Abstract
Purpose:
To compare the outcomes of photodynamic therapy (PDT) combined with intravitreal bevacizumab (IVB) with versus without intravitreal triamcinolone (IVT) in neovascular age-related macular degeneration (AMD).
Methods:
Eighty-four eyes with active CNV secondary to AMD with no prior treatment were enrolled and followed for 1-year. Eligible eyes were randomly assigned to either PDT/IVB or PDT/IVB/IVT. The main outcome measure was change in best-corrected visual acuity (BCVA).
Results:
Mean patient age was 71 ± 9 years. BCVA changes from baseline were statistically significant in both study arms at all follow-up intervals, however no significant difference was observed between the two groups regarding BCVA changes at week 12 (95% CI:-0.11–0.12 LogMAR) and other time points (all P > 0.6). Mixed model analysis revealed a significant effect from age (P < 0.001), pigment epithelial detachment (P = 0.009) and baseline BCVA (P < 0.001) on visual improvement. Significant central macular thickness (CMT) reduction occurred at all-time points as compared to baseline in both groups which was comparable between the study arms. There was no significant difference between the study arms in terms of retreatment rate (P = 0.1) and survival to the first repeat IVB injection (P = 0.065).
Conclusion:
Additional low-dose IVT to a PDT/IVB regimen for neovascular AMD provided no beneficial effects in terms BCVA or CMT, yet demonstrated a trend toward extending the injection-free period.
Keywords: Age-Related Macular Degeneration; Bevacizumab; Choroidal Neovascular Membrane; Combination (Combined) Therapy, Photodynamic Therapy; Triamcinolone
INTRODUCTION
Photodynamic therapy (PDT) supplanted thermal laser modalities in the management of neovascular age-related macular degeneration (AMD) during the 1990s.[1,2,3] PDT however, could only stabilize vision instead of improving it;[1,2,3] additionally, it has been associated with adverse effects such as transient hypoperfusion of the choriocapillaris and upregulation of vascular endothelial growth factor (VEGF).[4,5,6,7,8] Attempts were made to raise the efficacy of PDT for treatment of AMD by combining it with intravitreal injection of triamcinolone acetonide (TA).[9,10,11] Subsequently, with the introduction of anti-VEGF drugs, and on the grounds that PDT and anti-VEGFs exert their effects on choroidal neovascular membrane through different mechanisms, it was suggested that they could have additive effects.[12,13,14,15,16,17,18] The fact that anti-VEGF therapies could inhibit choroidal neovascularization (CNV) but were unable to destroy existing CNV, underpinned the rationale behind combining anti-VEGF drugs and PDT. On the other hand, anti-VEGF treatment might delay CNV recurrence by extending the period of decreased blood flow as well as inhibiting PDT induced upregulation of VEGF.[12] A triple therapy regimen including PDT, an intravitreal anti-VEGF drug and a corticosteroid was further recommended by some authors;[19,20,21,22] adding TA or dexamethasone to this regimen was attributed to their anti-inflammatory, anti-fibrotic and anti-permeability properties.[8,9,10,11]
This clinical trial aimed to compare the therapeutic effects of a dual therapy regimen combining single-session PDT and intravitreal bevacizumab injection with a triple therapy regimen comprising of single-session PDT with intravitreal injection of both bevacizumab and triamcinolone acetonide. Herein, we report the 1-year results of this study.
METHODS
This randomized clinical trial adheres to the tenets of the declaration of Helsinki and was approved by the Ethics Committees of the Ophthalmic Research Center at Shahid Beheshti University of Medical Sciences. Patients were examined at two centers, Labbafinejad Medical Center and Farabi Eye Hospital, Tehran. Written informed consent was obtained from all participants prior to enrollment. The study was registered at http://www.clinicaltrials.gov as NCT00370539.
Participants
Patients with subfoveal CNV of all types (predominantly classic, minimally classic, occult and retinal angiomatous proliferation) secondary to AMD and no history of prior treatment were recruited. CNV lesions were also classified into three subgroups according to lesion size; smaller than 2 disc areas, 2–4 disc areas, and larger than 4 disc areas. However, lesions did not exceed 8 disc areas (including blood, scar or atrophy and neovascularization), of which at least 50% had to be active CNV. Exclusion criteria comprised presence of diabetic retinopathy, glaucoma, or any macular disease other than AMD.
