Highlights
-
•
ERp29 regulates epithelial cell plasticity and the mesenchymal–epithelial transition.
-
•
ERp29 shows a tumor suppressive function in primary tumor development.
-
•
ERp29 is potentially associated with distant metastasis in cancer.
-
•
ERp29 modulates cell survival against genotoxic stress.
-
•
Thus, ERp29 displays dual functions as a “friend or foe” in epithelial cancer.
Keywords: ERp29, Epithelial cell plasticity, DNA stability, Metastasis, Chemo-/radio-resistance, Mesenchymal–epithelial transition
Abstract
The endoplasmic reticulum (ER) protein 29 (ERp29) is a molecular chaperone that plays a critical role in protein secretion from the ER in eukaryotic cells. Recent studies have also shown that ERp29 plays a role in cancer. It has been demonstrated that ERp29 is inversely associated with primary tumor development and functions as a tumor suppressor by inducing cell growth arrest in breast cancer. However, ERp29 has also been reported to promote epithelial cell morphogenesis, cell survival against genotoxic stress and distant metastasis. In this review, we summarize the current understanding on the biological and pathological functions of ERp29 in cancer and discuss the pivotal aspects of ERp29 as “friend or foe” in epithelial cancer.
1. Introduction
The endoplasmic reticulum (ER) is found in all eukaryotic cells and is complex membrane system constituting of an extensively interlinked network of membranous tubules, sacs and cisternae. It is the main subcellular organelle that transports different molecules to their subcellular destinations or to the cell surface [10,85].
The ER contains a number of molecular chaperones involved in protein synthesis and maturation. Of the ER chaperones, protein disulfide isomerase (PDI)-like proteins are characterized by the presence of a thioredoxin domain and function as oxido-reductases, isomerases and chaperones [33]. ERp29 lacks the active-site double-cysteine (CxxC) motif and does not belong to the redox-active PDIs [5,47]. ERp29 is recognized as a characterized resident of the cellular ER, and it is expressed ubiquitously and abundantly in mammalian tissues [50]. Protein structural analysis showed that ERp29 consists of N-terminal and C-terminal domains [5]: N-terminal domain involves dimerization whereas the C-terminal domain is essential for substrate binding and secretion [78]. The biological function of ERp29 in protein secretion has been well established in cells [8,63,67].
ERp29 is proposed to be involved in the unfolded protein response (UPR) as a factor facilitating transport of synthesized secretory proteins from the ER to Golgi [83]. The expression of ERp29 was demonstrated to be increased in cells exposed to radiation [108], sperm cells undergoing maturation [42,107], and in certain cell types both under the pharmacologically induced UPR and under the physiological conditions (e.g., lactation, differentiation of thyroid cells) [66,82]. Under ER stress, ERp29 translocates the precursor protein p90ATF6 from the ER to Golgi where it is cleaved to be a mature and active form p50ATF by protease (S1P and S2P) [48]. In most cases, ERp29 interacts with BiP/GRP78 to exert its function under ER stress [65].
ERp29 is considered to be a key player in both viral unfolding and secretion [63,67,77,78] Recent studies have also demonstrated that ERp29 is involved in intercellular communication by stabilizing the monomeric gap junction protein connexin43 [27] and trafficking of cystic fibrosis transmembrane conductance regulator to the plasma membrane in cystic fibrosis and non-cystic fibrosis epithelial cells [90]. It was recently reported that ERp29 directs epithelial Na(+) channel (ENaC) toward the Golgi, where it undergoes cleavage during its biogenesis and trafficking to the apical membrane [40]. ERp29 expression protects axotomized neurons from apoptosis and promotes neuronal regeneration [111]. These studies indicate a broad biological function of ERp29 in cells.
Recent studies demonstrated a tumor suppressive function of ERp29 in cancer. It was found that ERp29 expression inhibited tumor formation in mice [4,87] and the level of ERp29 in primary tumors is inversely associated with tumor development in breast, lung and gallbladder cancer [4,29].
However, its expression is also responsible for cancer cell survival against genotoxic stress induced by doxorubicin and radiation [34,76,109]. The most recent studies demonstrate other important roles of ERp29 in cancer cells such as the induction of mesenchymal–epithelial transition (MET) and epithelial morphogenesis [3,4]. MET is considered as an important process of transdifferentiation and restoration of epithelial phenotype during distant metastasis [23,52]. These findings implicate ERp29 in promoting the survival of cancer cells and also metastasis. Hence, the current review focuses on the novel functions of ERp29 and discusses its pathological importance as a “friend or foe” in epithelial cancer.
2. ERp29 regulates mesenchymal–epithelial transition
2.1. Epithelial–mesenchymal transition (EMT) and MET
The EMT is an essential process during embryogenesis [6] and tumor development [43,96]. The pathological conditions such as inflammation, organ fibrosis and cancer progression facilitate EMT [16]. The epithelial cells after undergoing EMT show typical features characterized as: (1) loss of adherens junctions (AJs) and tight junctions (TJs) and apical–basal polarity; (2) cytoskeletal reorganization and distribution; and (3) gain of aggressive phenotype of migration and invasion [98]. Therefore, EMT has been considered to be an important process in cancer progression and its pathological activation during tumor development induces primary tumor cells to metastasize [95]. However, recent studies showed that the EMT status was not unanimously correlated with poorer survival in cancer patients examined [92].
In addition to EMT in epithelial cells, mesenchymal-like cells have capability to regain a fully differentiated epithelial phenotype via the MET [6,35]. The key feature of MET is defined as a process of transdifferentiation of mesenchymal-like cells to polarized epithelial-like cells [23,52] and mediates the establishment of distant metastatic tumors at secondary sites [22]. Recent studies demonstrated that distant metastases in breast cancer expressed an equal or stronger E-cadherin signal than the respective primary tumors and the re-expression of E-cadherin was independent of the E-cadherin status of the primary tumors [58]. Similarly, it was found that E-cadherin is re-expressed in bone metastasis or distant metastatic tumors arising from E-cadherin-negative poorly differentiated primary breast carcinoma [81], or from E-cadherin-low primary tumors [25]. In prostate and bladder cancer cells, the nonmetastatic mesenchymal-like cells were interacted with metastatic epithelial-like cells to accelerate their metastatic colonization [20]. It is, therefore, suggested that the EMT/MET work co-operatively in driving metastasis.
2.2. Molecular regulation of EMT/MET
E-cadherin is considered to be a key molecule that provides the physical structure for both cell–cell attachment and recruitment of signaling complexes [75]. Loss of E-cadherin is a hallmark of EMT [53]. Therefore, characterizing transcriptional regulators of E-cadherin expression during EMT/MET has provided important insights into the molecular mechanisms underlying the loss of cell–cell adhesion and the acquisition of migratory properties during carcinoma progression [73].
