Abstract
Purpose:
To evaluate the efficacy of topical cyclosporine A (tCsA) for treatment of dry eye disease in patients suffering from chronic ocular complications of mustard gas (MG) injury.
Methods:
This interventional case series included patients with MG injury suffering from severe dry eye despite receiving artificial tears and punctal plugs. Patients were administered tCsA 0.05% twice daily for 3 months. Severity of the condition was evaluated by measuring tear osmolarity, ocular surface disease index (OSDI), tear break-up time (TBUT), and Schirmer's test at baseline and at the end of study.
Results:
A total of 34 patients with chronic MG injury and mean age of 47.1 ± 6.5 years were studied. Compared to baseline values, tear osmolarity (301.7 ± 11.5 vs. 286.3 ± 7.9 mOsmol/L, P < 0.001) and OSDI (47.5 ± 7.2 vs. 42.7 ± 7.1, P < 0.001) were significantly improved. Likewise, Schirmer's test (4.6 ± 1.3 vs. 5 ± 1.3 mm, P < 0.001) and TBUT (1.9 ± 1.4 vs. 2.7 ± 1.5 s, P < 0.001) also significantly recovered at the end of the study.
Conclusion:
TCsA 0.05% reduces tear osmolarity and improves dry eye symptoms and can serve as an efficacious treatment for ocular complications in patients with chronic MG injury.
Keywords: Cyclosporine A, Dry Eye Syndrome, Mustard Gas, Tear Osmolarity
INTRODUCTION
Late ocular complications of sulfur mustard gas (MG) are very common among intoxicated individuals. Ocular toxicity of MG often manifests as dry eye and decreased tear meniscus.[1,2,3,4] Victims of MG exposure experience symptoms of dry eye disease such as burning, itching, foreign body sensation, photophobia, red eye, and reduced visual acuity.[1,5] These symptoms have a negative impact on patients’ daily activities and quality of life, and thus require effective management.[6,7]
Susceptibility of the eyes to the toxic effects of MG is due to moistness of the ocular surface that allows rapid cyclization and activation of the agent, in addition to the high turnover and metabolic rate of corneal epithelial cells that increase their sensitivity to the lipophilic sulfur mustard trapped into the oily tear layer. The most frequent destructive ocular complications of MG injury include chronic blepharitis, decreased tear meniscus, limbal ischemia and conjunctival vascular abnormalities.[5] MG-induced keratitis may be persistent leading to corneal degeneration, or progress in a silent manner to severe ocular lesions.[5] While the pathophysiology of MG-induced keratitis remains obscure, an autoimmune reaction to corneal antigens has been suggested to play a central role in the development of this disease and causes severe limbal and corneal damage.[7,8,9]
Although various strategies are employed in the treatment and management of dry eye disease, currently available medications have limited efficacy in controlling the symptoms, especially in cases secondary to sulfur mustard (SM) exposure.[10] Hence, there is an urgent demand for novel therapeutic approaches in patients suffering from late ocular complications of SM. Recent studies have suggested a key role for an underlying inflammatory component in the pathogenesis of dry eye disease. This has led to the use of many anti-inflammatory-based therapies, such as short-term corticosteroids and long-term cyclosporine, to improve the efficacy of treatment in these patients.[11,12] Thus far, several lines of evidence have favored the effectiveness of topical cyclosporine A (tCsA) in the treatment of dry eye disease due to varying etiologies.[13,14,15,16,17] However, tCsA has not yet been tested in patients with late ocular complications of MG. The present study was undertaken to investigate the effects of tCsA in a group of MG-exposed patients suffering from severe dry eye disease.