Interventions
All patients underwent baseline evaluation including best-corrected visual acuity (BCVA) measurement using Snellen chart, slit lamp examination, intraocular pressure (IOP) measurement using Goldmann applanation tonometry, fundus examination with a noncontact 78-diopter lens, optical coherence tomography (Cirrus OCT; Carl Zeiss Meditec, Dublin, CA, USA) and fluorescein angiography (FA-HRAII, Heidelberg Engineering, Heidelberg, Germany). Lens opacities were graded from 0 to 4 + using the Lens Opacities Classification System III (LOCS III) for each of the three different categories including nuclear sclerosis, posterior subcapsular opacities, and cortical cataracts.[23]
Eligible eyes were randomly assigned to receive vertoporfin PDT plus intravitreal bevacizumab (IVB) or a combination of PDT and bevacizumab/triamcinolone (IVB/IVT). Subjects were initially allocated in a 1:1 ratio. Patients in the dual treatment group underwent standard PDT followed by intravitreal injection of 1.25 mg/0.05 ml bevacizumab (Avastin, made for F. Hoffmann-La Roche Ltd. Basel, Switzerland by Genentech Inc., San Francisco, USA) after 48 h. In the triple treatment group, 2 mg triamcinolone acetonide (Triamhexal, Hexal AG, Holzkirchen, Germany) was injected intravitreally in addition to the above. IVB injections were performed using a 30-gauge needle inserted through the superior temporal pars plana 3.75 mm and 3.25 mm from the limbus in phakic and pseudophakic eyes respectively. Triamcinolone acetonide (TA) was injected employing a separate syringe in the inferior temporal quadrant. Topical chloramphenicol eye drops was administered 6-hourly for 3 days following each injection. All patients were examined on the 1st day after injection particularly for signs of intraocular inflammation. IOP was re-evaluated at weeks 1 and 3; topical antiglaucoma medication was initiated in participants with IOP values of 21 mmHg or higher. Ophthalmic examinations and OCT were repeated at week 12 and then at 6-week intervals. Furthermore, FA was repeated upon the surgeon's discretion. Need for retreatment with IVB injection was first evaluated at week 12. Additional IVB injections were given in eyes with active CNV according to clinical findings (including decrease in VA and/or hemorrhage on fundus examinations), and/or fluid on OCT, and/or persistence or recurrence of dye leakage on FA. Neither PDT nor IVT injection were not repeated during the follow-up period.
Outcome Measures
The primary outcome measure was change in BCVA from baseline. Secondary outcome measures included changes in central macular thickness (CMT) during the study period, the need for additional injections, and time interval up to the first retreatment.
Sample Size
To reveal a presumed difference of 0.2 logarithm of the minimum angle of resolution (logMAR) change in BCVA between the two study groups, with study power of 80% and significance level of 0.05, when standard deviation of BCVA changes in each group was assumed to be 0.3 logMAR, a sample size of 72 eyes (36 eyes in each group) was required. To compensate for possible loss to follow-up, 84 eyes (42 in each group) were recruited.
Randomization
A computer generated random block of 4 was utilized to randomize participants between the two study groups. Random allocation sequence was performed by a biostatistician.
Masking
Visual acuity assessment and OCT were performed by an optometrist who was masked to the groups. In addition, the statistician who performed the analysis was also masked to details of the series.
Statistical Analysis
Data were evaluated by both intention to treat (ITT) and on-treatment (per-protocol) analyses. ITT analysis was performed employing the last observation carried forward (LOCF) method and the mixed model. Percentages, mean values ± standard deviations (SD), median, interquartile range and 95% confidence intervals (CI) were used to describe data. Chi-square, Fischer's exact and Mann–Whitney tests were adopted to compare qualitative data. Baseline-adjusted CMT and BCVA were compared between the two groups by employing analysis of covariance (ANCOVA). Mixed model allowed us to evaluate changes within each study group at different time points compared to baseline. Adjustment for multiple comparisons was performed by Hochberg method.[24]
The number (s) of required additional IVB injection (s) was evaluated by Mann–Whitney test. The survival time prior to the first retreatment was drawn by Kaplan–Meier curve and compared between the two groups by log rank test. At the final step, mixed model was used to evaluate and adjust the effects of the two treatment designs, baseline values, age, CNV type, pigment epithelial detachment (PED) and CNV size on BCVA and CMT throughout the study period. P <0.05 were considered as statistically significant. The statistical analysis was performed using SPSS software (version 17.0, SPSS Inc., Chicago, USA).