Several known signaling pathways, such as those involving transforming growth factor-β (TGF-β), Notch, fibroblast growth factor and Wnt signaling pathways, have been shown to trigger epithelial dedifferentiation and EMT [28,97,110]. These signals repress transcription of epithelial genes, such as those encoding E-cadherin and cytokeratins, or activate transcription programs that facilitate fibroblast-like motility and invasion [73,97].
The involvement of microRNAs (miRNAs) in controlling EMT has been emphasized [11,12,18]. MiRNAs are small non-coding RNAs (∼23 nt) that silence gene expression by pairing to the 3′UTR of target mRNAs to cause their posttranscriptional repression [7]. MiRNAs can be characterized as “mesenchymal miRNA” and “epithelial miRNA” [68]. The “mesenchymal miRNA” plays an oncogenic role by promoting EMT in cancer cells. For instance, the well-known miR-21, miR-103/107 are EMT inducer by repressing Dicer and PTEN [44].
The miR-200 family has been shown to be major “epithelial miRNA” that regulate MET through silencing the EMT-transcriptional inducers ZEB1 and ZEB2 [13,17]. MiRNAs from this family are considered to be predisposing factors for cancer cell metastasis. For instance, the elevated levels of the epithelial miR-200 family in primary breast tumors associate with poorer outcomes and metastasis [57]. These findings support a potential role of “epithelial miRNAs” in MET to promote metastatic colonization [15].
2.3. ERp29 promotes MET in breast cancer
The role of ERp29 in regulating MET has been established in basal-like MDA-MB-231 breast cancer cells. It is known that myosin light chain (MLC) phosphorylation initiates to myosin-driven contraction, leading to reorganization of the actin cytoskeleton and formation of stress fibers [55,56]. ERp29 expression in this type of cells markedly reduced the level of phosphorylated MLC [3]. These results indicate that ERp29 regulates cortical actin formation through a mechanism involved in MLC phosphorylation (Fig. 1). In addition to the phenotypic change, ERp29 expression leads to: expression and membranous localization of epithelial cell marker E-cadherin; expression of epithelial differentiation marker cytokeratin 19; and loss of the mesenchymal cell marker vimentin and fibronectin [3] (Fig. 1). In contrast, knockdown of ERp29 in epithelial MCF-7 cells promotes acquisition of EMT traits including fibroblast-like phenotype, enhanced cell spreading, decreased expression of E-cadherin and increased expression of vimentin [3,4]. These findings further substantiate a role of ERp29 in modulating MET in breast cancer cells.
Fig. 1.
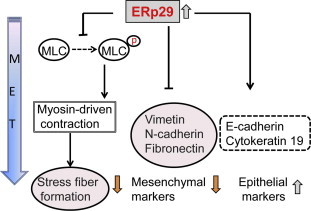
ERp29 triggers mesenchymal–epithelial transition. Exogenous expression of ERp29 in mesenchymal MDA-MB-231 breast cancer cells inhibits stress fiber formation by suppressing MLC phosphorylation. In addition, the overexpressed ERp29 decreases the expression of mesenchymal markers (e.g., vimentin, N-cadherin and fibronectin) and reactivates the expression of epithelial marker (e.g., E-cadherin) and epithelial cell differentiation marker (e.g., cytokeratin 19).
2.4. ERp29 targets E-cadherin transcription repressors
The transcription repressors such as Snai1, Slug, ZEB1/2 and Twist have been considered to be the main regulators for E-cadherin expression [19,26,32]. Mechanistic studies revealed that ERp29 expression significantly down-regulated transcription of these repressors, leading to their reduced nuclear expression in MDA-MB-231 cells [3,4] (Fig. 2). Consistent with this, the extracellular signal-regulated kinase (ERK) pathway which is an important up-stream regulator of Slug and Ets1 was highly inhibited [4]. Apparently, ERp29 up-regulates the expressions of E-cadherin transcription repressors through repressing ERK pathway. Interestingly, ERp29 over-expression in basal-like BT549 cells resulted in incomplete MET and did not significantly affect the mRNA or protein expression of Snai1, ZEB2 and Twist, but increased the protein expression of Slug [3]. The differential regulation of these transcriptional repressors of E-cadherin by ERp29 in these two cell-types may occur in a cell-context-dependent manner.
Fig. 2.
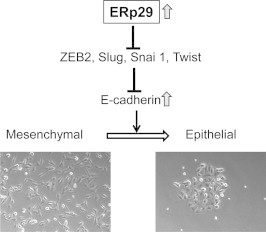
ERp29 decreases the expression of EMT inducers to promote MET. Exogenous expression of ERp29 in mesenchymal MDA-MB-231 breast cancer cells suppresses transcription and protein expression of E-cadherin transcription repressors (e.g., ZEB2, SNAI1 and Twist), resulting in re-expression of E-cadherin and re-establishment of epithelial cell phenotype.
2.5. ERp29 antagonizes Wnt/ β-catenin signaling
Wnt proteins are a family of highly conserved secreted cysteine-rich glycoproteins. The Wnt pathway is activated via a binding of a family member to a frizzled receptor (Fzd) and the LDL-Receptor-related protein co-receptor (LRP5/6). There are three different cascades that are activated by Wnt proteins: namely canonical/β-catenin-dependent pathway and two non-canonical/β-catenin-independent pathways that include Wnt/Ca2+ and planar cell polarity [84]. Of note, the Wnt/β-catenin pathway has been extensively studied, due to its important role in cancer initiation and progression [79]. The presence of Wnt promotes formation of a Wnt–Fzd–LRP complex, recruitment of the cytoplasmic protein Disheveled (Dvl) to Fzd and the LRP phosphorylation-dependent recruitment of Axin to the membrane, thereby leading to release of β-catenin from membrane and accumulation in cytoplasm and nuclei. Nuclear β-catenin replaces TLE/Groucho co-repressors and recruits co-activators to activate expression of Wnt target genes. The most important genes regulated are those related to proliferation, such as Cyclin D1 and c-Myc [46,94], which are over-expressed in most β-catenin-dependent tumors. When β-catenin is absent in nucleus, the transcription factors T-cell factor/lymphoid enhancer factors (TCF/LEF) recruits co-repressors of the TLE/Groucho family and function as transcriptional repressors.
