Abstract
Untreated obstructive sleep apnea (OSA) is an independent risk factor for hypertension, myocardial infarction, and stroke. The repetitive hypoxia/reoxygenation and sleep fragmentation associated with OSA impair endothelial function. Endothelial dysfunction, in turn, may mediate increased risk for cardiovascular diseases. Specifically, in OSA, endothelial nitric oxide availability and repair capacity are reduced, whereas oxidative stress and inflammation are enhanced. Treatment of OSA improves endothelial vasomotor tone and reduces inflammation. We review the evidence and possible mechanisms of endothelial dysfunction as well as the effect of treatment on endothelial function in OSA.
Obstructive sleep apnea (OSA), a condition that affects 9% to 25% of the American adult population, is characterized by repetitive apneas, causing hypoxemia and arousals from sleep.1,2 Untreated OSA is an independent risk factor for hypertension, myocardial ischemia, and stroke3–5; however, the mechanisms underlying the association between OSA and cardiovascular diseases are not well understood. More recent research suggests that OSA directly affects the endothelium, providing a possible link between OSA and the development of cardiovascular disease.
Endothelial dysfunction is an early marker of vascular abnormality preceding clinically overt cardiovascular disease. The intact endothelium regulates vascular tone and repair capacity, maintaining proinflammatory, anti-inflammatory, and coagulation homeostasis.6 Alteration of these homeostatic pathways results in endothelial dysfunction before structural changes in the vasculature.7
Abnormal endothelial function may play an important role in increased cardiovascular risk associated with OSA. For example, OSA patients who are otherwise free of cardiovascular comorbidities have increased endothelial oxidative stress, inflammation, and reduced endothelial repair capacity, strongly suggesting that OSA independently impairs endothelial function.8–13 Endothelium-dependent vasodilation is impaired in otherwise healthy patients with OSA, suggesting reduced nitric oxide (NO) bioavailability.14,15 Furthermore, treatment of OSA improves endothelial function and appears to reduce the risk of fatal and nonfatal cardiovascular events.16,17 Reversal of endothelial dysfunction may play an important role in treatment-mediated reduction of cardiovascular risk in these patients.
The present review will first focus on evidence of endothelial dysfunction in patients with untreated OSA. Possible mechanisms of endothelial dysfunction in OSA will then be considered. Lastly, the effect of OSA treatment on endothelial function will be discussed.
Evidence of Endothelial Dysfunction in Untreated OSA
The evaluation of endothelial function in patients with OSA includes assessment of vasomotor tone, endothelial proinflammatory/anti-inflammatory activity, coagulation homeostasis, and endothelial repair capacity.
Regulation of Vasomotor Tone
The healthy endothelium maintains balance between vasodilation and vasoconstriction in response to physical and biochemical stimuli6. Endothelial dysfunction is characterized by impaired endothelium-dependent vascular relaxation in response to mediators such as acetylcholine or to increased blood flow.7 This can be clinically measured by ultrasound studies of forearm blood flow responses to various endothelial stimuli. Flow-mediated dilation, which measures the change of the brachial artery diameter after a brief period of forearm ischemia, is a noninvasive method commonly used to assess endothelium-dependent vasodilation.18 The transient brachial artery dilation that follows ischemia appears to be primarily regulated by NO bioavailability, although endothelium-derived prostanoids may play an adjunctive role.19–21
The cardiology and diabetes literature has shown that impaired endothelial-dependent vasodilation in response to acetylcholine or reactive hyperemia has prognostic value based on correlations with increased risk of subsequent cardiovascular events (such as myocardial infarction and stroke). Researchers in the sleep apnea field have shown that OSA, like coronary artery disease and diabetes, can independently contribute to impaired endothelial function as well. For example, endothelium-dependent vasodilation measured by forearm blood flow after intraarterial infusion of acetylcholine, an endothelium-dependent vasodilator, is reduced in otherwise healthy patients with severe OSA compared with age- and body mass index (BMI)–matched controls.14,15 Similarly, flow-mediated dilation is reduced in otherwise healthy patients with untreated OSA, indicating reduced NO bioavailability.16 Among 1,037 elderly patients with OSA who participated in the Sleep Heart Health/Cardiovascular Health Study, flow-mediated dilation remained impaired even after adjustment for BMI and cardiovascular comorbidities.22 Brachial artery reactivity correlated with the degree of hypoxemia rather than apnea-hypopnea index (AHI), suggesting that hypoxemia/reoxygenation plays a crucial role in reducing NO bioavailability and promoting endothelial dysfunction in OSA.22 However, we are also aware of studies showing no major association between OSA and endothelial dysfunction that have not been published, presumably as a result of publication biases. Thus, further work is required.
Although impaired flow-mediated dilation suggests that there is a decrease in NO bioavailability, further studies have shown that, in the plasma and in endothelial cells, there is indeed less NO in OSA patients compared with controls. Initial studies show that circulating NO levels are decreased in untreated OSA.23,24 Furthermore, plasma levels of asymmetric NG,NG-dimethylarginine, an endogenous inhibitor of endothelial NO synthase (eNOS), are increased and correlate inversely with flow-mediated dilation in patients with untreated OSA.25 In freshly harvested venous endothelial cells, there are decreased eNOS activity and increased nitrotyrosine production, a byproduct of NO degradation.13 In aggregate, these studies provide direct evidence that NO bioavailability is reduced in OSA patients without overt cardiovascular disease.