METHODS
This prospective interventional case series included 36 MG-exposed male veterans suffering from symptoms of dry eye disease despite receiving artificial tear and punctal plugs. Inclusion criteria were ocular surface disease index (OSDI) score of ≥ 0.25 and Schirmer's test (with anesthesia) <10 mm. All subjects underwent a thorough ocular examination including slit lamp biomicroscopy and visual acuity measurement using a logMAR chart (at 3 m testing distance). For each patient, only the more severely affected eye was included in the study. Exclusion criteria were the presence of active ocular infection, history of ocular surgery within the past 3 months, and a history of hypersensitivity to cyclosporine A. Individuals with any systemic diseases that may cause dry eye, such as diabetes mellitus, rheumatologic disorders and Sjögren's syndrome were also excluded. The Ethics Committee at Baqiyatallah University of Medical Sciences approved the study protocol and written informed consent was obtained from all participants.
All participants were asked to stop using any previous topical medication. One drop of tCsA 0.05% (Restasis, Allergan Inc, Irvine, CA, USA) twice daily and one drop of preservative-free artificial tear (Artelac Advanced, Bausch and Lomb GmbH, Germany) every 6 hours were prescribed for each eye for a period of 3 months. Patients were encouraged to continue their medication and were advised to report any adverse effect during the follow-up period. Participants were informed that they might experience a burning sensation during the initial weeks of therapy. During the study period, patients were contacted every 3 weeks to assure their compliance with the medication and lack of adverse effects. After 3 months, all patients were re-examined using the same tests as the first visit.
Assessment of dry eye severity was performed at baseline and at the end of the study using tear osmolarity assay, OSDI, Schirmer's test and tear break-up time (TBUT). Ocular surface staining with rose bengal or fluorescein was not performed in this study. The reason is that apart from the subjective nature of staining results, many patients with MG-induced keratitis have varying degrees of limbal stem cell deficiency which can affect the staining result independent of dry eye disease. Therefore, staining results may not be reliable in patients with MG-induced dry eye.
Tear Osmolarity Measurement
Patients were asked not to instill any drop in their eyes at least 1 hour prior to the test. Measurement of tear osmolarity was performed using an automated device (TearLab Osmolarity System, TearLab Corp., San Diego, CA, USA). This device can determine tear osmolarity with sample volumes as low as 50 nL with no need for additional calibration of the instrument as a coefficient of variation of 1.5% and analytical standard variation of ± 5.0 mOsmol/L allows performing the test just once with a high level of validity.
Ocular Surface Disease Index
A Persian translation of OSDI was applied which consists of 12 questions measuring the severity of dry eye symptoms. The OSDI score was calculated using methods described previously.[18]
Schirmer's Test
To determine the Schirmer's score, a standard Schirmer's test strip was placed in the temporal third of the lower eyelid for 5 minutes and the length of the wet part was measured in millimeters. Prior to the test, one drop of proparacaine (Alcaine, Alcon Laboratories, TX, USA) was instilled in the eyes to measure basic tear secretion.
Tear Break-Up Time Test
To conduct the TBUT test, a fluorescein strip moistened with a drop of saline solution was placed on the inferior palpebral conjunctiva. The elapsed time before the initial break-up of tear film was counted in seconds. The test was repeated three times and the mean was reported as TBUT.
Statistical Analysis
Statistical analysis was performed using SPSS software version 18 (SPSS, Inc., Chicago, IL, USA). Given the normal distribution of data, paired-samples t-test was used for pre-and post-treatment comparisons. Bivariate associations between baseline and post-treatment values of the evaluated parameters as well as their changes during the course of the study were assessed using Pearson's (in case of normally distributed data) or Spearman's (in case of non-normally distributed data) correlation coefficients. P < 0.05 were considered as statistically significant.
RESULTS
A total of 36 eyes from 36 patients with mean age of 47.1 ± 6.5 years were included in this study. Two patients (5.6%) failed to complete the treatment period with tCsA. Reasons for drop-out were respiratory complications due to MG exposure which made it impossible to continue the study in one case and severe burning sensation and blurred vision after instillation of tCsA in another patient. These two cases were excluded from statistical analysis. Mean time from exposure to MG was 26.5 ± 0.7 years. All subjects were previously using preservative-free artificial tears for treatment of dry eye and 23 (63.9%) of them had undergone punctal plugging. The most common symptom was blurred vision (83.3%) followed by ocular itching (72.2%). Slit lamp examination of the conjunctiva and cornea revealed abnormal findings such as corneal opacity in 13 (36.1%) cases, limbal ischemia affecting at least one-third of the limbus in 11 (30.1%) cases, and corneal vascularization in 10 (27.7%) cases.