RESULTS
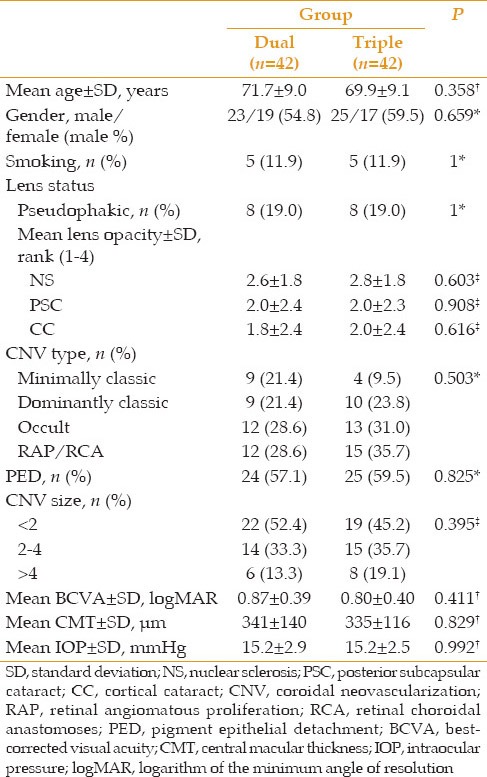
Eighty-four eyes of 84 patients with mean age of 71.7 ± 9.0 years (median: 71.5, range: 45–85) were initially enrolled. Forty-two eyes were assigned to each of the dual and triple treatment groups. Sixty-three patients including 33 (52.4%) male and 30 (47.6%) female subjects with the mean age of 70.5 ± 8.2 years completed the 6-month follow-up course. The corresponding figure was 51 patients at month 12 (54 weeks); 24 and 27 patients in the dual and triple treatment groups respectively. Participation of the subjects has been displayed in Figure 1. There was no statistically significant difference between the two groups in terms of baseline characteristics [Table 1].
Figure 1.
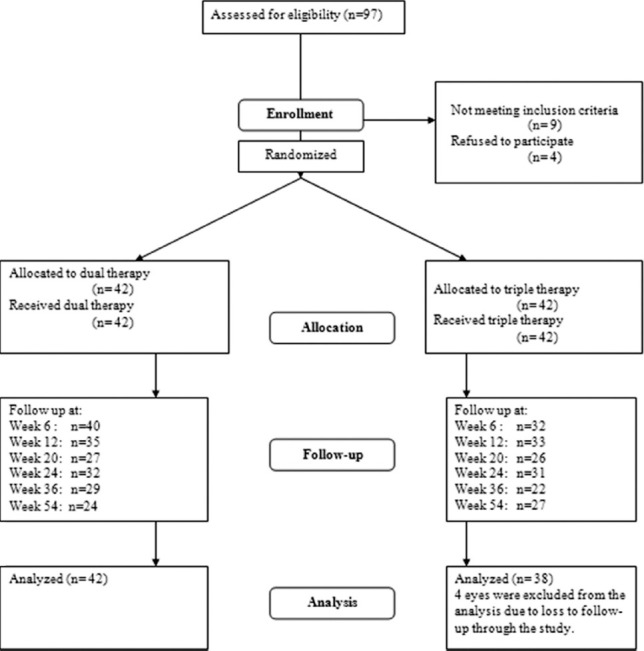
Study flow chart.
Table 1.
