Abstract
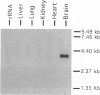
Sec1 is a hydrophilic protein that plays an essential role in exocytosis from the yeast Saccharomyces cerevisiae. Two high copy suppressors of mutations in the Sec1 gene, SSO1 and SSO2, were recently identified that encode proteins of the syntaxin family. Syntaxin (a T-SNARE), together with SNAP-25 and synaptobrevin/VAMP (a T- and a V-SNARE, respectively), is thought to form the core of the docking-fusion complex in synaptic vesicle exocytosis. Proteins that exhibit similarity to Sec1 were identified in the nervous system of Drosophila melanogaster (Rop) and Caenorhabditis elegans (UNC18). Based on the amino acid sequence alignment of Sec1, Rop, and UNC18, we have used a PCR-based approach to isolate a rat brain cDNA encoding a Sec1 homologue. The cDNA hybridizes to a 3.5-kb brain-specific mRNA by Northern blot analysis and encodes a protein of 593 amino acids (rbSec1). Antibodies raised against a central portion of rbSec1 recognize a 67.5-kDa protein in total homogenates of rat brain but not of nonneuronal tissues. When incubated with a Triton X-100 brain extract, rbSec1-glutathione S-transferase (GST) fusion protein, but not GST protein alone, specifically interacts with syntaxin but not with SNAP-25 or synaptobrevin/VAMP. We conclude that the function of proteins of the Sec1 family in membrane fusion involves an interaction with a T-SNARE.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto M. K., Ronne H., Keränen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993 Nov;12(11):4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalto M. K., Ruohonen L., Hosono K., Keränen S. Cloning and sequencing of the yeast Saccharomyces cerevisiae SEC1 gene localized on chromosome IV. Yeast. 1991 Aug-Sep;7(6):643–650. doi: 10.1002/yea.320070613. [DOI] [PubMed] [Google Scholar]
- Aalto M. K., Ruohonen L., Hosono K., Keränen S. Cloning and sequencing of the yeast Saccharomyces cerevisiae SEC1 gene localized on chromosome IV. Yeast. 1992 Jul;8(7):587–588. doi: 10.1002/yea.320080710. [DOI] [PubMed] [Google Scholar]
- Adams M. D., Dubnick M., Kerlavage A. R., Moreno R., Kelley J. M., Utterback T. R., Nagle J. W., Fields C., Venter J. C. Sequence identification of 2,375 human brain genes. Nature. 1992 Feb 13;355(6361):632–634. doi: 10.1038/355632a0. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Hofstein R., Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985 Jun;352(2):286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Baumert M., Maycox P. R., Navone F., De Camilli P., Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989 Feb;8(2):379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992 Jul 10;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., García-Arrarás J. E., Elferink L. A., Peterson K., Fleming A. M., Hazuka C. D., Scheller R. H. The syntaxin family of vesicular transport receptors. Cell. 1993 Sep 10;74(5):863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhathena S. J., Oie H. K., Gazdar A. F., Voyles N. R., Wilkins S. D., Recant L. Insulin, glucagon, and somatostatin receptors on cultured cells and clones from rat islet cell tumor. Diabetes. 1982 Jun;31(6 Pt 1):521–531. doi: 10.2337/diab.31.6.521. [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., De Camilli P., Südhof T. C., Niemann H., Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993 Sep 9;365(6442):160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Yamasaki S., Binz T., Niemann H., Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993 Dec;12(12):4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J., Roberts D., Montaldi M., Novick P., De Camilli P. A mammalian guanine-nucleotide-releasing protein enhances function of yeast secretory protein Sec4. Nature. 1993 Feb 4;361(6411):464–467. doi: 10.1038/361464a0. [DOI] [PubMed] [Google Scholar]
- Cameron P. L., Südhof T. C., Jahn R., De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991 Oct;115(1):151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Ossig R., Gallwitz D., Schmitt H. D. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991 Feb;11(2):872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P. Exocytosis goes with a SNAP. Nature. 1993 Jul 29;364(6436):387–388. doi: 10.1038/364387a0. [DOI] [PubMed] [Google Scholar]