Figure 1 shows the outcome measures before and after treatment. Best corrected visual acuity improved from 0.5 ± 0.2 LogMAR at baseline to 0.4 ± 0.2 LogMAR at the end of the study (P = 0.001). Mean OSDI score was 47.5 ± 7.2 at baseline and 42.7 ± 7.1 at the end of study (P < 0.001). There was a significant increase in Schirmer test scores from baseline (4.6 ± 1.3 mm) to the end of the study (5.0 ± 1.3 mm) (P < 0.001). tCsA therapy was associated with a significant decrease in tear osmolarity (from 301.7 ± 11.5–286.3 ± 7.9 mOsmol/L, P < 0.001), and a significant increase in TBUT (from 1.9 ± 1.4–2.7 ± 1.5 s, P < 0.001).
Figure 1.
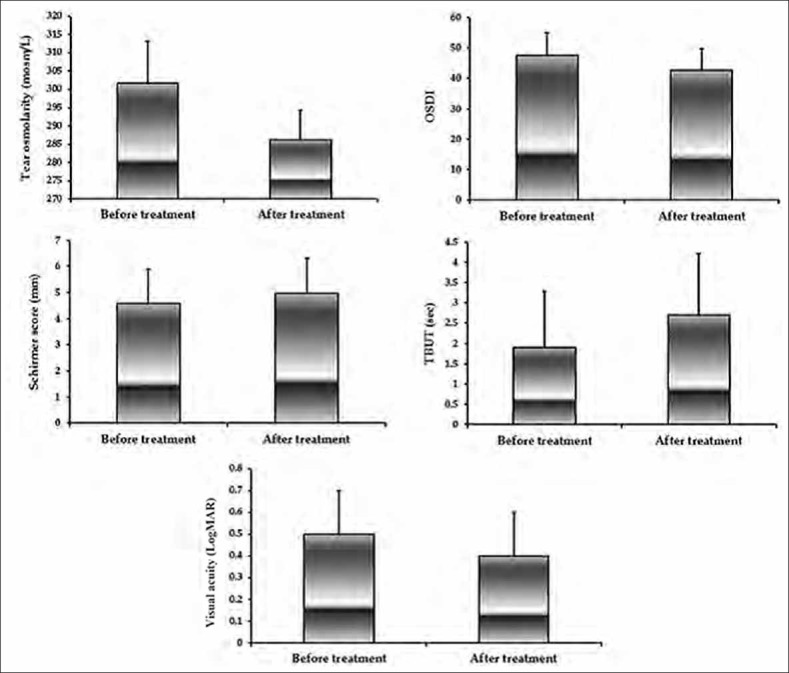
Dry eye parameters before and after treatment with topical cyclosporine A. Values are mean±standard deviation. OSDI, ocular surface disease index; TBUT, tear break-up time; LogMAR, logarithm of the minimum angle of resolution.
Overall, the majority of patients experienced improved symptoms associated with each evaluated efficacy measure. The proportion of patients with an improved score in Schirmer's test, visual acuity and TBUT at the end of the study were 61.8%, 35.3% and 50%, respectively. The proportion of patients with an increased score in Schirmer's test, visual acuity and TBUT at the end of the study were 61.8%, 35.3% and 50%, respectively. Also, the majority of patients (85.3%) had decreased tear osmolarity and OSDI score at the end of study as compared to baseline [Figure 2].
Figure 2.