Patients’ baseline characteristics
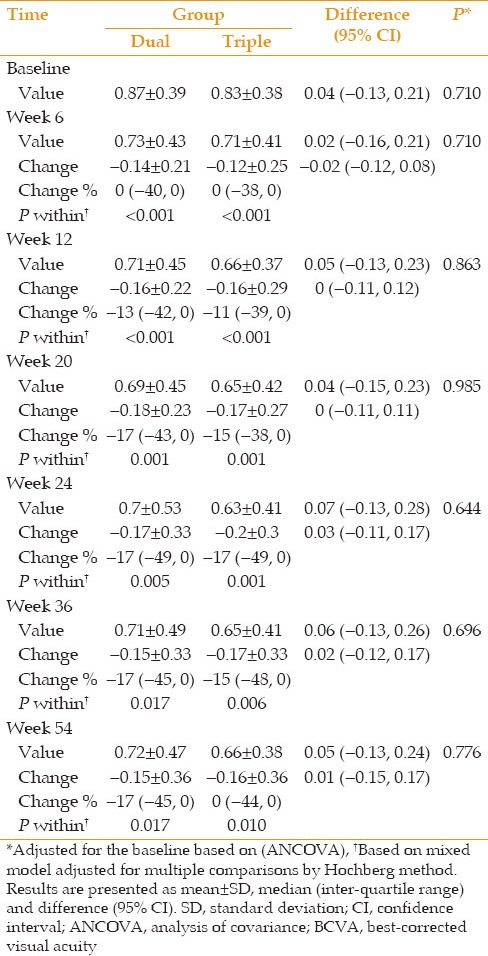
BCVA and CMT data from each study group at baseline and weeks 6, 12, 20, 24, 36 and 54 after intervention are demonstrated in Figures 2 and 3. In addition, BCVA values, changes in BCVA from baseline and corresponding percentages at different time points are presented in Table 2. Mean changes in BCVA from baseline were statistically significant in both groups at all follow-up sessions. Based on ITT analysis, there was no difference between the two groups regarding the values and changes adjusted for baseline values at different time points (minimum of P > 0.6) [Table 2].
Figure 2.
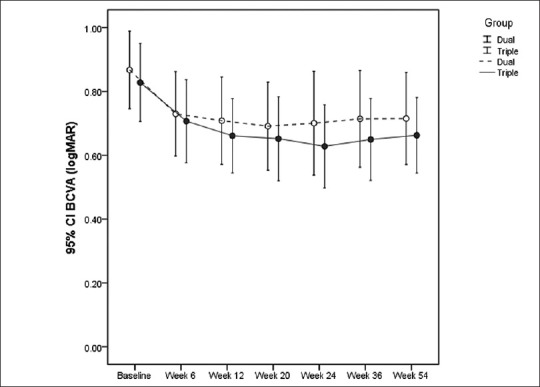
Best-corrected visual acuity for each treatment group before and at weeks 6, 12, 20, 24, 36 and 54 (mean±95% confidence interval).
Figure 3.
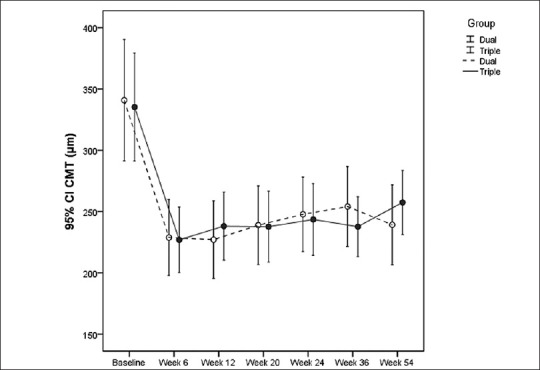
Central macular thickness for each treatment group before and at weeks 6, 12, 20, 24, 36 and 54 (mean±95% confidence interval).
Table 2.
