of glycogen (glycogenesis), on the one hand, and glucose production through glycogen breakdown (glycogenolysis) or de novo synthesis of glucose (gluconeogenesis), on the other hand. Of note, quercetin is a known nonspecific inhibitor of glycogen phosphorylase, the enzyme that catalyzes the rate-limiting step of glycogenolysis.[38,39]
Gluconeogenesis is under the control of two main enzymes; G6Pase and phosphoenolpyruvate carboxykinase (PEPCK). Activation of AMPK in primary culture of hepatocytes was shown to reduce the gene expression of the latter two enzymes and to inhibit hepatic glucose output. The data of the present study demonstrates that quercetin also activates AMPK and can significantly reduce G6Pase activity in H4IIE hepatocytes to a level nearly equivalent to an optimal concentration of insulin. Hence, quercetin possesses the potential to reduce hepatic glucose production.
Finally, aside from reducing liver gluconeogenesis, hepatic glucose production can also be attenuated through the increased storage of glucose in the form of glycogen. The rate-limiting enzyme for hepatic glycogenesis is GS. The activity of this enzyme is modulated by allosteric factors such as intracellular G6P and by phosphorylation/dephosphorylation states under the control of kinases and phosphatases. Under basal condition, GS is kept in its inactive form through phosphorylation by the serine/threonine protein kinase, glycogen synthase kinase 3 (GSK-3).[40] The activity of the latter enzyme is inhibited by insulin through phosphorylation, thus relieving its inhibition of GS. Several studies have reported that certain flavonoids such as kaempherol glycosides and rutin can stimulate glycogen synthesis.[41,42] In the present study, quercetin had a tendency to increase GS activity in HepG2 cells, but the effect failed to reach statistical significance as opposed to the insulin positive control. Further studies will be necessary to determine if a significant action can be obtained at other concentrations or periods of stimulation.
Collectively, the data of the present study confirm that quercetin can influence glucose homeostasis at the level of both skeletal muscle and liver. Indeed, we have established that the flavonoid stimulates GLUT4 translocation and expression in skeletal muscle, by mechanisms associated with the activation of AMPK rather than insulin-dependent pathways such as Akt. Similarly, in cultured hepatocytes, quercetin also activated AMPK and this was associated with a significant inhibition of G6Pase. Therefore our findings provide further insight into the cellular and molecular mechanisms of the antidiabetic activity of quercetin and a rationale for its use in the management of T2DM.
ACKNOWLEDGMENTS
This work was supported by a Team Grant from the Canadian Institutes of Health Research (CTP-79855, CIHR Team in Aboriginal Antidiabetic Medicines) to PSH and was conducted with the consent and support of the Cree Nation of Eeyou Istchee (Eastern James Bay in Quebec, Canada) as well as that of the Cree Board of Health and Social Services of James Bay (Quebec, Canada).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: Peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clinl Endocrinol Metab. 2004;89:463–78. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.IDF Diabetes Atlas, Sixth edition. [Last accessed on 2014 Sep 3]. Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf .
- 4.Kasbia GS. Functional foods and nutraceuticals in the management of obesity. Nutr Food Sci. 2005;35:344–52. [Google Scholar]
- 5.Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, et al. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 6.Teixeira S, Siquet C, Alves C, Boal I, Marques MP, Borges F, et al. Structure-property studies on the antioxidant activity of flavonoids present in diet. Free Radic Biol Med. 2005;39:1099–108. doi: 10.1016/j.freeradbiomed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Cushnie TP, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Emerit I, Huang CY, Serejo F, Filipe P, Fernandes A, Costa A, et al. Oxidative stress in chronic hepatitis C: A preliminary study on the protective effects of antioxidant flavonoids. Hepatogastroenterology. 2005;52:530–6. [PubMed] [Google Scholar]
- 9.Orhan DD, Ozcelik B, Ozgen S, Ergun F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol Res. 2010;165:496–504. doi: 10.1016/j.micres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Gallego J, Sanchez-Campos S, Tunon MJ. Anti-inflammatory properties of dietary flavonoids. Nutr Hosp. 2007;22:287–93. [PubMed] [Google Scholar]
- 11.Cook NC, Samman S. Flavonoids: Chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7:66–76. [Google Scholar]
- 12.Lamson DW, Brignall MS. Antioxidants and cancer, part 3: Quercetin. Altern Med Rev. 2000;5:196–208. [PubMed] [Google Scholar]
- 13.Zheng SY, Li Y, Jiang D, Zhao J, Ge JF. Anticancer effect and apoptosis induction by quercetin in the human. Mol Med Rep. 2012;5:822–6. doi: 10.3892/mmr.2011.726. [DOI] [PubMed] [Google Scholar]
- 14.Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY, et al. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218:44–52. doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Beretz A, Cazenave JP, Anton R. Inhibition of aggregation and secretion of human platelets by quercetin and other flavonoids: Structure-activity relationships. Agents Actions. 1982;12:382–7. doi: 10.1007/BF01965408. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep. 2009;61:67–75. doi: 10.1016/s1734-1140(09)70008-8. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Oue E. Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci Biotechnol Biochem. 2006;70:933–9. doi: 10.1271/bbb.70.933. [DOI] [PubMed] [Google Scholar]