- Gengyo-Ando K., Kamiya Y., Yamakawa A., Kodaira K., Nishiwaki K., Miwa J., Hori I., Hosono R. The C. elegans unc-18 gene encodes a protein expressed in motor neurons. Neuron. 1993 Oct;11(4):703–711. doi: 10.1016/0896-6273(93)90080-b. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K. G., Pelham H. R. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992 Nov;119(3):513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono R., Hekimi S., Kamiya Y., Sassa T., Murakami S., Nishiwaki K., Miwa J., Taketo A., Kodaira K. I. The unc-18 gene encodes a novel protein affecting the kinetics of acetylcholine metabolism in the nematode Caenorhabditis elegans. J Neurochem. 1992 Apr;58(4):1517–1525. doi: 10.1111/j.1471-4159.1992.tb11373.x. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990 May 18;61(4):723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matteoli M., Takei K., Cameron R., Hurlbut P., Johnston P. A., Südhof T. C., Jahn R., De Camilli P. Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. J Cell Biol. 1991 Nov;115(3):625–633. doi: 10.1083/jcb.115.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ossig R., Dascher C., Trepte H. H., Schmitt H. D., Gallwitz D. The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol Cell Biol. 1991 Jun;11(6):2980–2993. doi: 10.1128/mcb.11.6.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989 Dec;109(6 Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Neurotransmission and secretion. Nature. 1993 Aug 12;364(6438):582–582. doi: 10.1038/364582a0. [DOI] [PubMed] [Google Scholar]
- Protopopov V., Govindan B., Novick P., Gerst J. E. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell. 1993 Sep 10;74(5):855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Pryer N. K., Wuestehube L. J., Schekman R. Vesicle-mediated protein sorting. Annu Rev Biochem. 1992;61:471–516. doi: 10.1146/annurev.bi.61.070192.002351. [DOI] [PubMed] [Google Scholar]
- Riezman H. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell. 1985 Apr;40(4):1001–1009. doi: 10.1016/0092-8674(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. S., Klionsky D. J., Banta L. M., Emr S. D. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988 Nov;8(11):4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Salzberg A., Cohen N., Halachmi N., Kimchie Z., Lev Z. The Drosophila Ras2 and Rop gene pair: a dual homology with a yeast Ras-like gene and a suppressor of its loss-of-function phenotype. Development. 1993 Apr;117(4):1309–1319. doi: 10.1242/dev.117.4.1309. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Rossetto O., Santucci A., DasGupta B. R., Montecucco C. Botulinum neurotoxins are zinc proteins. J Biol Chem. 1992 Nov 25;267(33):23479–23483. [PubMed] [Google Scholar]
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993 Nov 5;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., De Camilli P., Niemann H., Jahn R. Membrane fusion machinery: insights from synaptic proteins. Cell. 1993 Oct 8;75(1):1–4. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Cowan D. M., Scheller R. H. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia A., Chardin P., Wittinghofer A., Sander C. The ras protein family: evolutionary tree and role of conserved amino acids. Biochemistry. 1991 May 14;30(19):4637–4648. doi: 10.1021/bi00233a001. [DOI] [PubMed] [Google Scholar]
- Wada Y., Kitamoto K., Kanbe T., Tanaka K., Anraku Y. The SLP1 gene of Saccharomyces cerevisiae is essential for vacuolar morphogenesis and function. Mol Cell Biol. 1990 May;10(5):2214–2223. doi: 10.1128/mcb.10.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteheart S. W., Griff I. C., Brunner M., Clary D. O., Mayer T., Buhrow S. A., Rothman J. E. SNAP family of NSF attachment proteins includes a brain-specific isoform. Nature. 1993 Mar 25;362(6418):353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Whiteheart S. W., Wiedmann M., Brunner M., Rothman J. E. A multisubunit particle implicated in membrane fusion. J Cell Biol. 1992 May;117(3):531–538. doi: 10.1083/jcb.117.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]