BCVA values and changes compared to baseline and percentage of these changes at different time points according to treatment group (dual vs. triple)
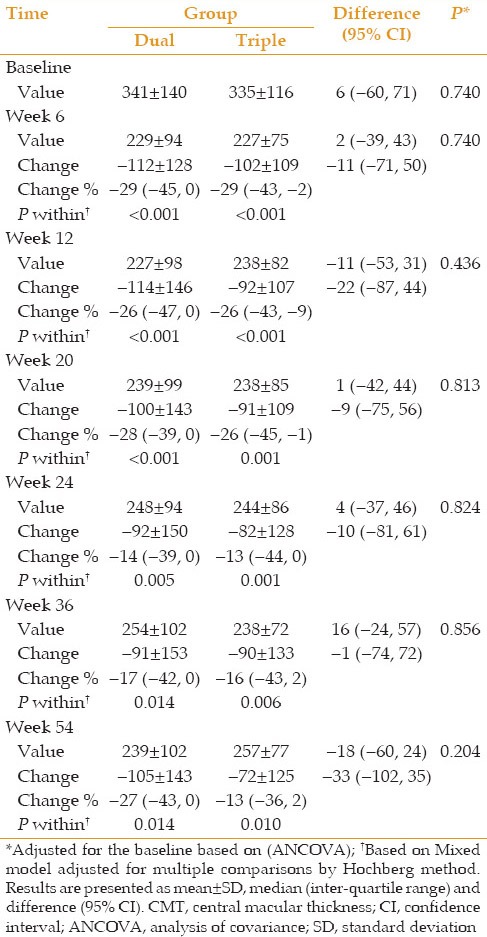
There was a statistically significant reduction in CMT at all time points compared to baseline values in both groups. These reductions were comparable between the two groups at all follow-up sessions [Table 3].
Table 3.
CMT values and changes compared to baseline and percentage of changes at different time points according to treatment groups (dual vs. triple)
The above-mentioned evaluations were repeated by per-protocol analysis; the results of between and within groups analyses regarding BCVA did not reveal any specific difference. Similarly, mean BCVA changes from baseline in both groups were significant at all time points but the differences between the two groups were not significant at any follow-up session. No difference appeared considering the per-protocol analysis results for CMT except for weeks 12 and 54 at which baseline adjusted values of CMT were statistically different in the two groups with more reduction in the IVB group (P = 0.018 and P = 0.010, respectively).
Mixed model analysis was used to investigate the concurrent effects of the two treatment designs, baseline values, age, CNV type, PED and CNV size on BCVA and CMT, and to obtain variations in the trend of alterations of these two outcome measures. Mixed model also implied that there were no meaningful differences between the two groups regarding BCVA values (P = 0.86) and the trend of its alterations during the study period (P = 0.58). The effects of CNV type (P = 0.241) and size (P = 0.229) were not statistically significant, however significant effects were observed from age (P < 0.001), PED (P = 0.009) and baseline BCVA (P < 0.001): an inverse relationship was observed between visual improvement and increasing age, the absence of PED had a positive effect on BCVA, and there was a direct correlation between baseline BCVA and visual improvement.
Mixed model analysis was likewise employed to evaluate the effects of the aforementioned parameters on CMT; the differences between the two groups were not statistically significant in terms of CMT values and the trend of CMT alterations (P = 0.712 and P = 0.415, respectively). PED did not reveal a statistically significant effect on CMT (P = 0.457); nevertheless the effects of age (P < 0.001), CNV type (P < 0.001), CNV size (P = 0.009) and baseline CMT (P < 0.001) were statistically significant in this regard. Among the different CNV types, retinal angiomatous proliferation (RAP) was associated with increased CMT values; while other choroidal neovascularization types did not significantly affect CMT values. There was a direct relationship between CMT changes and pre-treatment CMT values; similarly, there was a correlation between visual improvement and CMT reduction.
Patterns of retreatment were not similar in the two study groups within the first 12 months. In the IVB group, 11 (26.2%) eyes were needless of retreatment within 12 months whereas 14 (33.3%), 13 (31%), 2 (4.8%) and 2 (4.8%) eyes needed one, two, three and four extra injections, respectively. Meanwhile 16 (38.1%) eyes from the IVB/IVT group did not require retreatment up to week 54 while one, two and three extra injections were required in 17 (40.5%), 5 (11.9%) and 4 (9.5%) eyes, correspondingly; a fourth injection was totally nonexistent among the latter group. The average number of retreatments was 1.3 ± 1.1 injections versus 0.9 ± 0.9 injections in the IVB and IVB/IVT groups respectively; nevertheless this difference was not statistically significant (P = 0.1, Mann–Whitney test).
The median time to the first retreatment was 15.6 (95% CI: 14.7–16.4) weeks in the IVB group and 25.1 (95% CI: 17.1–33.2) weeks in the IVB/IVT group; the survival time to the first required additional injection was not different between the two groups [P = 0.065, log rank test, Figure 4].
