Abstract
Purpose:
To evaluate the effect of two intravitreal bevacizumab (IVB) injections on peripapillary retinal nerve fiber layer (RNFL) thickness in patients with wet type age-related macular degeneration (ARMD).
Methods:
This prospective interventional case series included 18 eyes of 18 patients receiving two IVB injections within a 6 weeks interval for treatment of wet type ARMD. Peripapillary RNFL thickness was measured prior to the first injection, and 12 and 24 weeks afterwards by optical coherence tomography (3D OCT-1000, Topcon Corporation, Tokyo, Japan). Mean RNFL thickness and values in the four peripapillary quadrants were compared at baseline, and 12 and 24 weeks after initial injection.
Results:
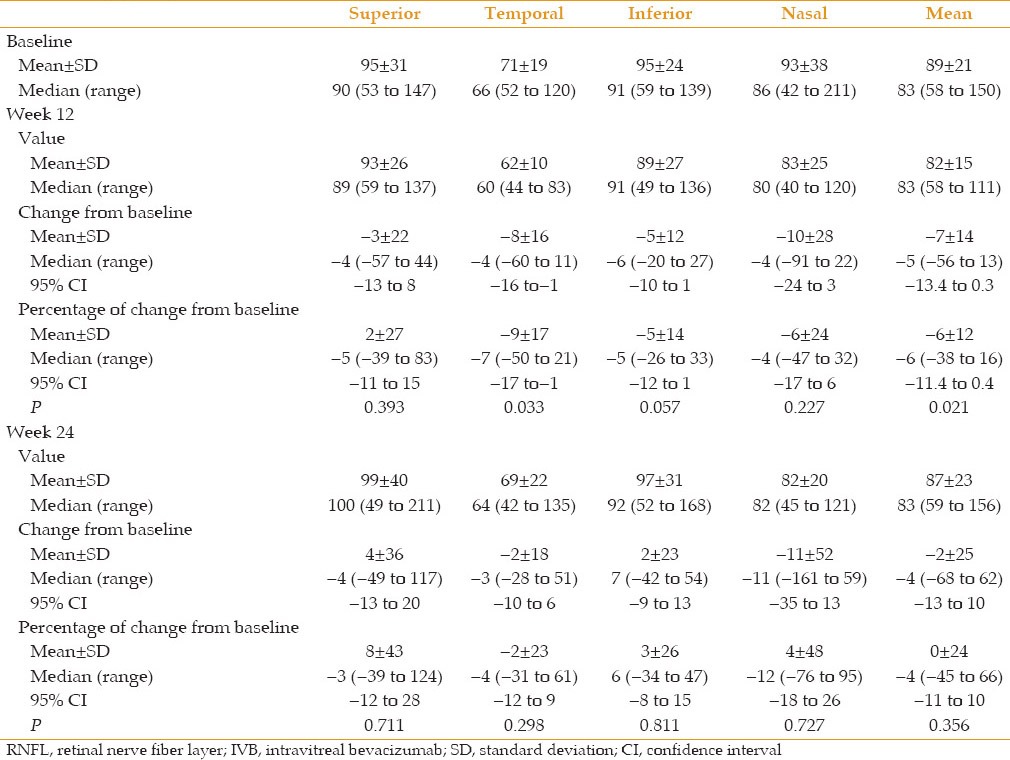
Mean RNFL thickness was 89 ± 21 μm at baseline which was significantly reduced to 82 ± 15 μm at 12 weeks (P = 0.021). At final follow-up (week 24), mean RNFL thickness reached 87 ± 23 μm and was comparable to baseline values (P = 0.356). Only the temporal quadrant showed a significant reduction in RNFL thickness at 12 weeks (P = 0.033); this quadrant followed the same pattern of change as the mean RNFL thickness, becoming comparable to pre-injection values at 24 weeks (P = 0. 298).
Conclusion:
RNFL thickness may decrease temporarily following two IVB injections in patients with wet type ARMD; however, in the long-term no significant change was detectable from baseline values.