β-catenin is highly expressed in the nucleus of mesenchymal MDA-MB-231 cells. ERp29 over-expression in this type of cells led to translocation of nuclear β-catenin to membrane where it forms complex with E-cadherin [3] (Fig. 3). This causes a disruption of β-catenin/TCF/LEF complex and abolishes its transcription activity. Indeed, ERp29 significantly decreased the expression of cyclin D1/D2 [36], one of the downstream targets of activated Wnt/β-catenin signaling [94], indicating an inhibitory effect of ERp29 on this pathway. Meanwhile, expression of ERp29 in this cell type increased the nuclear expression of TCF3, a transcription factor regulating cancer cell differentiation while inhibiting self-renewal of cancer stem cells [102,106]. Hence, ERp29 may play dual functions in mesenchymal MDA-MB-231 breast cancer cells by: (1) suppressing activated Wnt/β-catenin signaling via β-catenin translocation; and (2) promoting cell differentiation via activating TCF3 (Fig. 3). Because β-catenin serves as a signaling hub for the Wnt pathway, it is particularly important to focus on β-catenin as the target of choice in Wnt-driven cancers. Though the mechanism by which ERp29 expression promotes the disassociation of β-catenin/TCF/LEF complex in MDA-MB-231 cells remains elusive, activating ERp29 expression may exert an inhibitory effect on the poorly differentiated, Wnt-driven tumors.
Fig. 3.
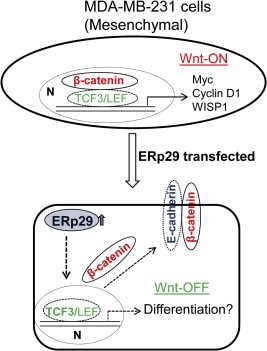
ERp29 over-expression “turns-off” activated Wnt/β-catenin signaling. In mesenchymal MDA-MB-231 cells, high expression of nuclear β-catenin activates its downstream signaling involved in cell cycles and cancer stem cell self-renewal. When ERp29 is over-expressed in this cell model, nuclear β-catenin is relocated at the membrane where it binds to E-cadherin, and Wnt/β-catenin signaling is switched off. Meanwhile, over-expression of ERp29 results in up-regulation of TCF3 and increases expression of genes involved in differentiation. N: Nucleus.
3. ERp29 regulates epithelial cell integrity
3.1. Cell adherens and tight junctions
Adherens junctions (AJs) and tight junctions (TJs) are composed of transmembrane proteins that adhere to similar proteins in the adjacent cell [69]. The transmembrane region of the TJs is composed mainly of claudins, tetraspan proteins with two extracellular loops [1]. AJs are mediated by Ca2+-dependent homophilic interactions of cadherins [71] which interact with cytoplasmic catenins that link the cadherin/catenin complex to the actin cytoskeleton [74].
The cytoplasmic domain of claudins in TJs interacts with occludin and several zona occludens proteins (ZO1-3) to form the plaque that associates with the cytoskeleton [99]. The AJs form and maintain intercellular adhesion, whereas the TJs serve as a diffusion barrier for solutes and define the boundary between apical and basolateral membrane domains [21]. The AJs and TJs are required for integrity of the epithelial phenotype, as well as for epithelial cells to function as a tissue [75].
The TJs are closely linked to the proper polarization of cells for the establishment of epithelial architecture [86]. During cancer development, epithelial cells lose the capability to form TJs and correct apico–basal polarity [59]. This subsequently causes the loss of contact inhibition of cell growth [91]. In addition, reduction of ZO-1 and occludin were found to be correlated with poorly defined differentiation, higher metastatic frequency and lower survival rates [49,64]. Hence, TJs proteins have a tumor suppressive function in cancer formation and progression.
3.2. Apical–basal cell polarity
The apical–basal polarity of epithelial cells in an epithelium is characterized by the presence of two specialized plasma membrane domains: namely, the apical surface and basolateral surface [30]. In general, the epithelial cell polarity is determined by three core complexes. These protein complexes include: (1) the partitioning-defective (PAR) complex; (2) the Crumbs (CRB) complex; and (3) the Scribble complex [2,30,45,51]. PAR complex is composed of two scaffold proteins (PAR6 and PAR3) and an atypical protein kinase C (aPKC) and is localized to the apical junction domain for the assembly of TJs [31,39]. The Crumbs complex is formed by the transmembrane protein Crumbs and the cytoplasmic scaffolding proteins such as the homologue of Drosophila Stardust (Pals1) and Pals-associated tight junction protein (Patj) and localizes to the apical [38]. The Scribble complex is comprised of three proteins, Scribble, Disc large (Dlg) and Lethal giant larvae (Lgl) and is localized in the basolateral domain of epithelial cells [100].
3.3. ERp29 restores the establishment of AJs and TJs
ERp29 is involved the establishment of the apical–junctional complex, which is formed by AJs and TJs [3]. These complexes are located in the upper portion of a polarized epithelial cell and are composed of trans-membrane proteins that interact with molecules in adjacent cells [69]. In MDA-MB-231 cells, β-catenin is expressed and localized in nuclear. ERp29 over-expression resulted in an increased expression and membrane localization of E-cadherin and translocation of β-catenin from the nucleus to the cell membrane [3] (Fig. 3). The ERp29-mediated membrane localization of β-catenin facilitates the assembly of E-cadherin/β-catenin complex and formation of AJs [45].
ERp29 over-expression led to an increase of TJ components such as ZO-1 and occludin at the membrane and cell–cell junctions in breast cancer cells [3] (Fig. 4). The increased expression of ZO-1 and occludin is regulated at translational level, as ERp29 over-expression did not affect their mRNA levels [3]. The role of ERp29 in ZO-1 protein expression and trafficking was further demonstrated in the ERp29-knockdown MCF-7 cells. Translational up-regulation of ZO-1 and occludin by ERp29 in these cell models may provide a mechanism of how ERp29 induces tumor suppression in breast cancer [4]. In addition, the formation of cortical actin filaments is critical for the establishment of AJs and TJs and the regulation of epithelial cell apical–basal polarity [75]. Reorganization of the actin cytoskeleton induces recruitment of ZO-1 to cell periphery before the assembly of junctional complexes between adjacent cells [37]. The ERp29-induced restoration of ZO-1 expression may be associated with actin reorganization. Hence, ERp29 plays a critical role in restoration of an epithelial-like phenotype by establishing cell–cell contact.
Fig. 4.
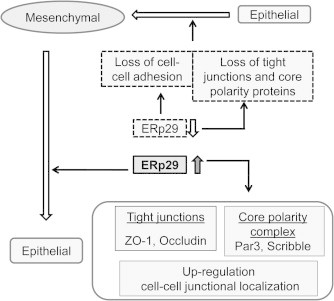
ERp29 regulates epithelial cell morphogenesis. Over-expression of ERp29 in breast cancer cells induces the transition from a mesenchymal-like to epithelial-like phenotype and the restoration of tight junctions and cell polarity. Up-regulation and membrane distribution of these molecules result in re-establishment of cell–cell contacts and epithelial-like phenotypic features. By contrast, ERp29 knockdown by shRNA in epithelial breast cancer cells reduces the expression and membrane localization of these “identity” proteins and disrupts cell–cell adhesion [3].