Figure 4.
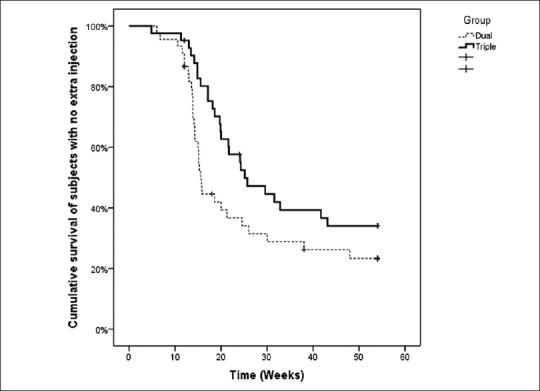
Cumulative survival of subjects with no extra injections.
Changes in IOP at weeks 12 and 24 were also evaluated; there were no statistically significant changes in IOP values in the two study groups (mean corresponding value for IOP changes were less than 0.1 and P values were above 0.8). In addition, the difference between the two groups was not statistically significant at any time point (P > 0.7). There was no significant change in the scores of lens opacity after 1-year. No case of RPE tear and endophthalmitis was seen and no systemic adverse event was observed.
Post-hoc Power Analysis
Considering the primary outcome measure and with regard to the observed standard deviation and sample size in each group, our study had an 88% power to detect 0.2 logMAR difference between the two study groups at week 12. The corresponding figures were 81%, 69%, 55% and 50% at weeks 20, 24, 36 and 54, respectively.
DISCUSSION
We previously evaluated the effects of dual and triple therapies for neovascular AMD in two separate interventional case series; both studies established significant visual improvement and CMT reduction.[14,19] The present randomized clinical trial was implemented to compare the outcomes of dual and triple combination therapies and showed that both approaches yield comparable results in terms of visual improvement and CMT reduction. Triple therapy demonstrated a trend toward obviating the need for retreatment and lengthening the injection-free period; the difference between the two groups however did not reach a statistically significant level. In a study by Tatar et al., IVT injection contributed to enhanced levels of endostatin as an endogenous angiogenesis inhibitor but could not suppress VEGF. On the other hand, bevacizumab was found to be a more potent angiogenesis inhibitor reducing VEGF expression besides enhancing endostatin.[25] These findings may explain why IVT added to the combination of PDT and IVB could not demonstrate significant additional benefit in our study.
PDT leads to selective closure of vessels within a CNV by non-thermal photothrombosis.[26] In addition, PDT results in choriocapillaris hypoperfusion in the irradiated area which recovers within 3 months.[5,7,26,27,28,29,30] These effects may play important roles in the closure of CNV and reducing retreatment rate.[30] Yet, they may result in atrophy of the RPE and neurosensory retina and therefore induce subsequent central scotomata.[31,32,33] In the current study, a single session PDT administration strategy was adopted to minimize possible adverse effects associated with repeated PDT sessions.[5]
Combination therapy with anti-VEGF drugs may reduce the risk of CNV recurrence by combating PDT-induced upregulation of VEGF. Furthermore, recent studies have shown that combined use of bevacizumab may prolong the duration of choroidal hypofluorescence following PDT administration.[34] This may carry a paradoxical consequence; it may lessen the need for retreatment despite increasing the risk of atrophic changes. The reduction of the blood flow at CNV may also delay its recurrence.[34] Similar results have been reported in combination therapy with PDT and intravitreal/subtenon triamcinolone.[33,34,35,36]
In the study by Hatta et al.,[34] the rate of retreatment was significantly lower when choroidal hypofluorescence was induced by a combination of PDT and bevacizumab compared with PDT plus subtenon TA. In the present study, adding IVT to the study regimen did not worsen the functional results nor significantly reduce retreatment rate; all of which suggest that it did not accentuate the possible hypofluorescence induced by the combination of IVB and PDT.