On the other hand, the evidence for increased production of vasoconstricting substances such as endothelin-1 and angiotensin II in patients with OSA is inconsistent. Plasma levels of aldosterone and angiotensin II in patients with OSA have been reported to be elevated or similar to controls.26,27 Endothelin-1 levels, however, have been variable; and the effect of OSA itself on endothelin-1 appears complicated. For instance, nocturnal plasma levels of endothelin-1 are increased in patients with untreated OSA and unspecified comorbidities compared with their bedtime values, indicating an acute adverse effect of OSA on nocturnal regulation of vasomotor tone.28 Similarly, both nocturnal and diurnal endothelin-1 levels are higher in patients with OSA compared with slightly younger and less obese healthy controls.28 However, coexistent cardiovascular diseases may influence endothelin-1 levels independently from OSA; and studying patients with comorbidities who may not be well matched by age and BMI can be problematic. In patients with OSA and coexistent cardiovascular diseases, plasma levels of the endothelin-1 precursor big endothelin-1 were elevated, whereas levels of endothelin-1 were similar, compared with healthy age-matched controls,29 suggesting that measuring endothelin-1 levels alone may not be reflective of increased production in general. Another study showed that untreated OSA patients do have greater morning plasma endothelin-1 levels than controls with lower BMI and systolic blood pressure.30 Similarly, endothelin-1 levels are elevated in 9 normotensive OSA patients compared with healthy controls; however, the small sample size limits the strength of this finding.31 In contrast, plasma levels of endothelin-1 were not different in either otherwise healthy OSA patients or those with coexistent cardiovascular diseases compared with their matched controls.26,32 Furthermore, nocturnal and diurnal endothelin-1 plasma levels were greater in hypertensive but not in normo-tensive OSA patients compared with healthy controls, suggesting that OSA does not affect plasma endothelin-1 levels in the absence of coexistent cardiovascular diseases.27 These inconsistent findings of plasma endothelin-1 levels in patients with OSA likely reflect the predominantly abluminal release of endothelin-1.33 Vascular production of endothelin-1 is elevated, whereas circulating levels are similar to controls, in a rat model of arterial hypertension.34 Therefore, normal circulating levels of endothelin-1 do not exclude its elevated vascular production in OSA.
In summary, the published literature suggests that OSA adversely affects endothelial regulation of peripheral vasomotor tone. Endothelial dysfunction identified in the peripheral vasculature strongly predicts coronary endothelial dysfunction.35 Impaired endothelial vasomotor tone may mediate increased risk for cardiovascular diseases in patients with OSA.
Endothelial Proinflammatory/Anti-Inflammatory Homeostasis
The repetitive hypoxia/reoxygenation associated with apneas and hypopneas in OSA up-regulates the production of inflammatory mediators and the expression of adhesion molecules. In general, accumulation and adhesion of circulating leukocytes to the vascular endothelium lead to vessel inflammation and progression of atherosclerosis.6,36 In otherwise healthy patients with OSA, levels of circulating soluble adhesion molecules that mediate adhesion of leukocytes to the vascular endothelium are elevated compared with age-matched healthy controls.8 In addition, monocyte expression of the adhesion molecules CD15 and CD11c is increased in patients with OSA compared with controls matched for age and cardiovascular comorbidities.9 Enhanced oxidative stress and adhesion to cultured endothelial cells in monocytes collected in the morning from OSA patients suggest an adverse effect of OSA on diurnal vascular proinflammatory/anti-inflammatory homeostasis.9 Lymphocytic production of interleukin (IL)-4, a proinflammatory cytokine, is greater, whereas production of IL-10, a potent anti-inflammatory cytokine, is decreased, in otherwise healthy patients with moderate to severe OSA compared with subjects with an AHI of less than 10/h.10 Levels of the circulating proinflammatory cytokines IL-6 and IL-18 are greater in otherwise healthy OSA patients compared with controls and are correlated with severity of OSA.12 Thus, endothelial proinflammatory/anti-inflammatory homeostasis is shifted toward vascular inflammation in patients with untreated OSA.
Coagulation Homeostasis
Shifting of coagulation homeostasis toward a procoagulable state contributes to the progression of atherosclerosis.37 Obstructive sleep apnea has been linked to altered coagulation homeostasis and excessive platelet activation.38 However, the role of OSA as an independent procoagulable stimulus remains uncertain. Coagulation homeostasis has been assessed predominantly in OSA patients with coexistent cardiovascular disease that itself adversely affects coagulation homeostasis.38 Levels of plasminogen activator inhibitor type 1, a marker of procoagulability, were similar in OSA patients and healthy controls after adjustment for blood pressure and BMI.39 Increased levels of hypercoagulability markers such as thrombin/antithrombin III complex and d-dimer are related to coexistent hypertension rather than to OSA.40 Furthermore, the AHI is not a significant predictor of plasminogen activator inhibitor type 1 levels in the presence of coexistent metabolic syndrome in OSA patients.41 Coexistent cardiovascular diseases, and hypertension in particular, rather than OSA itself appear to alter coagulation homeostasis in these patients.
Endothelial Repair Capacity
Endothelial dysfunction can result from direct damage to the endothelium itself or, alternatively, can be caused by reduced endothelial repair in response to damage. In general, reduced levels of bone marrow–derived endothelial progenitor cells, a marker of endothelial repair capacity, are associated with impaired vascular endothelial function and increased cardiovascular risk.42,43 Endothelial progenitor cells enter the systemic circulation to replace defective or injured mature endothelial cells.44
There is evidence to suggest that OSA alters the ability of the endothelium to repair itself. For example, levels of circulating endothelial progenitor cells are reduced in patients with OSA who are free of overt cardiovascular disease.13 Reduced endothelial progenitor cell levels may exacerbate endothelial dysfunction in patients with OSA because these cells are the major repository of eNOS at the site of ischemia/reperfusion-induced endothelial injury.34
Not only are endothelial progenitor cells reduced in OSA, but it appears that endothelial cells themselves are damaged and apoptotic. Levels of circulating apoptotic endothelial cells are reported to be greater in obese patients with untreated OSA who are free of overt cardiovascular diseases compared with age-matched non-obese controls, suggesting an increased rate of endothelial apoptosis.45 Reduced endothelial progenitor cell levels and possible enhanced endothelial apoptosis compromise endothelial repair capacity and are likely to contribute to OSA-related increase in cardiovascular risk.