3.4. ERp29 restores cell polarity
In line with the role of ERp29 in regulating MET and re-establishment of the epithelial-like phenotype, ERp29 over-expression restores epithelial polarity [3] (Fig. 4). In mesenchymal MDA-MB-231 and basal-like BT549 cells, ERp29 expression did not affect mRNA levels of Par3 and Scribble, but increased their protein translation and membrane distribution. It was reported that Cdc42, a small GTPase, is one of the key regulators modulating the expression of Par6 and aPKC [54,60] and has a critical role in establishing cell polarity in epithelial cells [72]. However, ERp29 over-expression did not affect both the expression and localization of Cdc42, Par6 and aPKC, indicating these PAR complex members are not involved in ERp29-regulated apical polarity. Thus, ERp29 is likely to specifically up-regulate Par3 protein expression during epithelial morphogenesis. ERp29 over-expression did not markedly alter the expression and distribution of Crumb1 [3], a member of the Crumbs complex [93], Similar to that observed for Par3, ERp29 over-expression resulted in a significant increase of protein expression of Scribble in both MDA-MB-231 and BT549 cells [3]. Suppression of ERp29 by shRNA in epithelial MCF-7 cells resulted in reduction of these core polarity proteins, leading to the disruption of cell–cell contact and increased cell spreading [3].
Previous studies demonstrated that polarity proteins are synthesized in the endoplasmic reticulum, transported to the Golgi complex and sorted at the trans-Golgi network into distinct apical and basolateral vesicular routes [88]. Given that ERp29 mediates the folding and secretion of newly synthesized proteins in the ER system [8], it is plausible that, in addition to increased protein expression of TJs and the core polarity complex, ERp29 may also have a critical role in protein trafficking and the maintenance of protein stability to modulate epithelial cell integrity. In agreement with this, the ERp29-induced tumor suppression in breast cancer cells is linked to the integrity of apical–basal polarity that is crucial for the prevention of tumor development [80].
4. ERp29 and primary tumor progression
The association of ERp29 with primary tumor development has been studied in only a few types of cancer. In lung tumors, ERp29 expression was observed to be variable within and between tumor stages, and inversely correlated with tumor progression [87]. A tissue array study in 98 breast tumors showed that ERp29 expression was reduced with the progression of tumor stage and grade [4]. In gallbladder adenocarcinoma, ERp29 positive rate is significant lower in poorly differentiated tumors (vs well differentiated tumors) and tumors at T4 stage (vs T1 stage) [29]. Taken together, these results indicate a negative association of ERp29 expression with primary tumor progression in these cancers. However, to further substantiate ERp29’s role in primary tumor development, extensive studies are needed in a large cohort of clinical specimens.
5. ERp29 and metastasis
The association of MET and distant metastasis has been well studied. For instance, analysis of MDA-MB-468 xenografts revealed that some tumor cells exist a metastable phenotype, characterized by the expression of both vimentin and E-cadherin [9], The cells at the invasive front showed a positive expression for vimentin and negative expression for E-cadherin, consistent with an EMT. On the other hand, the lymphovascular-invaded tumor cells showed a gradual transition of invaded tumor cells from mesenchymal to metastable and then to the epithelial phenotype, indicating that a MET process occurs as an early event in the metastatic process.
Given the function of ERp29 in promoting MET in breast cancer cells [4], the role of ERp29 in cancer cell metastasis has been examined. Recent studies showed that ERp29 was significantly increased in the highly metastatic variant of parental MDA-MB-231 cells compared to the parental cells [104]. Similarly, ERp29 was found to be one of the proteins that were highly expressed in the metastatic tissues compared to the primary uveal melanoma tissues [61]. In colon cancer, ERp29, together with CLIC4 and Smac/DIABLO, was integrated into a novel panel associated with metastasis and was stratified for the prognostic risks of colorectal cancer [29]. These results may implicate an important role of ERp29 in cancer cell metastasis and disease recurrence. Indeed, our recent studies revealed that high expression of ERp29 in breast tumors strongly associated with reduced relapse time of disease and short survival time of patients (unpublished data). The role of MET in facilitating distant metastasis has been clinically recognized by the observation that MET is able to reversibly convert the disseminated mesenchymal cancer cells to an epithelial cell state [23]. Hence, ERp29 may have a critical role in promoting distant metastasis during cancer progression, although this needs to be investigated further. Consequently, understanding the association of ERp29 with disease recurrence and distant metastasis is of significance in assessing its prognostic value in clinical applications.
The tumor microenvironment is an important factor in regulating cancer metastasis via MET [41,89]. The interplays between tumor cells, host cells, and the extracellular matrix in tumor ecosystem endow cancer cells with malignant properties, leading to metastatic dissemination. It has been reported that the expression of ERp29 was significantly affected by the culture conditions, where ERp29 expression was significantly increased in xenografts compared with the same cell types cultured in monolayer or spheroid condition [87]. This indicates that ERp29 could be physiologically regulated in the tumor ecosystem. When MDA-MB-231 cells were co-cultured with hepatocytes, E-cadherin was re-expressed, resulting in an increased chemo-resistance [24]. In vivo studies demonstrated that MDA-MB-231 cells formed E-cadherin-negative primary tumors, but showed a re-activated E-cadherin expression in lung metastatic site via MET, suggesting an effect of the microenvironment on cells at the metastatic site [25]. However, it is uncertain whether ERp29 was increased in parallel with metastasis in this in vivo experiment. Although the tumor microenvironment-induced MET and metastasis is a complex process, investigating the involvement of ERp29 in MET and metastasis may enhance our understanding of its pathological functions in cancer progression.
6. ERp29 confers resistance to genotoxic stress in cancer cells
To survive from the stress environment, cells have developed a variety of responsive mechanisms to cope with stress-induced cell death, such as cell cycle arrest and activation of the DNA repair. Recent studies have demonstrated that ERp29 is a novel molecule protecting cells from the genotoxic stress induced by doxorubicin and radiation [76,108,109].
Doxorubicin is one of the conventional chemotherapeutic drugs for cancer intervention via the intercalation of DNA and subsequent activation of the tumor suppressor p53 [62]. While most cancer cells are sensitive to doxorubicin and eventually killed by this drug, some cells develop an adaptive response to doxorubicin-induced genotoxic stress and survive. Clinically, chemo-resistance of cancer cells is a predominant cause of cancer recurrence after long-term treatment. It has been found that doxorubicin induced ERp29 expression and ERp29 expression is causally linked to resistance to this drug by a mechanism that requires PERK [34]. PERK activation promotes the phosphorylation of a general translation factor eIF2α and attenuates translation of global proteins including cyclin D1 [14], thereby resulting in inhibition of cell cycle. Apparently, the doxorubicin-induced ERp29 facilitates cell’s response to genotoxic stress that ultimately results in resistance against chemotherapy by doxorubicin. Indeed, when ERp29 was over-expressed in MDA-MB-231 cells, these cells showed a significant G0/G1 growth arrest and resistant to doxorubicin treatment, whereas knockdown of ERp29 in MCF-7 cells led to an enhanced sensitivity of these cells to doxorubicin [109]. Mechanistic studies revealed a critical role of up-regulated Hsp27 in the ERp29-induced doxorubicin resistance in these cell models. In addition, the ERp29-induced activation of ER stress-related XBP-1/p58IPK cell survival pathway also plays a pivotal role in this aspect [36]. In support of this, silencing of p58IPK in MCF-7 cells and ERp29-overexpressing MDA-MB-231 clones re-sensitizes them to doxorubicin by activating ATF4/CHOP/caspase-3 pro-apoptotic signaling [36].