In a recent prospective open-label interventional trial, Sivaprassad et al.[37] assessed the safety and efficacy of a quadruple combination treatment for management of neovascular AMD, which included reduced fluence PDT, intravitreal ranibizumab, intravitreal dexamethasone and oral minocycline. Intravitreal injection of ranibizumab was exclusively performed as a retreatment and was administered in cases of CNV recurrence. After 12 months, vision stabilization was achieved in most cases rather than visual improvement. They applied both single-session and half fluence PDT to reduce the risks of choroidal hypoperfusion, inflammation, vascular leakage and VEGF upregulation. Dexamethasone, being more rapidly cleared from circulation than triamcinolone, was administered to lower the risk of raised IOP and cataract formation. The TA dose employed in our study did not result in significant adverse events either. Intravitreal injection of steroid was performed for only a single session in both studies. Sivaprassad et al. attributed the absence of an initial steep gradient of visual gain in their series to the use of only one intravitreal ranibizumab injection at baseline instead of repeated injections during the induction phase. The results of our study however did not support this assumption; significant functional and structural improvement was observed in both groups of our series despite a single intravitreal injection of bevacizumab at baseline. The major difference between these two studies was the method used for photodynamic therapy, standard versus reduced fluence. The mean number of ranibizumab injections was 3.4 (range: 2.6) within 1-year in Sivaprasad's study while the corresponding figures were 1.3 ± 1.1 and 0.9 ± 0.9 injections in the IVB and IVB/IVT groups respectively in our study.
In a retrospective analysis of triple combination therapy with IVB, posterior sub-Tenon's TA injection and low-fluence PDT in patients with neovascular AMD, significant 3- and 6-month visual improvement and CMT reduction was observed by Kovacs et al.[22] At the 3-month time point, 16.7% of eyes required retreatment and the corresponding figures were 40.9% by 6 months and 61.1% by 12 months.
The results of the above-mentioned study were comparable to our findings although they applied half-fluence PDT as well. The discrepancy between the outcomes of these two studies, despite similarities of the employed methods, may originate from varieties in the presenting lesions.
Yip et al.[20] followed a triple combination therapy containing single session standard PDT, 1.25 mg IVB, and 4 mg IVT; they repeated IVB injection at month 3 for residual leakage. From a total of 36 eyes, 28 (77.8%) achieved CNV resolution by a single course of triple therapy. At 6 months, 61.1% of eyes had stable or improving vision. The rate of IVT induced side-effects was relatively high; 3 eyes developed significant cataracts requiring surgery and 2 demonstrated persistent raised IOP at 6 months. In discussing the cause of retreatment frequencies in Yip's study[20] compared to our previous report,[19] Kovacs[22] pointed to the possible effect of higher IVT dose in the former study. The same high dose may conversely explain the higher complication rates in the Yip's study[20] compared with our triple treatment protocol.
Mataix et al.[38] recently performed a prospective interventional study on combined ranibizumab and PDT for treatment of AMD and considered it as an option for improving treatment efficiency. They used a single initial dose of PDT similar to our method combined with intravitreal ranibizumab and employed the same criteria as ours for retreatment. However, their retreatment method consisted of monthly intravitreal injections of ranibizumab and performing PDT every 3 months. There was a significant visual improvement after 12 months with mean gain of 7.2 letters, the mean number of PDT sessions per patient was 1.22 and that for ranibizumab injections per patient was 2.37 at 12 months. Nevertheless, we did not repeat PDT to minimize potential side-effects of this treatment modality.
Spielberg and Leys[39] performed a prospective nonrandomized, open-label study on 27 eyes with neovascular AMD. They used a single-time reduced-fluence PDT combined with intravitreal injection of ranibizumab on the same day. Second and third intravitreal injections were also given as a mandatory regimen. Additional treatment with ranibizumab was performed as needed. There was a significant visual improvement with an average of 5.1 injections during the 1st year and 7.1 injections over 24 months. This study lacked a control group but they applied a method similar to one of our study arms and their 2-year results were comparable with our 1-year findings. In addition, they used a single-session PDT technique with reduced fluence. The other difference between these two studies was the number of mandatory injections of an anti-VEGF drug which was 3 in Spielberg's study against 1 in our trial.