Whether OSA independently up-regulates vascular endothelial growth factor (VEGF), a regulator of angiogenesis, is unclear. Levels of VEGF have been assessed mostly in obese OSA patients with coexistent cardiovascular diseases.46–48 Hypertension, aging, and obesity are themselves associated with elevated VEGF concentrations.48–51 Plasma levels of VEGF are similar in normotensive patients with OSA and age- and BMI-matched controls, whereas hypertensive patients with OSA have greater VEGF levels.51 Concentrations of VEGF appear to better correlate with advancing age than OSA severity.48
Mechanisms of Endothelial Dysfunction in OSA
Repetitive hypoxia/reoxygenation and sleep fragmentation associated with transient cessation of breathing in OSA adversely affect endothelial function.
Hypoxia/Reoxygenation
Repetitive episodes of hypoxia/reoxygenation can impair endothelial function by directly reducing endothelial NO production at the transcriptional and posttranscriptional levels and increasing production of reactive oxygen species (ROS) in experimental models.52,53 Increased ROS causes more oxidative stress, which also reduces and destabilizes eNOS messenger RNA while limiting the availability of cofactors required for NO production.54–58 The story is further complicated by the differing effects on the endothelium of short-term and long term exposure to oxidative stress. Prolonged oxidative stress as observed in untreated OSA reduces eNOS activity by suppressing its phosphorylation.59,60 Hypoxia/reoxygenation, therefore, not only affects NO production, but causes oxidative stress that decreases NO and eNOS levels by altering production of NO, transcription of eNOS, and activation of eNOS.
Among the ROS, the role of superoxide has been more extensively researched in OSA than other ROS. Superoxide rapidly scavenges NO, generating peroxynitrite, a toxic metabolite that nitrosylates tyrosine residues, forming nitrotyrosine.61 However, levels of circulating free nitrotyrosine were similar in patients with OSA and in healthy subjects.62 In contrast, expression of nitrotyrosine in endothelial cells harvested from otherwise healthy patients with OSA was greater than controls, suggesting enhanced endothelial oxidative stress.13 Endothelial expression of nitrotyrosine more closely reflects endothelial oxidative stress in OSA than levels of circulating free nitrotyrosine because the in vivo half-life of nitrotyrosine is short and its volume of distribution is 20-fold greater than the plasma volume, indicating its extensive distribution in the extravascular compartment.63 As endothelial oxidative stress increases and fewer cofactors are available for NO synthesis, eNOS preferentially promotes superoxide production that hastens NO degradation and reduces its availability,56,64,65 causing a vicious cycle. Reduced NO availability results in endothelial dysfunction, thereby increasing the risk for vascular diseases in patients with OSA.
Up-regulation of cyclooxygenase-2 (COX-2) and inducible NOS in venous endothelial cells suggest increased vascular inflammation in patients with untreated OSA.13 Inducible NOS plays an essential role in vascular inflammation and oxidative stress.66,67 Endothelial COX-2 up-regulation may have dual pathogenic implications in patients with OSA.68,69 It may contribute to oxidative stress by promoting superoxide generation and endothelial activation via increased production of vasoconstricting/inflammatory prostanoids.69 Alternatively, COX-2 upregulation may be a protective mechanism against repetitive episodes of hypoxemia/reoxygenation.70 Similarly, VEGF upregulation may be an adaptive response to repetitive hypoxia/reoxygenation in OSA71–74 or a marker of accelerated atherogenesis in OSA.75
Whether OSA-related hypoxia/reoxygenation activates an adaptive hypoxia-inducible factor–1 (HIF-1) pathway is unclear. Elevated levels of VEGF and nocturnal erythropoietin, both mediated by the HIF-1 pathway, provide indirect evidence of HIF-1 activation in patients with OSA.46,76 However, coexisting cardiovascular diseases may increase VEGF levels independently from OSA.46 Prolonged and/or severe hypoxia upregulates HIF-1 in endothelial cells in vitro.77,78 However, experimental models of sustained severe hypoxia may not be applicable to OSA. More relevant models of repetitive hypoxia/reoxygenation suggest that the proinflammatory transcription factor nuclear factor–κB is activated selectively over the HIF-1–mediated adaptive pathway, suggesting a maladaptive response to hypoxic stimulus in OSA.79,80 Nuclear factor–κB up-regulates several proinflammatory genes including tumor necrosis factor (TNF)–α, IL-8, and IL-6.81 Neutrophil counts and circulating TNF-α and IL-6 levels are elevated in OSA patients compared with controls.12,79 The initial sensing and signaling event for nuclear factor–κB activation remains unknown because it does not appear to be influenced by oxidative stress.82
Adequate recruitment and activity of endothelial progenitor cells are required to maintain endothelial repair capacity. Adhesion of endothelial progenitor cells to ischemic endothelium in vitro is mediated by adaptive HIF-1 up-regulation in response to hypoxic stimulus.83 Maladaptive response to repetitive hypoxia/reoxygenation may decrease adhesion capacity of endothelial progenitor cells in OSA.79 Impaired recruitment of endothelial progenitor cells from the bone marrow is likely to be related to depressed NO production and activity in patients with OSA.84 Hypoxia/reoxygenation injury that triggers programmed cell death may lead to increased endothelial apoptosis in OSA.85 Decreased endothelial repair capacity in conjunction with increased endothelial apoptosis suggests altered vascular remodeling in OSA.