Keywords: Age-related Macular Degeneration, Bevacizumab, Optical Coherence Tomography, Retinal Nerve Fiber Layer
INTRODUCTION
Bevacizumab is a full-length humanized monoclonal antibody which binds to all subtypes of the vascular endothelial growth factor (VEGF).[1] Intravitreal injection of bevacizumab (IVB), as an off-label agent, has shown beneficial therapeutic effects on several retinal diseases such as neovascular age-related macular degeneration (ARMD).[2,3,4,5,6,7] However, IVB has a limited duration of effect, hence repeated injections are required to maintain its therapeutic effect. While the use of IVB has gained increased popularity, the long-term safety of repeated injections of this drug remains unclear.[8] Moreover, there are a few reports on reduction of visual acuity, enlargement of the foveal avascular zone and aggravation of macular ischemia following IVB injections.[9]
Vascular endothelial growth factor is known to be neurotrophic. Theoretically, chronic suppression of a neurotrophic cytokine may result in deleterious downstream effects on the retinal nerve fiber layer (RNFL). Furthermore, there are several reports on both transient and sustained elevation of intraocular pressure (IOP) following IVB.[10,11,12,13,14,15,16] Therefore, repeated IVB injections, through both inhibition of the neurotrophic properties of VEGF and IOP fluctuations, may alter RNFL thickness. Herein, we report changes in RNFL thickness in the peripapillary region in the eyes with neovascular ARMD following two IVB injections 6 weeks apart.
METHODS
This prospective interventional case series was performed from August 2008 to February 2011 and included patients with neovascular ARMD who received two IVB injections within a 6 weeks interval. Subjects with other major retinal disorders including diabetic retinopathy, retinal occlusive disease, inflammatory retinal disease, toxic retinopathies and neuropathies, glaucoma and media haziness were excluded. Patient characteristics such as age, gender, laterality, history of diabetes mellitus, hypertension, cardiac disease, smoking, fellow eye condition, as well as visual acuity and IOP were recorded.
In addition to ancillary tests commonly performed to evaluate eyes with neovascular ARMD, such as optical coherence tomography (OCT), fluorescein angiography and indocyanine green angiography, all eyes underwent measurement of peripapillary RNFL thickness which was performed on a circle 3.4 mm in diameter centered around the optic disc in the superior, temporal, inferior and nasal quadrants at baseline (prior to IVB injections) and repeated 12 and 24 weeks after the first injection. All measurements were performed using a spectral domain OCT machine (3D OCT-1000, Topcon Corporation, Tokyo, Japan). The thickness of each quadrant and global mean values were used for analysis.
All eyes received two 1.25 mg (0.05 cc) IVB injections (Avastin, Genentech, Inc., San Francisco, CA, USA) within a 6 weeks interval. The injections were performed under sterile conditions in the operating room by one ophthalmologist. The patients were visited on day 1, and 1 and 6 weeks after each injection, and 18 weeks after the second injection.
Based on a pilot study, we observed a standard deviation (SD) of 7 microns for the change in RNFL thickness at week 24, thus, we included 18 patients to be able to detect a 5 microns change in RNFL with study power of 85% when type I error was assumed to be 0.05. To describe data, we used mean ± standard deviation (SD), median (range) and frequency (percent) values. To evaluate changes within the same eyes, we used Wilcoxon signed-ranked test. All statistical analyses were performed using SPSS software (Version 17.0, SPSS Inc., Chicago, IL, USA). Statistical level of significance was present at 0.05.