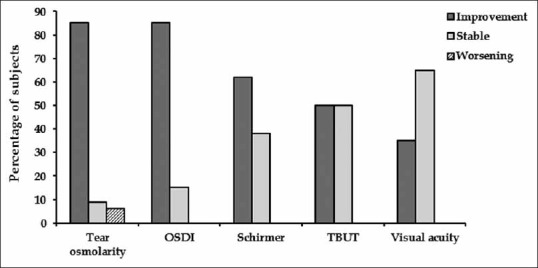
Proportion of subjects with improved stable or worsened score for each efficacy measure. OSDI, ocular surface disease index; TBUT, tear break-up time.
DISCUSSION
The morbidity associated with chronic complications of MG injury in different organs of the body necessitates attempts to find effective therapies to control the symptoms and correct the underlying biochemical imbalances following intoxication.[19,20,21,22,23,24,25,26,27,28,29] The present study aimed to investigate the efficacy of tCsA 0.05% for treatment of severe dry eye in a group of veterans suffering from late ocular complications due to MG exposure. Our primary efficacy measure was the change in tear osmolarity as the most specific and accurate test for monitoring treatment efficacy. There is compelling evidence supporting that tear osmolarity has the highest correlation with the severity of dry eye disease.[30,31,32,33] Tear film hyperosmolarity has been shown to play a critical role in the pathophysiology of aqueous tear deficiency and evaporative dry eye, and is directly connected to the etiology of disease manifestations.[34]
In this study, all patients had previous history of treatment with preservative-free artificial tears. After 3 months of tCsA 0.05% (twice daily) treatment plus preservative-free artificial tears (four times daily), all subjective (OSDI score) and objective (Schirmer score, TBUT, tear osmolarity) parameters used for assessing dry eye improved significantly. As a late complication of exposure to MG, severe dry eye is common among exposed individuals and affects their quality of life.[1,4] Two important elements in the pathogenesis of dry eye disease are eyelid inflammation and loss of conjunctival goblet cells, both leading to tear film instability and decreased tear meniscus.
Several studies have supported the efficacy of tCsA in improving signs and symptoms in different stages of dry eye disease.[13,16,17] Nevertheless, to the best of our knowledge, no study has yet been conducted on patients with MG-induced disease. Stevenson et al[13] showed the efficacy of tCsA at different concentrations in decreasing rose bengal staining, superficial punctuate keratitis, and symptoms of ocular discomfort in patients with moderate to severe keratoconjunctivitis sicca. They reported that the 0.05% concentration is associated with the highest rate of improvement. Sall et al[14] compared treatment with tCsA 0.05%, 0.1%, or vehicle twice daily in 877 patients with moderate to severe dry eye. They found that both objective (corneal fluorescein staining and Schirmer's values) and subjective measures of dry eye significantly improved in patients treated with tCsA (0.05 or 0.1%) as compared to those treated with vehicle. Perry et al[16] also reported that tCsA is effective in all stages of dry eye disease, with most improvement of symptoms in mild stages and greatest alleviation of signs in severe stages. There is also evidence indicating that tCsA is effective in increasing goblet cell density in patients with dry eye disease.[35] In a study by Moon et al[36] cyclosporine was found to be more effective in improving objective parameters of dry eye such as TBUT and goblet cell density than artificial tears. Also, the results of a trial by Pflugfelder et al[37] revealed that cyclosporine emulsion, but not artificial tears, was effective in increasing goblet cell density in patients with dry eye. The use of tCsA for the treatment of dry eye has also been evaluated in different situations associated with this syndrome i.e., in patients undergoing LASIK or in patients with dry eye associated with graft versus host disease after stem cell transplantation.[14,15]
Objective measurement of the effectiveness of an applied medication on dry eye has always been a great challenge, as many of the tests do not reflect the patients’ symptoms.[38,39] In many of these methods such as Schirmer's test or rose bengal staining, physical endpoints are used which reflect end-stage disease and have low positive predictive values (PPV). Researchers have shown that tear osmolarity is a test with the highest PPV and the most accurate one for diagnosis as well as follow-up of dry eye disease.[30,31,40] The automated TearLab osmolarity system that was employed in the current study is an easy-to-use system which requires a very small amount (about 50 nL) of tear and can appropriately address concerns about effectiveness and rapidity of tear osmolarity measurement. It is well known that tear hyperosmolarity plays an important role in progression of inflammation and corneal epithelial damage in dry eye conditions by activating an inflammatory cascade.[41,42,43] Hence, change in tear osmolarity is an important underlying factor that, along with ocular inflammation, is responsible for symptoms of the disease.[43]
The main limitation of the present study was lack of any control group receiving artificial tears alone. In addition, although the participants were within the same age range and had a comparable time interval since exposure to MG, they were not completely homogeneous in terms of the frequency of different ocular abnormalities such as limbal ischemia, corneal opacity and corneal vascularization at baseline. It may also be argued that concurrent administration of artificial tears with tCsA is responsible, at least in part, for the favorable effects that were observed in this study. However, it must be taken into account that all enrolled patients had been unresponsive to artificial tear treatment and sought additional medications. Therefore, it is less likely that improvement in signs and symptoms were related to the use of artificial tear.