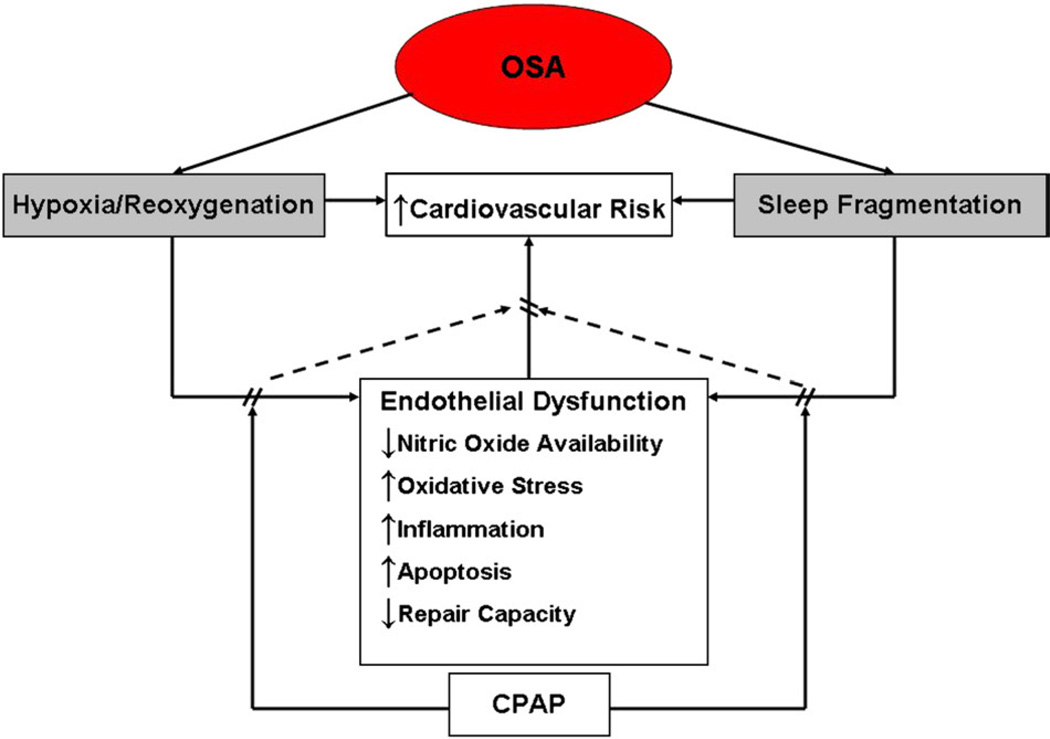
Thus, repetitive hypoxia/reoxygenation as observed in OSA adversely impacts endothelial function by promoting oxidative stress and inflammation, and reducing NO availability and repair capacity (Fig 1).
Fig 1.
Intermediary mechanisms associated with endothelial dysfunction in patients with OSA that potentially contribute to increased cardiovascular risks. Repetitive hypoxia/reoxygenation and sleep fragmentation associated with transient cessation of breathing in OSA adversely impact endothelial function by promoting oxidative stress, inflammation, and apoptosis, and reducing NO availability and repair capacity. Continuous positive airway pressure therapy ameliorates these alterations. Continuous positive airway pressure–induced reversal of vascular endothelial dysfunction may reduce and/or reverse increased cardiovascular risk in OSA.
Sleep Fragmentation and Deprivation
Not only does hypoxia/reoxygenation of OSA affect endothelial function, but the repetitive arousals associated with OSA result in chronic sleep fragmentation that in and of itself may adversely affect endothelial function. Sleep deprivation appears to increase cardiovascular risk independently from upper airway obstruction. For example, self-reported reduced sleep duration in 70,000 participants in the Nurses Health Study was associated with increased incidence of myocardial infarction over a 10-year period after adjustment for age, snoring, BMI, and other known potential confounding variables.86 In healthy subjects, chronic sleep deprivation is associated with a 50% decline in flow-mediated vasodilation, a surrogate outcome for cardiovascular disease.87 Other studies have shown generally increased inflammatory markers in sleep-deprived subjects, suggesting overall increased inflammation, sympathetic tone, and hypercoagulability that can all affect cardiovascular disease outcomes. Levels of proinflammatory markers such as C-reactive protein (CRP), IL-6, and TNF-α are elevated after partial and sustained sleep deprivation in healthy subjects.88–90 Increased awake time after sleep onset is associated with elevated levels of norepinephrine and plasma d-dimer in elderly caregivers.91,92 Arousal index is correlated with plasma levels of von Willebrand factor, a key mediator of platelet adhesion.93 Wake after sleep onset time is correlated with levels of soluble tissue factor, an initiator of the coagulation cascade, after adjustment for AHI.93 Chronic sleep deprivation and fragmentation may compound the adverse effects of hypoxia/reoxygenation on endothelial function in OSA (Fig 1).
Effects of OSA Treatment on Endothelial Function
A beneficial effect of continuous positive airway pressure (CPAP) on endothelial function in OSA is reasonably well established. Evidence of improvement in endothelial function with mandibular advancement device therapy, upper airway surgical procedures, and adjunct pharmacotherapy for OSA has also recently emerged.