In an early study, when cells were exposed to ionization radiation, ERp29 expression was elevated in several types of cultured cells [108]. Concomitantly, splicing of XBP-1 mRNA under radiation was increased, suggesting the involvement of ER stress sensor might be a reason to induce ERp29 gene expression [108]. In nasopharyngeal carcinoma (NPC) cells, ERp29 knockdown attenuated radio-resistance of NPC CNE-1 cells, whereas ERp29 over-expression enhanced radio-resistance of NPC CNE-2 cells. Hence, ERp29 could potentiate resistance to radiation in NPC cells [76]. Furthermore, ERp29 was significantly expressed in radio-resistant nasopharyngeal carcinoma (NPC) tissues compared to radio-sensitive NPC tissues, indicating a potential role ERp29 in radio-resistance in NPC tumors [103]. Our recent studies in MBA-MD-231 and MCF-7 breast cancer cells indicated that ERp29 expression increased post-irradiation survival rate, whereas ERp29 repression by siRNA reduced post-irradiation survival rate and increased γ-H2AX expression and DNA damage induced by irradiation (unpublished data). These findings further indicate a protective role of ERp29 in DNA integrity and stability.
Mechanistic studies revealed that ERp29 over-expression in MDA-MB-231 cells significantly up-regulated the expression of the DNA repair gene, O6-methylguanine-DNA methyltransferase (MGMT). MGMT repairs the mutagenic and cytotoxic interstrand DNA cross-links via rapidly reversing alkylation, including methylation, at the O6 position of guanine by transferring the alkyl group to the active site of the enzyme [101]. In addition to DNA repair function, MGMT plays a role in integrating DNA damage/repair-related signals with replication, cell cycle progression and genomic stability [70,105]. Hence, MGMT is also an important factor in ERp29-induced anti-genotoxic stress and cell survival. The ERp29-upregulated DNA repair pathway might cause resistance to chemo- and radio- therapy, and thus targeting this pathway might have a potential to develop alternative strategy for efficient treatment of chemo- and/or radio-resistant cancer cells.
7. Conclusion
The current data from breast cancer cells supports the idea that ERp29 can function as a tumor suppressive protein, in terms of suppression of cell growth and primary tumor formation and inhibition of signaling pathways that facilitate EMT. Nevertheless, the significant role of ERp29 in cell survival against drugs, induction of cell differentiation and potential promotion of MET-related metastasis may lead us to re-assess its function in cancer progression, particularly in distant metastasis. Hence, it is important to explore in detail the ERp29’s role in cancer as a “friend or foe” and to elucidate its clinical significance in breast cancer and other epithelial cancers. Targeting ERp29 and/or its downstream molecules might be an alternative molecular therapeutic approach for chemo/radio-resistant metastatic cancer treatment.
Contributor Information
Shaohua Chen, Email: brenda77@163.com.
Daohai Zhang, Email: dave6503@gmail.com.
References
- 1.Anderson J.M., Van Itallie C.M., Fanning A.S. Setting up a selective barrier at the apical junction complex. Curr. Opin. Cell Biol. 2004;16:140–145. doi: 10.1016/j.ceb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Assemat E., Bazellieres E., Pallesi-Pocachard E., Le Bivic A., Massey-Harroche D. Polarity complex proteins. Biochim. Biophys. Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Bambang I.F., Lee Y.K., Richardson D.R., Zhang D. Endoplasmic reticulum protein 29 regulates epithelial cell integrity during the mesenchymal–epithelial transition in breast cancer cells. Oncogene. 2013;32:1240–1251. doi: 10.1038/onc.2012.149. [DOI] [PubMed] [Google Scholar]
- 4.Bambang I.F., Xu S., Zhou J., Salto-Tellez M., Sethi S.K., Zhang D. Overexpression of endoplasmic reticulum protein 29 regulates mesenchymal–epithelial transition and suppresses xenograft tumor growth of invasive breast cancer cells. Lab. Invest. 2009;89:1229–1242. doi: 10.1038/labinvest.2009.87. [DOI] [PubMed] [Google Scholar]
- 5.Barak N.N., Neumann P., Sevvana M., Schutkowski M., Naumann K., Malesevic M., Reichardt H., Fischer G., Stubbs M.T., Ferrari D.M. Crystal structure and functional analysis of the protein disulfide isomerase-related protein ERp29. J. Mol. Biol. 2009;385:1630–1642. doi: 10.1016/j.jmb.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 6.Barrallo-Gimeno A., Nieto M.A. The snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 7.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baryshev M., Sargsyan E., Mkrtchian S. ERp29 is an essential endoplasmic reticulum factor regulating secretion of thyroglobulin. Biochem. Biophys. Res. Commun. 2006;340:617–624. doi: 10.1016/j.bbrc.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 9.Bonnomet A., Syne L., Brysse A., Feyereisen E., Thompson E.W., Noel A., Foidart J.M., Birembaut P., Polette M., Gilles C. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene. 2012;31:3741–3753. doi: 10.1038/onc.2011.540. [DOI] [PubMed] [Google Scholar]
- 10.Braakman I., Bulleid N.J. Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- 11.Brabletz S., Brabletz T. The ZEB/miR-200 feedback loop–a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bracken C.P., Gregory P.A., Khew-Goodall Y., Goodall G.J. The role of microRNAs in metastasis and epithelial–mesenchymal transition. Cell. Mol. Life Sci. CMLS. 2009;66:1682–1699. doi: 10.1007/s00018-009-8750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bracken C.P., Gregory P.A., Kolesnikoff N., Bert A.G., Wang J., Shannon M.F., Goodall G.J. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial–mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 14.Brewer J.W., Diehl J.A. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl. Acad. Sci. USA. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullock M.D., Sayan A.E., Packham G.K., Mirnezami A.H. MicroRNAs: critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biol. Cell. 2012;104:3–12. doi: 10.1111/boc.201100115. [DOI] [PubMed] [Google Scholar]
- 16.Burden S.J., DePalma R.L., Gottesman G.S. Crosslinking of proteins in acetylcholine receptor-rich membranes: association between the beta-subunit and the 43 kd subsynaptic protein. Cell. 1983;35:687–692. doi: 10.1016/0092-8674(83)90101-0. [DOI] [PubMed] [Google Scholar]