Finally, it must be noted that although the efficacy measures in this study were significantly improved by tCsA, the effect size was generally small and thus may not be clinically significant. Nevertheless, it is plausible to obtain clinically relevant effects after longer durations of treatment.
In summary, our study showed that tCsA 0.05% is effective in reducing tear osmolarity as well as improving symptoms in patients suffering from MG-induced dry eye disease. Given the role of tear hyperosmolarity as a major determinant of the progression and severity of dry eye disease,[44] and a high frequency of chronic ocular complications in MG-exposed individuals, it is recommended that tCsA 0.05% be considered as a potential treatment for reduction of symptoms and improvement of quality of life. The interesting results of the present pilot study on the efficacy of tCsA in decreasing MG-induced dry eye symptoms generates the basis for conducting future large-scale randomized controlled trials.
ACKNOWLEDGEMENTS
The authors would also like to thank all the subjects who participated in this study. Mostafa Mafi wishes to thank the National Elite Foundation of Iran for its support. Results of this study have been partly presented in the ISOPT Clinical Congress (7–10 March 2013, Paris, France).
Footnotes
Source of Support: Baqiyatallah University of Medical Sciences.
Conflict of Interest: Baqiyatallah University of Medical Sciences.
REFERENCES
- 1.Solberg Y, Alcalay M, Belkin M. Ocular injury by mustard gas. Surv Ophthalmol. 1997;41:461–466. doi: 10.1016/s0039-6257(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 2.Safarinejad MR, Moosavi SA, Montazeri B. Ocular injuries caused by mustard gas: Diagnosis, treatment, and medical defense. Mil Med. 2001;166:67–70. [PubMed] [Google Scholar]
- 3.Etezad-Razavi M, Mahmoudi M, Hefazi M, Balali-Mood M. Delayed ocular complications of mustard gas poisoning and the relationship with respiratory and cutaneous complications. Clin Experiment Ophthalmol. 2006;34:342–346. doi: 10.1111/j.1442-9071.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- 4.Baradaran-Rafii A, Eslani M, Tseng SC. Sulfur mustard-induced ocular surface disorders. Ocul Surf. 2011;9:163–178. doi: 10.1016/s1542-0124(11)70026-x. [DOI] [PubMed] [Google Scholar]
- 5.Javadi MA, Yazdani S, Sajjadi H, Jadidi K, Karimian F, Einollahi B, et al. Chronic and delayed-onset mustard gas keratitis: Report of 48 patients and review of literature. Ophthalmology. 2005;112:617–625. doi: 10.1016/j.ophtha.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Mertzanis P, Abetz L, Rajagopalan K, Espindle D, Chalmers R, Snyder C, et al. The relative burden of dry eye in patients’ lives: Comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005;46:46–50. doi: 10.1167/iovs.03-0915. [DOI] [PubMed] [Google Scholar]
- 7.Management and therapy of dry eye disease: Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:163–178. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 8.Kadar T, Turetz J, Fishbine E, Sahar R, Chapman S, Amir A. Characterization of acute and delayed ocular lesions induced by sulfur mustard in rabbits. Curr Eye Res. 2001;22:42–53. doi: 10.1076/ceyr.22.1.42.6975. [DOI] [PubMed] [Google Scholar]
- 9.Jampol LM, Axelrod A, Tessler H. Pathways of the eye's response to topical nitrogen mustard. Invest Ophthalmol. 1976;15:486–489. [PubMed] [Google Scholar]