Continuous Positive Airway Pressure
Continuous positive airway pressure delivers positive airway pressure that acts as a pneumatic splint to prevent airway collapse during sleep.94 Therapy with CPAP improves flow-mediated vasodilation in OSA patients without overt cardiovascular diseases.16 Endothelium-dependent vasodilation is increased, whereas endothelium-independent vasodilation remains unchanged, in otherwise healthy OSA patients after 3 months of CPAP therapy, suggesting increased NO availability.95 In addition, basal NO production is increased with CPAP therapy.95 Forearm vasoreactivity improves after as little as 2 weeks of CPAP therapy in both normotensive and hypertensive patients with OSA.96 Plasma levels of NO derivatives increase after 2 nights of CPAP therapy and remain constant at 5-month follow-up.24 Long-term CPAP therapy enhances endothelial repair capacity as evidenced by increased levels of circulating endothelial progenitor cells and reduced number of circulating apoptotic endothelial cells.13,45 Blood pressure control improves with long-term CPAP therapy in hypertensive patients with OSA.97,98 Reversal of endothelial dysfunction with effective long-term CPAP therapy may in part explain its beneficial effect on hypertension control in patients with OSA.
Continuous positive airway pressure therapy lowers levels of circulating soluble adhesion molecules and TNF-α, and reduces monocyte adhesion capacity to cultured endothelial cells, indicating decreased inflammation, leukocyte activation, and leukocyte-endothelial interaction.9,10,79,99 Levels of oxidative stress normalize with 4 months of effective CPAP therapy, whereas antioxidant defense remains partially impaired after 1 year of therapy, in OSA patients.11,100 Plasma levels of the inflammatory markers CRP, IL-6, and TNF-α as well as VEGF remain unchanged after withdrawal of CPAP therapy for 7 days despite immediate return of OSA.101 Pretreatment values were not reported in this cohort of OSA patients who were treated with CPAP for at least 1 year, precluding a definitive conclusion about an enduring beneficial effect of CPAP on vascular inflammation.
Overnight treatment with autoadjusting CPAP, which delivers variable positive airway pressure during sleep depending on the degree of upper airway obstruction, normalizes levels of circulating NO derivatives; however, the improvement in endothelial function does not correlate with change in morning blood pressure in hypertensive OSA patients.23 The long-term effect of autoadjusting CPAP on endothelial function in OSA is not known. Therapy with autoadjusting CPAP appears inferior to fixed CPAP in controlling blood pressure in OSA.102,103 Autoadjusting CPAP may be less effective than fixed CPAP in eliminating obstructive hypopneas.104 Persistent hypoxia/reoxygenation and sleep fragmentation associated with residual obstructive hypopneas may undermine blood pressure control in hypertensive OSA patients.
Upper Airway Surgery
Adenotonsillectomy reduces levels of the inflammatory markers CRP, IL-6, and CD40 ligand, and increases the levels of the anti-inflammatory marker IL-10 in children with OSA.105–107 Pulmonary artery pressures are reduced and hyperemic response after cuff-induced brachial artery occlusion improves after adenotonsillectomy in children with OSA.108,109 In adult patients with OSA, uvulopalatopharyngoplasty decreases plasma levels of the inflammatory mediators CRP and TNF-α.110,111 The effect of upper airway surgery on endothelial vasomotor function in adults with OSA remains unknown.
Mandibular Advancement Devices
Mandibular advancement splints increase oropharyngeal cross-sectional area and reduce the likelihood of airway collapse in OSA.112,113 A modest reduction in diastolic but not systolic blood pressure during wakefulness was noted in hypertensive OSA patients after a 4-week therapy with mandibular advancement device.114 Long-term therapy with mandibular advancement devices appears to reverse endothelial dysfunction in OSA despite incomplete elimination of obstructive events. Vascular reactivity, assessed by peripheral arterial tonometry, and lipid peroxidation, a marker of oxidative stress, were similar in patients with OSA and controls matched for age, BMI, and cardiovascular comorbidities after 1 year of suboptimal therapy with an oral device (mean residual AHI, 19/h).115
Emerging Pharmacotherapy
Pharmacotherapy targeted to endothelial dysfunction may be an important adjunct treatment modality in OSA, considering suboptimal long-term adherence with CPAP and dental devices and limited efficacy of upper airway surgery.116–118
Daily administration of allopurinol, an inhibitor of the hypoxia-activated enzyme xanthine oxidase, increases flow-mediated vasodilation and reduces oxidative stress in patients with severe untreated OSA.119 Allopurinol may inhibit the production of ROS by xanthine oxidase, thereby reducing oxidative stress and increasing NO bioavailability. Scavenging superoxide radicals by exogenous administration of antioxidants may reduce oxidative stress. Administration of vitamin C, an antioxidant, acutely normalizes impaired flow-mediated vasodilation in otherwise healthy patients with OSA.120 However, acute improvement in endothelial function may not be sustained. Flow-mediated vasodilation deteriorates to pretreatment levels at 8 weeks of therapy with vitamin C despite high serum levels of ascorbate in healthy smokers.121 No data are available on the long-term effects of antioxidant administration on the endothelial function in OSA.
Summary
Evidence strongly suggests that OSA independently impairs endothelial function by altering regulation of endothelial vasomotor tone and repair capacity while promoting vascular inflammation and oxidative stress. Possible mechanisms underlying altered endothelial function in OSA include repetitive hypoxia/reoxygenation and sleep fragmentation. Treatment of OSA with CPAP improves endothelial function and may play an important role in reduction of cardiovascular risk in these patients. Pharmacotherapy targeted to endothelial dysfunction may have an adjunct role in the treatment of OSA.