- 17.Burk U., Schubert J., Wellner U., Schmalhofer O., Vincan E., Spaderna S., Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cano A., Nieto M.A. Non-coding RNAs take centre stage in epithelial-to-mesenchymal transition. Trends Cell Biol. 2008;18:357–359. doi: 10.1016/j.tcb.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Cano A., Perez-Moreno M.A., Rodrigo I., Locascio A., Blanco M.J., del Barrio M.G., Portillo F., Nieto M.A. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 20.Celia-Terrassa T., Meca-Cortes O., Mateo F., de Paz A.M., Rubio N., Arnal-Estape A., Ell B.J., Bermudo R., Diaz A., Guerra-Rebollo M., Lozano J.J., Estaras C., Ulloa C., Alvarez-Simon D., Mila J., Vilella R., Paciucci R., Martinez-Balbas M., de Herreros A.G., Gomis R.R., Kang Y., Blanco J., Fernandez P.L., Thomson T.M. Epithelial–mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J. Clin. Invest. 2012;122:1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cereijido M., Valdes J., Shoshani L., Contreras R.G. Role of tight junctions in establishing and maintaining cell polarity. Annu. Rev. Physiol. 1998;60:161–177. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- 22.Chaffer C.L., Dopheide B., McCulloch D.R., Lee A.B., Moseley J.M., Thompson E.W., Williams E.D. Upregulated MT1-MMP/TIMP-2 axis in the TSU-Pr1-B1/B2 model of metastatic progression in transitional cell carcinoma of the bladder. Clin. Exp. Metastasis. 2005;22:115–125. doi: 10.1007/s10585-005-5141-3. [DOI] [PubMed] [Google Scholar]
- 23.Chaffer C.L., Thompson E.W., Williams E.D. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- 24.Chao Y., Wu Q., Shepard C., Wells A. Hepatocyte induced re-expression of E-cadherin in breast and prostate cancer cells increases chemoresistance. Clin. Exp. Metastasis. 2012;29:39–50. doi: 10.1007/s10585-011-9427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao Y.L., Shepard C.R., Wells A. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol. Cancer. 2010;9:179. doi: 10.1186/1476-4598-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comijn J., Berx G., Vermassen P., Verschueren K., van Grunsven L., Bruyneel E., Mareel M., Huylebroeck D., van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 27.Das S., Smith T.D., Sarma J.D., Ritzenthaler J.D., Maza J., Kaplan B.E., Cunningham L.A., Suaud L., Hubbard M.J., Rubenstein R.C., Koval M. ERp29 restricts Connexin43 oligomerization in the endoplasmic reticulum. Mol. Biol. Cell. 2009;20:2593–2604. doi: 10.1091/mbc.E08-07-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Craene B., van Roy F., Berx G. Unraveling signalling cascades for the Snail family of transcription factors. Cell. Signal. 2005;17:535–547. doi: 10.1016/j.cellsig.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Deng Y.J., Tang N., Liu C., Zhang J.Y., An S.L., Peng Y.L., Ma L.L., Li G.Q., Jiang Q., Hu C.T., Wang Y.N., Liang Y.Z., Bian X.W., Fang W.G., Ding Y.Q. CLIC4, ERp29, and Smac/DIABLO derived from metastatic cancer stem-like cells stratify prognostic risks of colorectal cancer. Clin. Cancer Res. 2014;20:3809–3817. doi: 10.1158/1078-0432.CCR-13-1887. [DOI] [PubMed] [Google Scholar]
- 30.Dow L.E., Humbert P.O. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int. Rev. Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- 31.Ebnet K. Organization of multiprotein complexes at cell-cell junctions. Histochem. Cell Biol. 2008;130:1–20. doi: 10.1007/s00418-008-0418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eger A., Aigner K., Sonderegger S., Dampier B., Oehler S., Schreiber M., Berx G., Cano A., Beug H., Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 33.Ellgaard L., Ruddock L.W. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmaki E., Mkrtchian S., Papazian I., Papavassiliou A.G., Kiaris H. ERp29 regulates response to doxorubicin by a PERK-mediated mechanism. Biochim. Biophys. Acta. 2011;1813:1165–1171. doi: 10.1016/j.bbamcr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Frisch S.M., Screaton R.A. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 36.Gao D., Bambang I.F., Putti T.C., Lee Y.K., Richardson D.R., Zhang D. ERp29 induces breast cancer cell growth arrest and survival through modulation of activation of p38 and upregulation of ER stress protein p58IPK. Lab. Invest. 2012;92:200–213. doi: 10.1038/labinvest.2011.163. [DOI] [PubMed] [Google Scholar]
- 37.Giannone G., Dubin-Thaler B.J., Dobereiner H.G., Kieffer N., Bresnick A.R., Sheetz M.P. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 38.Gibson M.C., Perrimon N. Apicobasal polarization: epithelial form and function. Curr. Opin. Cell Biol. 2003;15:747–752. doi: 10.1016/j.ceb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein B., Macara I.G. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grumbach Y., Bikard Y., Suaud L., Chanoux R.A., Rubenstein R.C. ERp29 regulates epithelial sodium channel functional expression by promoting channel cleavage. Am. J. Physiol. Cell Physiol. 2014;307:C701–709. doi: 10.1152/ajpcell.00134.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunasinghe N.P., Wells A., Thompson E.W., Hugo H.J. Mesenchymal–epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 2012;31:469–478. doi: 10.1007/s10555-012-9377-5. [DOI] [PubMed] [Google Scholar]
- 42.Guo W., Qu F., Xia L., Guo Q., Ying X., Ding Z. Identification and characterization of ERp29 in rat spermatozoa during epididymal transit. Reproduction. 2007;133:575–584. doi: 10.1530/REP-06-0301. [DOI] [PubMed] [Google Scholar]
- 43.Gupta G.P., Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Harquail J., Benzina S., Robichaud G.A. MicroRNAs and breast cancer malignancy: an overview of miRNA-regulated cancer processes leading to metastasis. Cancer Biomark. 2012;11:269–280. doi: 10.3233/CBM-120291. [DOI] [PubMed] [Google Scholar]
- 45.Hartsock A., Nelson W.J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He T.C., Sparks A.B., Rago C., Hermeking H., Zawel L., da Costa L.T., Morin P.J., Vogelstein B., Kinzler K.W. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 47.Hermann V.M., Cutfield J.F., Hubbard M.J. Biophysical characterization of ERp29. Evidence for a key structural role of cysteine 125. J. Biol. Chem. 2005;280:13529–13537. doi: 10.1074/jbc.M410889200. [DOI] [PubMed] [Google Scholar]