- 10.Asbell PA, Spiegel S. Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: Results of a physician survey. Eye Contact Lens. 2010;36:33–38. doi: 10.1097/ICL.0b013e3181c739ad. [DOI] [PubMed] [Google Scholar]
- 11.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: The interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: A dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000;107:967–974. doi: 10.1016/s0161-6420(00)00035-x. [DOI] [PubMed] [Google Scholar]
- 13.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 14.Salib GM, McDonald MB, Smolek M. Safety and efficacy of cyclosporine 0.05% drops versus unpreserved artificial tears in dry-eye patients having laser in situ keratomileusis. J Cataract Refract Surg. 2006;32:772–778. doi: 10.1016/j.jcrs.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Ogawa Y, Dogru M, Kawai M, Tatematsu Y, Uchino M, et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2008;41:293–302. doi: 10.1038/sj.bmt.1705900. [DOI] [PubMed] [Google Scholar]
- 16.Perry HD, Solomon R, Donnenfeld ED, Perry AR, Wittpenn JR, Greenman HE, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. 2008;126:1046–1050. doi: 10.1001/archopht.126.8.1046. [DOI] [PubMed] [Google Scholar]
- 17.Byun YS, Rho CR, Cho K, Choi JA, Na KS, Joo CK. Cyclosporine 0.05% ophthalmic emulsion for dry eye in Korea: A prospective, multicenter, open-label, surveillance study. Korean J Ophthalmol. 2011;25:369–374. doi: 10.3341/kjo.2011.25.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 19.Panahi Y, Davoudi SM, Beiraghdar F, Amiri M, Saadat A, Marzony ET, et al. Serum levels of interleukins 2, 4, 6, and 10 in veterans with chronic sulfur mustard-induced pruritus: A cross-sectional study. Skinmed. 2013;11:205–209. [PubMed] [Google Scholar]
- 20.Panahi Y, Davoudi SM, Beiraghdar F, Saadat A, Sahebkar A. Relationship between levels of IFNγ, TNFα, and TGFβ and pruritus in sulfur mustard-exposed veterans. J Immunotoxicol. 2013;10:173–177. doi: 10.3109/1547691X.2012.707697. [DOI] [PubMed] [Google Scholar]
- 21.Panahi Y, Taherzadeh ES, Davoudi SM, Sahebkar A, Ranjbar R. Investigation of serum substance P status in patients with chronic pruritic skin lesions due to sulfur mustard: A cross-sectional study. Cutan Ocul Toxicol. 2013;32:4–8. doi: 10.3109/15569527.2012.686077. [DOI] [PubMed] [Google Scholar]
- 22.Panahi Y, Sahebkar A, Parvin S, Saadat A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann Clin Biochem. 2012;49:580–588. doi: 10.1258/acb.2012.012040. [DOI] [PubMed] [Google Scholar]
- 23.Panahi Y, Sahebkar A, Amiri M, Davoudi SM, Beiraghdar F, Hoseininejad SL, et al. Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: Results of a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2012;108:1272–1279. doi: 10.1017/S0007114511006544. [DOI] [PubMed] [Google Scholar]
- 24.Sahebkar A. Baicalin as a potentially promising drug for the management of sulfur mustard induced cutaneous complications: A review of molecular mechanisms. Cutan Ocul Toxicol. 2012;31:226–234. doi: 10.3109/15569527.2011.633950. [DOI] [PubMed] [Google Scholar]
- 25.Panahi Y, Sarayani A, Beiraghdar F, Amiri M, Davoudi SM, Sahebkar A. Management of sulfur mustard-induced chronic pruritus: A review of clinical trials. Cutan Ocul Toxicol. 2012;31:220–225. doi: 10.3109/15569527.2011.631655. [DOI] [PubMed] [Google Scholar]