References
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976;27:465–484. doi: 10.1146/annurev.me.27.020176.002341. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Peker Y, Kraiczi H, Hedner J, et al. An independent association between obstructive sleep apnea and coronary artery disease. Eur Respir J. 1999;13:179–184. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 6.Aird WC. Phenotypic heterogeneity of the endothelium: representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 7.Huang PL. Unraveling the links between diabetes, obesity, and cardiovascular disease. Circ Res. 2005;96:1129–1131. doi: 10.1161/01.RES.0000170705.56583.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohga E, Nagase T, Tomita T, et al. Increased levels of circulating ICAM-1, VCAM-1, and l-selectin in obstructive sleep apnea syndrome. J Appl Physiol. 1999;87:10–14. doi: 10.1152/jappl.1999.87.1.10. [DOI] [PubMed] [Google Scholar]
- 9.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 10.Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci. 2005;1051:340–350. doi: 10.1196/annals.1361.076. [DOI] [PubMed] [Google Scholar]
- 11.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 12.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–630. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 13.Jelic S, Padeletti M, Higgins C, et al. Inflammation, oxidative stress and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 15.Carlson J, Rangemark C, Hedner J. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnea. J Hypertens. 1996;14:577–584. doi: 10.1097/00004872-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Ip MS, Tse HF, Lam B, et al. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 17.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnea-hypopnea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 18.Corretti M, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. J Am Coll Cardiol. 2000;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 19.Pohl U, Holtz J, Busse R, et al. Crucial role of the endothelium in the vasodilator response to flow in vivo. Hypertension. 1985;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- 20.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 21.Sun D, Huang A, Smith CJ, et al. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilatation in eNOS-knockout mice. Circ Res. 1999;85:288–293. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- 22.Nieto FJ, Herrington DM, Redline S, et al. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169:354–360. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 23.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 24.Schulz R, Schmidt D, Blum A, et al. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnea: response to CPAP therapy. Thorax. 2000;55:1046–1051. doi: 10.1136/thorax.55.12.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohike Y, Kozaki K, Iijima K, et al. Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure—possible involvement of nitric oxide and asymmetric NG,NG-dimethylarginine. Circ J. 2005;69:221–226. doi: 10.1253/circj.69.221. [DOI] [PubMed] [Google Scholar]
- 26.Møller DS, Lind P, Strunge B, et al. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274–280. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 27.Gjørup PH, Sadauskiene L, Wessels J, et al. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am J Hypertens. 2007;20:44–52. doi: 10.1016/j.amjhyper.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Phillips BG, Narkiewicz K, Pesek CA, et al. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17:61–66. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 29.Jordan W, Reinbacher A, Cohrs S, et al. Obstructive sleep apnea: plasma endothelin-1 precursor but not endothelin-1 levels are elevated and decline with nasal continuous positive airway pressure. Peptides. 2005;26:1654–1660. doi: 10.1016/j.peptides.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Zamarrón-Sanz C, Ricoy-Galbaldon J, Gude-Sampedro F, et al. Plasma levels of vascular endothelial markers in obstructive sleep apnea. Arch Med Res. 2006;37:552–555. doi: 10.1016/j.arcmed.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Saarelainen S, Seppälä E, Laasonen K, et al. Circulating endothelin-1 in obstructive sleep apnea. Endothelium. 1997;5:115–118. doi: 10.3109/10623329709079869. [DOI] [PubMed] [Google Scholar]
- 32.Grimpen F, Kanne P, Schulz E, et al. Endothelin-1 plasma levels are not elevated in patients with obstructive sleep apnea. Eur Respir J. 2000;15:320–325. doi: 10.1034/j.1399-3003.2000.15b17.x. [DOI] [PubMed] [Google Scholar]
- 33.Rossi GP, Pitter G. Genetic variation in the endothelin system: do polymorphisms affect the therapeutic strategies? Ann N Y Acad Sci. 2006;1069:34–50. doi: 10.1196/annals.1351.004. [DOI] [PubMed] [Google Scholar]
- 34.Ii M, Nishimura H, Iwakura A, et al. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 35.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 36.Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999;107:85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 37.Davies MJ. The contribution of thrombosis to the clinical expression of coronary atherosclerosis. Thromb Res. 1996;82:1–32. doi: 10.1016/0049-3848(96)00035-7. [DOI] [PubMed] [Google Scholar]
- 38.von Känel R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124:1956–1967. doi: 10.1378/chest.124.5.1956. [DOI] [PubMed] [Google Scholar]
- 39.Rångemark C, Hedner JA, Carlson JT, et al. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep. 1995;18:188–194. doi: 10.1093/sleep/18.3.188. [DOI] [PubMed] [Google Scholar]
- 40.von Kanel R, Le DT, Nelesen RA, et al. The hypercoagulable state in sleep apnea is related to comorbid hypertension. J Hypertens. 2001;19:1445–1451. doi: 10.1097/00004872-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 41.von Känel R, Loredo JS, Ancoli-Israel S, et al. Elevated plasminogen activator inhibitor 1 in sleep apnea and its relation to the metabolic syndrome: an investigation in 2 different study samples. Metabolism. 2007;56:969–976. doi: 10.1016/j.metabol.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 43.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 44.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 45.El Solh AA, Akinnusi ME, Baddoura FH, et al. Endothelial cell apoptosis in obstructive sleep apnea. A link to endothelial dysfunction. Am J Respir Crit Care Med. 2007;175:1186–1191. doi: 10.1164/rccm.200611-1598OC. [DOI] [PubMed] [Google Scholar]