- 18.Kim MR, Lee JY, Lee HH, Aryal DK, Kim YG, Kim SK, et al. Antioxidative effects of quercetin-glycosides isolated from the flower buds of Tussilago farfara L. Food Chem Toxicol. 2006;44:1299–307. doi: 10.1016/j.fct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Manzano S, Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol Nutr Food Res. 2010;54:1773–80. doi: 10.1002/mnfr.201000019. [DOI] [PubMed] [Google Scholar]
- 20.Youl E, Bardy G, Magous R, Cros G, Sejalon F, Virsolvy A, et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic beta-cells against oxidative damage via the ERK1/2 pathway. Br J Pharmacol. 2010;161:799–814. doi: 10.1111/j.1476-5381.2010.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011;5:107–11. doi: 10.4162/nrp.2011.5.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res. 2005;51:117–23. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Eid HM, Martineau LC, Saleem A, Muhammad A, Vallerand D, Benhaddou-Andaloussi A, et al. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol Nutr Food Res. 2010;54:991–1003. doi: 10.1002/mnfr.200900218. [DOI] [PubMed] [Google Scholar]
- 24.Pelletier A, Joly E, Prentki M, Coderre L. Adenosine 5’-monophosphate-activated protein kinase and p38 mitogen-activated protein kinase participate in the stimulation of glucose uptake by dinitrophenol in adult cardiomyocytes. Endocrinology. 2005;146:2285–94. doi: 10.1210/en.2004-1565. [DOI] [PubMed] [Google Scholar]
- 25.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Adenosine 5’-monophosphate-activated protein kinase and p38 mitogen-activated protein kinase participate in the stimulation of glucose uptake by dinitrophenol in adult cardiomyocytes. Endocrinology. 2005;146:2285–94. doi: 10.1210/en.2004-1565. [DOI] [PubMed] [Google Scholar]
- 26.Song GY, Gao Y, Wang C, Hu SG, Wang J, Qu DM, et al. Rosiglitazone reduces fatty acid translocase and increases AMPK in skeletal muscle in aged rats: A possible mechanism to prevent high-fat-induced insulin resistance. Chin Med J (Engl) 2010;123:2384–91. [PubMed] [Google Scholar]
- 27.Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004;53:3048–56. doi: 10.2337/diabetes.53.12.3048. [DOI] [PubMed] [Google Scholar]
- 28.Somwar R, Koterski S, Sweeney G, Sciotti R, Djuric S, Berg C, et al. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J Biol Chem. 2002;277:50386–95. doi: 10.1074/jbc.M205277200. [DOI] [PubMed] [Google Scholar]
- 29.Niu W, Huang C, Nawaz Z, Levy M, Somwar R, Li D, et al. Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis. J Biol Chem. 2003;278:17953–62. doi: 10.1074/jbc.M211136200. [DOI] [PubMed] [Google Scholar]
- 30.Heishi M, Ichihara J, Teramoto R, Itakura Y, Hayashi K, Ishikawa H, et al. Global gene expression analysis in liver of obese diabetic db/db mice treated with metformin. Diabetologia. 2006;49:1647–55. doi: 10.1007/s00125-006-0271-y. [DOI] [PubMed] [Google Scholar]
- 31.Lau CH, Chan CM, Chan YW, Lau KM, Lau TW, Lam FC, et al. in vitro antidiabetic activities of five medicinal herbs used in Chinese medicinal formulae. Phytother Res. 2008;22:1384–8. doi: 10.1002/ptr.2513. [DOI] [PubMed] [Google Scholar]
- 32.Simpson RJ. Quantifying protein by bicinchoninic Acid. CSH Protoc 2008. 2008 doi: 10.1101/pdb.prot4722. pdb.prot4722. [DOI] [PubMed] [Google Scholar]
- 33.Franch J, Aslesen R, Jensen J. Regulation of glycogen synthesis in rat skeletal muscle after glycogen-depleting contractile activity: Effects of adrenaline on glycogen synthesis and activation of glycogen synthase and glycogen phosphorylase. Biochem J. 1999;344:231–5. [PMC free article] [PubMed] [Google Scholar]
- 34.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. ix. [DOI] [PubMed] [Google Scholar]
- 35.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98:378–85. doi: 10.1172/JCI118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James DE, Strube M, Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989;338:83–7. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: Activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–31. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- 38.Gregus Z, Nemeti B. Glutathione-dependent reduction of arsenate by glycogen phosphorylase responsiveness to endogenous and xenobiotic inhibitors. Toxicol Sci. 2007;100:44–53. doi: 10.1093/toxsci/kfm212. [DOI] [PubMed] [Google Scholar]
- 39.Jakobs S, Fridrich D, Hofem S, Pahlke G, Eisenbrand G. Natural flavonoids are potent inhibitors of glycogen phosphorylase. Mol Nutr Food Res. 2006;50:52–7. doi: 10.1002/mnfr.200500163. [DOI] [PubMed] [Google Scholar]
- 40.Greenberg CC, Jurczak MJ, Danos AM, Brady MJ. Glycogen branches out: New perspectives on the role of glycogen metabolism in the integration of metabolic pathways. Am J Physiol Endocrinol Metab. 2006;291:E1–8. doi: 10.1152/ajpendo.00652.2005. [DOI] [PubMed] [Google Scholar]
- 41.Cazarolli LH, Folador P, Moresco HH, Brighente IM, Pizzolatti MG, Silva FR. Stimulatory effect of apigenin-6-C-beta-L-fucopyranoside on insulin secretion and glycogen synthesis. Eur J Med Chem. 2009;44:4668–73. doi: 10.1016/j.ejmech.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Cazarolli LH, Folador P, Pizzolatti MG, Mena Barreto Silva FR. Signaling pathways of kaempferol-3-neohesperidoside in glycogen synthesis in rat soleus muscle. Biochimie. 2009;91:843–9. doi: 10.1016/j.biochi.2009.04.004. [DOI] [PubMed] [Google Scholar]