- 26.Panahi Y, Eftekhari Milani A, Sahebkar A, Naderi M, Babaei M, Beiraghdar F, et al. Tear total protein analysis in patients with late sulfur mustard-induced ocular complications: A cross-sectional study. Cutan Ocul Toxicol. 2012;31:104–110. doi: 10.3109/15569527.2011.615359. [DOI] [PubMed] [Google Scholar]
- 27.Panahi Y, Davoudi SM, Sahebkar A, Beiraghdar F, Dadjo Y, Feizi I, et al. Efficacy of Aloe vera/olive oil cream versus betamethasone cream for chronic skin lesions following sulfur mustard exposure: A randomized double-blind clinical trial. Cutan Ocul Toxicol. 2012;31:95–103. doi: 10.3109/15569527.2011.614669. [DOI] [PubMed] [Google Scholar]
- 28.Panahi Y, Sahebkar A, Davoudi SM, Amiri M, Beiraghdar F. Efficacy and safety of immunotherapy with interferon-gamma in the management of chronic sulfur mustard-induced cutaneous complications: Comparison with topical betamethasone 1% ScientificWorldJournal 2012. 2012 doi: 10.1100/2012/285274. 285274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panahi Y, Ghanei M, Vahedi E, Ghazvini A, Parvin S, Madanchi N, et al. Effect of recombinant human IFNγ in the treatment of chronic pulmonary complications due to sulfur mustard intoxication. J Immunotoxicol. 2014;11:72–77. doi: 10.3109/1547691X.2013.797525. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Begley C, Chen M, Bradley A, Bonanno J, McNamara NA, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50:3671–3679. doi: 10.1167/iovs.08-2689. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz EH, Fan VC, Banday H, Ramanathan LV, Bitra RK, Garry E, et al. Evaluation of a new tear osmometer for repeatability and accuracy, using 0.5-μL (500-Nanoliter) samples. Cornea. 2009;28:677–680. doi: 10.1097/ICO.0b013e318198396b. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51:6125–30. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- 33.Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35:553–564. doi: 10.3109/02713683.2010.484557. [DOI] [PubMed] [Google Scholar]
- 34.Berta A, Higazy M, Petricek I. Red eye: Differential diagnosis and management. Int Ophthalmol. 2008;28:1–64. [Google Scholar]
- 35.Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: Effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118:1489–1496. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 36.Moon JW, Lee HJ, Shin KC, Wee WR, Lee JH, Kim MK. Short term effects of topical cyclosporine and viscoelastic on the ocular surfaces in patients with dry eye. Korean J Ophthalmol. 2007;21:189–194. doi: 10.3341/kjo.2007.21.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27:64–69. doi: 10.1097/ICO.0b013e318158f6dc. [DOI] [PubMed] [Google Scholar]
- 38.Smith J, Nichols KK, Baldwin EK. Current patterns in the use of diagnostic tests in dry eye evaluation. Cornea. 2008;27:656–662. doi: 10.1097/QAI.0b013e3181605b95. [DOI] [PubMed] [Google Scholar]
- 39.Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocul Surf. 2009;7:199–211. doi: 10.1016/s1542-0124(12)70187-8. [DOI] [PubMed] [Google Scholar]
- 40.Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47:4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- 41.Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 42.Li DQ, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 44.Benelli U, Nardi M, Posarelli C, Albert TG. Tear osmolarity measurement using the TearLab Osmolarity System in the assessment of dry eye treatment effectiveness. Cont Lens Anterior Eye. 2010;33:61–67. doi: 10.1016/j.clae.2010.01.003. [DOI] [PubMed] [Google Scholar]