- 46.Lavie L, Kraiczi H, Hefetz A, et al. Plasma vascular endothelial growth factor in sleep apnea syndrome. Am J Respir Crit Care Med. 2002;165:1624–1628. doi: 10.1164/rccm.20110-040OC. [DOI] [PubMed] [Google Scholar]
- 47.Schulz R, Hummel C, Heinemann S, et al. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165:67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 48.Peled N, Shitrit D, Bendayan D, et al. Association of elevated levels of vascular endothelial growth factor in obstructive sleep apnea syndrome with patient age rather than with obstructive sleep apnea syndrome severity. Respiration. 2007;74:50–55. doi: 10.1159/000095675. [DOI] [PubMed] [Google Scholar]
- 49.Belgore FM, Blann AD, Li-Saw-Hee FL, et al. Plasma levels of vascular endothelial growth factor and its soluble receptor (SFlt-1) in essential hypertension. Am J Cardiol. 2001;87:805–807. doi: 10.1016/s0002-9149(00)01512-5. [DOI] [PubMed] [Google Scholar]
- 50.Miyazawa-Hoshimoto S, Takahashi K, Bujo J, et al. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologica. 2003;46:1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 51.Valipour A, Litschauer B, Mittermayer F, et al. Circulating plasma levels of vascular endothelial growth factor in patients with sleep disordered breathing. Respir Med. 2004;98:1180–1186. doi: 10.1016/j.rmed.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 52.McQuillan LP, Leung GK, Marsden PA, et al. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol. 1994;267:H1921–H1927. doi: 10.1152/ajpheart.1994.267.5.H1921. [DOI] [PubMed] [Google Scholar]
- 53.Liao JK, Zulueta JJ, Yu FS, et al. Regulation of bovine endothelial constitutive nitric oxide synthase by oxygen. J Clin Invest. 1995;96:2661–2666. doi: 10.1172/JCI118332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takemoto M, Sun J, Hiroki J, et al. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 55.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 56.Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in ApoE deficient mice Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 57.Kuzkaya N, Weissmann N, Harrison DG, et al. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 58.Antoniades C, Shirodaria C, Warrick N, et al. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 59.Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka T, Nakamura H, Yodoi J, et al. Redox regulation of the signaling pathways leading to eNOS phosphorylation. Free Radic Biol Med. 2005;38:1231–1242. doi: 10.1016/j.freeradbiomed.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Knepler JL, Jr, Taher LN, Gupta MP, et al. Peroxynitrite causes endothelial cell monolayer barrier dysfunction. Am J Physiol Cell Physiol. 2001;281:C1064–C1075. doi: 10.1152/ajpcell.2001.281.3.C1064. [DOI] [PubMed] [Google Scholar]
- 62.Svatikova A, Wolk R, Wang HH, et al. Circulating free nitrotyrosine in obstructive sleep apnea. Am J Physiol Regul Integr Comp Physiol. 2004;287:R284–R287. doi: 10.1152/ajpregu.00241.2004. [DOI] [PubMed] [Google Scholar]
- 63.Tabrizi-Fard MA, Maurer TS, Fung HL. In vivo disposition of 3-nitro-l-tyrosine in rats: implications on tracking systemic peroxynitrite exposure. Drug Metab Dispos. 1999;27:429–431. [PubMed] [Google Scholar]
- 64.Vasquez-Vivar J, Kalyanaraman B, Martasek P, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia Y, Tsai AL, Berka V, et al. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 66.Kleinert H, Pautz A, Linker K, et al. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 67.Xia Y, Roman LJ, Masters BS, et al. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 68.Gilroy DW, Colville-Nash PR, Willis D, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 69.Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005;112:759–770. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]
- 70.Bolli R, Shinmura K, Tang XL, et al. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res. 2002;55:506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shweiki D, Itin A, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 72.Dor Y, Porat R, Keshet E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol. 2001;280:C1367–C1374. doi: 10.1152/ajpcell.2001.280.6.C1367. [DOI] [PubMed] [Google Scholar]
- 73.Marti HH, Risau W. Systemic hypoxia changes the organ specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci. 1998;95:15809–15814. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Forsythe JA, Jiang BH, Lyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inoue M, Itoh H, Ueda M, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98:108–116. doi: 10.1161/01.cir.98.20.2108. [DOI] [PubMed] [Google Scholar]
- 76.Winnicki M, Shamsuzzaman A, Lanfranchi P, et al. Erythropoietin and obstructive sleep apnea. Am J Hypertens. 2004;17:783–786. doi: 10.1016/j.amjhyper.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 77.Toffoli S, Feron O, Raes M, et al. Intermittent hypoxia changes HIF-1α phosphorylation pattern in endothelial cells: unraveling of a new PKA-dependent regulation of HIF-1α. Biochim Biophys Acta. 2007;1773:1558–1571. doi: 10.1016/j.bbamcr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Yuan G, Nanduri J, Bhasker CR, et al. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–4328. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 79.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 80.Greenberg H, Ye X, Wilson D, et al. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006;343:591–596. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 81.Williams A, Scharf SM. Obstructive sleep apnea, cardiovascular disease, and inflammation—is NF-kappaB the key? Sleep Breath. 2007;11:69–76. doi: 10.1007/s11325-007-0106-1. [DOI] [PubMed] [Google Scholar]