RESULTS
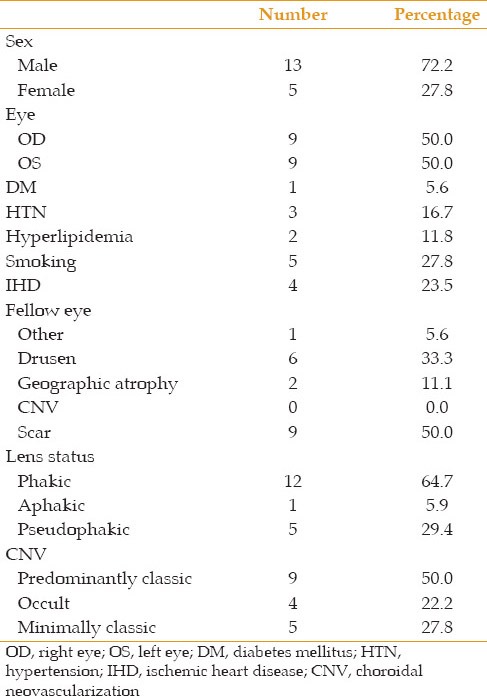
Initially 25 eyes of 25 patients with neovascular ARMD entered the study. One eye developed sterile endophthalmitis which was treated successfully with topical and systemic steroids but excluded from the study; six other cases did not attend follow-up visits and were thus excluded. Eventually, data from 18 eyes of 18 subjects including, 5 female and 13 male patients, with mean age of 76.1 ± 1.6 years were analyzed. During the study period, no subject developed ocular hypertension. Baseline patient of the patients are detailed in Table 1.
Table 1.
Baseline characteristics of patients
Mean peripapillary RNFL thickness decreased significantly from 89 ± 21 μm at baseline to 82 ± 15 μm at 12 weeks (P = 0.021). However, mean thickness increased to 87 ± 23 μm at 24 weeks which was comparable to baseline values (P = 0.356). Comparing RNFL thickness in four quadrants reveals that changes in RNFL thickness in each quadrant were not significant except in the temporal quadrant at 12 weeks in which the thickness diminished significantly by − 8 ± 16 μm (P = 0.033) [Table 2]. This quadrant followed the same pattern of change as mean RNFL thickness, becoming comparable to pre-injection values at 24 weeks (P = 0. 298).
Table 2.
Peripapillary RNFL thickness in microns at baseline, and 12 and 24 weeks after the first IVB injection in 4 quadrants
DISCUSSION
In addition to its angiogenic role, VEGF-A has a neuroprotective function. Zachary described both neurotrophic and neuroprotective effects for VEGF-A in glial cells.[17] Another study has implicated that decreased levels of VEGF may cause neuronal degeneration possibly due to decreased perfusion as well as reduced neuroprotective properties. Therefore, the chronic use of anti-VEGF agents could potentially lead to RNFL thinning by inhibiting VEGF-A.[18,19] The present study showed a temporary decline in peripapillary RNFL thickness at 12 weeks in eyes with neovascular ARMD following two IVB injections but thickness was restored to pre-intervention levels at 24 weeks.
In a retrospective observational case series, Horsley et al demonstrated that repeated intravitreal injections (more than 10 episodes) of anti-VEGF drugs, including pegaptanip, bevacizumab and ranibizumab, did not have any significant effect on RNFL thickness in patients with neovascular ARMD. However, they compared the initial thickness with one measured at final follow-up which was not <12 months, therefore they were unable to detect early transient changes.[8]
It has been shown that IOP elevation is associated with intraocular injections of anti-VEGF agents. This IOP elevation falls below 30 mmHg by 30 min and returns to baseline values by 30–60 min in most patients. These episodic IOP fluctuations can be a potential risk factor for the progression of glaucomatous damage to the optic nerve head. Therefore, repeat injections of bevacizumab at 4–6 weeks intervals, for instance, could potentially damage the RNFL.[13,14,15] The present study, however, did not support this theory since no permanent effect of bevacizumab injections was observed on RNFL thickness. Nonetheless, it should be noted that 2 injections may be insufficient to cause lasting damage.
There have been some reports on sustained IOP elevations weeks and months after initial or frequent intravitreal injections of anti-VEGF agents. It has been postulated that sustained IOP elevation could be related to trabecular meshwork obstruction due to anti-VEGF agents or impurities within the injection fluid or the result of an inflammatory response.[9,10,11,12,13,14,15,16] None of the patients in our study had episodes of sustained increase in IOP.