- 48.Hirsch I., Weiwad M., Prell E., Ferrari D.M. ERp29 deficiency affects sensitivity to apoptosis via impairment of the ATF6-CHOP pathway of stress response. Apoptosis. 2014;19:801–815. doi: 10.1007/s10495-013-0961-0. [DOI] [PubMed] [Google Scholar]
- 49.Hoover K.B., Liao S.Y., Bryant P.J. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am. J. Pathol. 1998;153:1767–1773. doi: 10.1016/S0002-9440(10)65691-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hubbard M.J., McHugh N.J., Carne D.L. Isolation of ERp29, a novel endoplasmic reticulum protein, from rat enamel cells. Evidence for a unique role in secretory-protein synthesis. Eur. J. Biochem./FEBS. 2000;267:1945–1957. doi: 10.1046/j.1432-1327.2000.01193.x. [DOI] [PubMed] [Google Scholar]
- 51.Humbert P.O., Grzeschik N.A., Brumby A.M., Galea R., Elsum I., Richardson H.E. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- 52.Itoh M., Bissell M.J. The organization of tight junctions in epithelia: implications for mammary gland biology and breast tumorigenesis. J. Mammary Gland Biol. Neoplasia. 2003;8:449–462. doi: 10.1023/B:JOMG.0000017431.45314.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeanes A., Gottardi C.J., Yap A.S. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joberty G., Petersen C., Gao L., Macara I.G. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 55.Katoh K., Kano Y., Amano M., Kaibuchi K., Fujiwara K. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblasts. Am. J. Physiol. Cell Physiol. 2001;280:C1669–1679. doi: 10.1152/ajpcell.2001.280.6.C1669. [DOI] [PubMed] [Google Scholar]
- 56.Klemke R.L., Cai S., Giannini A.L., Gallagher P.J., de Lanerolle P., Cheresh D.A. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korpal M., Ell B.J., Buffa F.M., Ibrahim T., Blanco M.A., Celia-Terrassa T., Mercatali L., Khan Z., Goodarzi H., Hua Y., Wei Y., Hu G., Garcia B.A., Ragoussis J., Amadori D., Harris A.L., Kang Y. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 2011;17:1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kowalski P.J., Rubin M.A., Kleer C.G. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217–222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Latorre I.J., Roh M.H., Frese K.K., Weiss R.S., Margolis B., Javier R.T. Viral oncoprotein-induced mislocalization of select PDZ proteins disrupts tight junctions and causes polarity defects in epithelial cells. J. Cell Sci. 2005;118:4283–4293. doi: 10.1242/jcs.02560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin D., Edwards A.S., Fawcett J.P., Mbamalu G., Scott J.D., Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 61.Linge A., Kennedy S., O’Flynn D., Beatty S., Moriarty P., Henry M., Clynes M., Larkin A., Meleady P. Differential expression of fourteen proteins between uveal melanoma from patients who subsequently developed distant metastases versus those who did Not. Invest. Ophthalmol. Vis. Sci. 2012;53:4634–4643. doi: 10.1167/iovs.11-9019. [DOI] [PubMed] [Google Scholar]
- 62.Lowe S.W., Bodis S., McClatchey A., Remington L., Ruley H.E., Fisher D.E., Housman D.E., Jacks T. P53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 63.Magnuson B., Rainey E.K., Benjamin T., Baryshev M., Mkrtchian S., Tsai B. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol. Cell. 2005;20:289–300. doi: 10.1016/j.molcel.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 64.Martin T.A., Watkins G., Mansel R.E., Jiang W.G. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur. J. Cancer. 2004;40:2717–2725. doi: 10.1016/j.ejca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 65.Mkrtchian S., Baryshev M., Matvijenko O., Sharipo A., Sandalova T., Schneider G., Ingelman-Sundberg M. Oligomerization properties of ERp29, an endoplasmic reticulum stress protein. FEBS Lett. 1998;431:322–326. doi: 10.1016/s0014-5793(98)00786-8. [DOI] [PubMed] [Google Scholar]
- 66.Mkrtchian S., Baryshev M., Sargsyan E., Chatzistamou I., Volakaki A.A., Chaviaras N., Pafiti A., Triantafyllou A., Kiaris H. ERp29, an endoplasmic reticulum secretion factor is involved in the growth of breast tumor xenografts. Mol. Carcinog. 2008;47:886–892. doi: 10.1002/mc.20444. [DOI] [PubMed] [Google Scholar]
- 67.Mkrtchian S., Sandalova T. ERp29, an unusual redox-inactive member of the thioredoxin family. Antioxid. Redox Signal. 2006;8:325–337. doi: 10.1089/ars.2006.8.325. [DOI] [PubMed] [Google Scholar]
- 68.Moyret-Lalle C., Ruiz E., Puisieux A. Epithelial–mesenchymal transition transcription factors and miRNAs: “Plastic surgeons” of breast cancer. World J. Clin. Oncol. 2014;5:311–322. doi: 10.5306/wjco.v5.i3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niessen C.M. Tight junctions/adherens junctions: basic structure and function. J. Invest. Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 70.Niture S.K., Doneanu C.E., Velu C.S., Bailey N.I., Srivenugopal K.S. Proteomic analysis of human O6-methylguanine-DNA methyltransferase by affinity chromatography and tandem mass spectrometry. Biochem. Biophys. Res. Commun. 2005;337:1176–1184. doi: 10.1016/j.bbrc.2005.09.177. [DOI] [PubMed] [Google Scholar]
- 71.Nollet F., Kools P., van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 72.Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr. Opin. Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 73.Peinado H., Olmeda D., Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 74.Perez-Moreno M., Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev. Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez-Moreno M., Jamora C., Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 76.Qi L., Wu P., Zhang X., Qiu Y., Jiang W., Huang D., Liu Y., Tan P., Tian Y. Inhibiting ERp29 expression enhances radiosensitivity in human nasopharyngeal carcinoma cell lines. Med. Oncol. 2012;29:721–728. doi: 10.1007/s12032-011-9929-5. [DOI] [PubMed] [Google Scholar]
- 77.Rainey-Barger E.K., Mkrtchian S., Tsai B. Dimerization of ERp29, a PDI-like protein, is essential for its diverse functions. Mol. Biol. Cell. 2007;18:1253–1260. doi: 10.1091/mbc.E06-11-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rainey-Barger E.K., Mkrtchian S., Tsai B. The C-terminal domain of ERp29 mediates polyomavirus binding, unfolding, and infection. J. Virol. 2009;83:1483–1491. doi: 10.1128/JVI.02057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 80.Royer C., Lu X. Epithelial cell polarity: a major gatekeeper against cancer? Cell Death Differ. 2011;18:1470–1477. doi: 10.1038/cdd.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saha B., Chaiwun B., Imam S.S., Tsao-Wei D.D., Groshen S., Naritoku W.Y., Imam S.A. Overexpression of E-cadherin protein in metastatic breast cancer cells in bone. Anticancer Res. 2007;27:3903–3908. [PubMed] [Google Scholar]