- 82.Hayakawa M, Miyashita H, Sakamoto I, et al. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 84.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 85.Dhar-Mascareno M, Carcamo JM, Golde DW. Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Radic Biol Med. 2005;38:1311–1322. doi: 10.1016/j.freeradbiomed.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 86.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 87.Takase B, Akima T, Uehata A, et al. Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin Cardiol. 2004;27:223–227. doi: 10.1002/clc.4960270411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 89.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 90.Irwin M, Wang M, Campomayor CO, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 91.von Känel R, Dimsdale JE, Ancoli-Israel S, et al. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin d-dimer in older caregivers of people with Alzheimer’s disease. J Am Geriatr Soc. 2006;54:431–437. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 92.Mausbach BT, Ancoli-Israel S, von Känel R, et al. Sleep disturbance, norepinephrine, and d-dimer are all related in elderly caregivers of people with Alzheimer disease. Sleep. 2006;29:1347–1352. doi: 10.1093/sleep/29.10.1347. [DOI] [PubMed] [Google Scholar]
- 93.von Känel R, Loredo JS, Ancoli-Israel S, et al. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131:733–739. doi: 10.1378/chest.06-2006. [DOI] [PubMed] [Google Scholar]
- 94.Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 95.Lattimore JL, Wilcox I, Skilton M, et al. Treatment of obstructive sleep apnoea leads to improved micro-vascular endothelial function in the systemic circulation. Thorax. 2006;61:491–495. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Imadojemu VA, Gleeson K, Quraishi SA, et al. Impaired vasodilator responses in obstructive sleep apnea are improved with continuous positive airway pressure. Am J Respir Crit Care Med. 2002;165:950–953. doi: 10.1164/ajrccm.165.7.2102003. [DOI] [PubMed] [Google Scholar]
- 97.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 98.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 99.Chin K, Nakamura T, Shimizu K, et al. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med. 2000;109:562–567. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 100.Barcelo A, Barbe F, de la Pena M, et al. Antioxidant status in patients with sleep apnea and impact of continuous positive airway pressure treatment. Eur Respir J. 2006;27:756–760. doi: 10.1183/09031936.06.00067605. [DOI] [PubMed] [Google Scholar]
- 101.Phillips CL, Yang Q, Williams A, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnea. J Sleep Res. 2007;16:217–225. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 102.Robinson GV, Smith DM, Langford BA, et al. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27:1229–1235. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 103.Patruno V, Aiolfi S, Costantino G, et al. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest. 2007;131:1393–1399. doi: 10.1378/chest.06-2192. [DOI] [PubMed] [Google Scholar]
- 104.Farré R, Montserrat JM, Rigau J, et al. Response of automatic continuous positive airway pressure devices to different sleep breathing patterns: a bench study. Am J Respir Crit Care Med. 2002;166:469–473. doi: 10.1164/rccm.2111050. [DOI] [PubMed] [Google Scholar]
- 105.Kheirandish-Gozal L, Capdevila OS, Tauman R, et al. Plasma C-reactive protein in nonobese children with obstructive sleep apnea before and after adenotonsillectomy. J Clin Sleep Med. 2006;2:301–304. [PMC free article] [PubMed] [Google Scholar]
- 106.Gozal D, Serpero LD, Sans Capdevila O, et al. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9:254–259. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gozal D, Kheirandish-Gozal L, Serpero LD, et al. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116:2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 108.Yilmaz MD, Onrat E, Altuntas A, et al. The effects of tonsillectomy and adenoidectomy on pulmonary arterial pressure in children. Am J Otolaryngol. 2005;26:18–21. doi: 10.1016/j.amjoto.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 109.Tezer MS, Karanfil A, Aktas D. Association between adenoidal-nasopharyngeal ratio and right ventricular diastolic functions in children with adenoid hypertrophy causing upper airway obstruction. Int J Pediatr Otorhinolaryngol. 2005;69:1169–1173. doi: 10.1016/j.ijporl.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Kinoshita H, Shibano A, Sakoda T, et al. Uvulopalatopharyngoplasty decreases levels of C-reactive protein in patients with obstructive sleep apnea syndrome. Am Heart J. 2006;152:692.e1–692.e5. doi: 10.1016/j.ahj.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 111.Kataoka T, Enomoto F, Kim R, et al. The effect of surgical treatment of obstructive sleep apnea syndrome on the plasma TNF-alpha levels. Tohuku J Exp Med. 2004;204:267–272. doi: 10.1620/tjem.204.267. [DOI] [PubMed] [Google Scholar]
- 112.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 113.Ng AT, Qian J, Cistulli PA. Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep. 2006;29:666–671. [PubMed] [Google Scholar]
- 114.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27:934–941. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- 115.Itzhaki S, Dorchin H, Clark G, et al. The effects of 1-year treatment with a Herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest. 2007;131:740–749. doi: 10.1378/chest.06-0965. [DOI] [PubMed] [Google Scholar]
- 116.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 117.Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofac Orthop. 2006;129:214–221. doi: 10.1016/j.ajodo.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 118.Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnea. Cochrane Database Syst Rev. 2005:CD001004. doi: 10.1002/14651858.CD001004.pub2. [DOI] [PubMed] [Google Scholar]
- 119.El Solh AA, Saliba R, Bosinski T, et al. Allopurinol improves endothelial function in sleep apnea: a randomized controlled study. Eur Respir J. 2006;27:997–1002. doi: 10.1183/09031936.06.00101005. [DOI] [PubMed] [Google Scholar]
- 120.Grebe M, Eisele HJ, Weissmann N, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- 121.Raitakari OT, Adams MR, McCredie RJ, et al. Oral vitamin C and endothelial function in smokers: short-term improvement, but no sustained beneficial effect. J Am Coll Cardiol. 2000;35:1616–1621. doi: 10.1016/s0735-1097(00)00576-3. [DOI] [PubMed] [Google Scholar]