Any intravitreal injection, through transient increase of IOP may be harmful to the optic nerve. However, this might not be the case in the present study, since our cases received only two injections and the possible short period of ocular hypertension following the injections probably was not severe enough to cause RNFL damage. In a case report, Chen et al noticed acute loss of visual acuity and an enlargement of the foveal avascular zone following IVB injection for treatment of diabetic macular edema. They postulated that bevacizumab may have disturbed an already tenuous state of vascular perfusion leading to worsening of macular ischemia and the resultant loss of visual acuity.[1] In our study, no acute visual acuity loss following IVB injections was observed.
In the current study, changes in mean RNFL thickness in the four quadrants was not significant except in the temporal quadrant at week 12 in which RNFL thickness was diminished significantly but restored to normal levels over 24 weeks. This change may be due to decreased retinal edema in response to bevacizumab at 12 weeks.
The present study was limited by small sample size and the few numbers of IVB injections; however, it demonstrated a temporary decline in RNFL thickness even after two consecutive IVB injections. This finding may raise caution in the era of growing interest for intravitreal drug injections. Whatever the exact cause of this transient damage is, anti-VEGF drug or the injection per se, it would be noteworthy for ophthalmologists to be cautious about administering multiple intravitreal injections. Furthermore, use of higher bevacizumab doses as well as sustained-release formulations which might affect the RNFL more drastically and in ways not observed with current treatment regimens, should be undertaken with care. Further studies with larger sample size looking at more frequent injections and comparing various drugs are warranted. They should address whether certain patient groups, such as those with open angle glaucoma, may be more susceptible to such damage.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Chen E, Hsu J, Park CH. Acute visual acuity loss following intravitreal bevacizumab for diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2009;40:68–70. doi: 10.3928/15428877-20090101-04. [DOI] [PubMed] [Google Scholar]
- 2.Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695–1705. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 3.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372.e5. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: A short-term study. Retina. 2006;26:279–284. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Mason JO, 3rd, Albert MA, Jr, Vail R. Intravitreal bevacizumab (Avastin) for refractory pseudophakic cystoid macular edema. Retina. 2006;26:356–357. doi: 10.1097/00006982-200603000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 7.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M, et al. Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: Results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–750. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Horsley MB, Mandava N, Maycotte MA, Kahook MY. Retinal nerve fiber layer thickness in patients receiving chronic anti-vascular endothelial growth factor therapy. Am J Ophthalmol. 2010;150:558–561.e1. doi: 10.1016/j.ajo.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Sabet-Peyman EJ, Heussen FM, Thorne JE, Casparis H, Patel SJ, Do DV. Progression of macular ischemia following intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2009;40:316–318. doi: 10.3928/15428877-20090430-17. [DOI] [PubMed] [Google Scholar]
- 10.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011;95:1111–1114. doi: 10.1136/bjo.2010.180729. [DOI] [PubMed] [Google Scholar]
- 12.Bakri SJ, Pulido JS, McCannel CA, Hodge DO, Diehl N, Hillemeier J. Immediate intraocular pressure changes following intravitreal injections of triamcinolone, pegaptanib, and bevacizumab. Eye (Lond) 2009;23:181–185. doi: 10.1038/sj.eye.6702938. [DOI] [PubMed] [Google Scholar]
- 13.Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin) Retina. 2007;27:1044–1047. doi: 10.1097/IAE.0b013e3180592ba6. [DOI] [PubMed] [Google Scholar]
- 14.Frenkel RE, Mani L, Toler AR, Frenkel MP. Intraocular pressure effects of pegaptanib (Macugen) injections in patients with and without glaucoma. Am J Ophthalmol. 2007;143:1034–1035. doi: 10.1016/j.ajo.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 15.Kim JE, Mantravadi AV, Hur EY, Covert DJ. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular. Graefes Arch Clin Exp Ophthalmol. 2008;246:955–958. doi: 10.1016/j.ajo.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Bakri SJ, McCannel CA, Edwards AO, Moshfeghi DM. Persisent ocular hypertension following intravitreal ranibizumab. Endothelial growth factor agents. Am J Ophthalmol. 2008;146:930–934. doi: 10.1007/s00417-008-0819-2. [DOI] [PubMed] [Google Scholar]
- 17.Zachary I. Neuroprotective role of vascular endothelial growth factor: Signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- 18.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: Once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]