- 82.Sargsyan E., Baryshev M., Mkrtchian S. The physiological unfolded protein response in the thyroid epithelial cells. Biochem. Biophys. Res. Commun. 2004;322:570–576. doi: 10.1016/j.bbrc.2004.07.155. [DOI] [PubMed] [Google Scholar]
- 83.Sargsyan E., Baryshev M., Szekely L., Sharipo A., Mkrtchian S. Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex. J. Biol. Chem. 2002;277:17009–17015. doi: 10.1074/jbc.M200539200. [DOI] [PubMed] [Google Scholar]
- 84.Sastre-Perona A., Santisteban P. Role of the wnt pathway in thyroid cancer. Front. Endocrinol. 2012;3:31. doi: 10.3389/fendo.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schonthal A.H. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem. Pharmacol. 2013;85:653–666. doi: 10.1016/j.bcp.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 86.Shin K., Fogg V.C., Margolis B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 87.Shnyder S.D., Mangum J.E., Hubbard M.J. Triplex profiling of functionally distinct chaperones (ERp29/PDI/BiP) reveals marked heterogeneity of the endoplasmic reticulum proteome in cancer. J. Proteome Res. 2008;7:3364–3372. doi: 10.1021/pr800126n. [DOI] [PubMed] [Google Scholar]
- 88.Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- 89.Sounni N.E., Noel A. Targeting the tumor microenvironment for cancer therapy. Clin. Chem. 2013;59:85–93. doi: 10.1373/clinchem.2012.185363. [DOI] [PubMed] [Google Scholar]
- 90.Suaud L., Miller K., Alvey L., Yan W., Robay A., Kebler C., Kreindler J.L., Guttentag S., Hubbard M.J., Rubenstein R.C. ERp29 regulates DeltaF508 and wild-type cystic fibrosis transmembrane conductance regulator (CFTR) trafficking to the plasma membrane in cystic fibrosis (CF) and non-CF epithelial cells. J. Biol. Chem. 2011;286:21239–21253. doi: 10.1074/jbc.M111.240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swisshelm K., Macek R., Kubbies M. Role of claudins in tumorigenesis. Adv. Drug Deliv. Rev. 2005;57:919–928. doi: 10.1016/j.addr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 92.Tan T.Z., Miow Q.H., Miki Y., Noda T., Mori S., Huang R.Y., Thiery J.P. Epithelial–mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol. Med. 2014;6:1279–1293. doi: 10.15252/emmm.201404208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tanos B., Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 94.Tetsu O., McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 95.Thiery J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 96.Thiery J.P. Epithelial–mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 97.Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 98.Tsuji T., Ibaragi S., Hu G.F. Epithelial–mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69:7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsukita S., Furuse M., Itoh M. Molecular architecture of tight junctions: occludin and ZO-1. Soc. Gen. Physiol. Ser. 1997;52:69–76. [PubMed] [Google Scholar]
- 100.Vasioukhin V. Lethal giant puzzle of Lgl. Dev. Neurosci. 2006;28:13–24. doi: 10.1159/000090749. [DOI] [PubMed] [Google Scholar]
- 101.Verbeek B., Southgate T.D., Gilham D.E., Margison G.P. O6-Methylguanine-DNA methyltransferase inactivation and chemotherapy. Br. Med. Bull. 2008;85:17–33. doi: 10.1093/bmb/ldm036. [DOI] [PubMed] [Google Scholar]
- 102.Wu C.I., Hoffman J.A., Shy B.R., Ford E.M., Fuchs E., Nguyen H., Merrill B.J. Function of Wnt/beta-catenin in counteracting Tcf3 repression through the Tcf3-beta-catenin interaction. Development. 2012;139:2118–2129. doi: 10.1242/dev.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu P., Zhang H., Qi L., Tang Q., Tang Y., Xie Z., Lv Y., Zhao S., Jiang W. Identification of ERp29 as a biomarker for predicting nasopharyngeal carcinoma response to radiotherapy. Oncol. Rep. 2012;27:987–994. doi: 10.3892/or.2011.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu S.G., Yan P.J., Shao Z.M. Differential proteomic analysis of a highly metastatic variant of human breast cancer cells using two-dimensional differential gel electrophoresis. J. Cancer Res. Clin. Oncol. 2010;136:1545–1556. doi: 10.1007/s00432-010-0812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan L., Donze J.R., Liu L. Inactivated MGMT by O6-benzylguanine is associated with prolonged G2/M arrest in cancer cells treated with BCNU. Oncogene. 2005;24:2175–2183. doi: 10.1038/sj.onc.1208250. [DOI] [PubMed] [Google Scholar]
- 106.Yi F., Pereira L., Hoffman J.A., Shy B.R., Yuen C.M., Liu D.R., Merrill B.J. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat. Cell Biol. 2011;13:762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ying X., Liu Y., Guo Q., Qu F., Guo W., Zhu Y., Ding Z. Endoplasmic reticulum protein 29 (ERp29), a protein related to sperm maturation is involved in sperm-oocyte fusion in mouse. Reprod. Biol. Endocrinol. 2010;8:10. doi: 10.1186/1477-7827-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang B., Wang M., Yang Y., Wang Y., Pang X., Su Y., Wang J., Ai G., Zou Z. ERp29 is a radiation-responsive gene in IEC-6 cell. J. Radiat. Res. 2008;49:587–596. doi: 10.1269/jrr.08014. [DOI] [PubMed] [Google Scholar]
- 109.Zhang D., Putti T.C. Over-expression of ERp29 attenuates doxorubicin-induced cell apoptosis through up-regulation of Hsp27 in breast cancer cells. Exp. Cell Res. 2010;316:3522–3531. doi: 10.1016/j.yexcr.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 110.Zhang J., Tian X.J., Zhang H., Teng Y., Li R., Bai F., Elankumaran S., Xing J. TGF-beta-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci. Signal. 2014;7:ra91. doi: 10.1126/scisignal.2005304. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y.H., Belegu V., Zou Y., Wang F., Qian B.J., Liu R., Dai P., Zhao W., Gao F.B., Wang L., Cao L.M., McDonald J.W., Liu S., Lin N., Wang T.H. Endoplasmic reticulum protein 29 protects axotomized neurons from apoptosis and promotes neuronal regeneration associated with Erk signal. Mol. Neurobiol. 2014 doi: 10.1007/s12035-014-8840-4. Sep 10 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