In a multivariate analysis using mixed model, the simultaneous effects of various factors on BCVA and CMT were studied.[40] The possible effects of these parameters on the alteration trends of study outcome measures were also dynamically evaluated; increasing age had a negative effect on BCVA as expected.[40] The possible deleterious effects of PED on the outcomes of pharmacological treatments in AMD patients have also been reported.[15,41] While there was a correlation between baseline and post-treatment BCVA in our study, the results of DENALI study showed that lower baseline BCVA was associated with higher BCVA gains.[42] Regarding CMT changes, there was a correlation between structural and functional outcomes in our study which is consistent with previous reports.[22] RAP showed a deleterious effect on CMT which is in accordance with previous studies.[43] The mixed model in this RCT correlated with subgroup analyses in the DENALI study.[42]
Ophthalmic complications including sustained ocular hypertension, endophthalmitis, significant progression of lens opacity necessitating cataract surgery, RPE rupture, and subretinal hemorrhage were not observed in our series. No thromboembolic event was observed either.
When the primary outcome measure was taken into account, the post-hoc power of this RCT was above 80% before month 5. However, this figure dropped gradually up to month 12. This limitation of our clinical trial could be attributed to loss to follow-up.
In conclusion, in patients with neovascular AMD, adding a low dose intravitreal triamcinolone to the combined regimen of single session PDT and intravitreal bevacizumab does not reveal beneficial effects on BCVA and CMT. However, a trend towards extending the injection-free period may be achieved using triple therapy.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials--TAP report. Arch Ophthalmol. 1999;117:1329–1345. [PubMed] [Google Scholar]
- 2.Bressler NM Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- 3.Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: Two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – Verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131:541–560. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 4.Peyman GA, Kazi AA, Unal M, Khoobehi B, Yoneya S, Mori K, et al. Problems with and pitfalls of photodynamic therapy. Ophthalmology. 2000;107:29–35. doi: 10.1016/s0161-6420(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt-Erfurth U, Michels S, Barbazetto I, Laqua H. Photodynamic effects on choroidal neovascularization and physiological choroid. Invest Ophthalmol Vis Sci. 2002;43:830–841. [PubMed] [Google Scholar]
- 6.Schmidt-Erfurth U, Schlötzer-Schrehard U, Cursiefen C, Michels S, Beckendorf A, Naumann GO. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003;44:4473–4480. doi: 10.1167/iovs.02-1115. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Erfurth UM, Michels S. Changes in confocal indocyanine green angiography through two years after photodynamic therapy with verteporfin. Ophthalmology. 2003;110:1306–1314. doi: 10.1016/S0161-6420(03)00452-4. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchihashi T, Mori K, Peyman G, Shimada Y, Yoneya S. Photodynamic effects on retinal oxygen saturation, blood flow, and electrophysiological function in patients with neovascular age-related macular degeneration. Retina. 2009;29:1450–1456. doi: 10.1097/IAE.0b013e3181ac2403. [DOI] [PubMed] [Google Scholar]
- 9.Spaide RF, Sorenson J, Maranan L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2005;112:301–304. doi: 10.1016/j.ophtha.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Augustin AJ, Schmidt-Erfurth U. Verteporfin and intravitreal triamcinolone acetonide combination therapy for occult choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;141:638–645. doi: 10.1016/j.ajo.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Moreno JM, Montero JA, Barile S. Triamcinolone and PDT to treat exudative age-related macular degeneration and submacular hemorrhage. Eur J Ophthalmol. 2006;16:426–434. doi: 10.1177/112067210601600311. [DOI] [PubMed] [Google Scholar]
- 12.Kiss CG, Simader C, Michels S, Schmidt-Erfurth U. Combination of verteporfin photodynamic therapy and ranibizumab: Effects on retinal anatomy, choroidal perfusion and visual function in the protect study. Br J Ophthalmol. 2008;92:1620–1627. doi: 10.1136/bjo.2007.135335. [DOI] [PubMed] [Google Scholar]
- 13.Antoszyk AN, Tuomi L, Chung CY, Singh A FOCUS Study Group. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): Year 2 results. Am J Ophthalmol. 2008;145:862–874. doi: 10.1016/j.ajo.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadieh H, Taei R, Soheilian M, Riazi-Esfahani M, Ahadi H. Single-session photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Eur J Ophthalmol. 2008;18:297–300. doi: 10.1177/112067210801800222. [DOI] [PubMed] [Google Scholar]
- 15.Lazic R, Gabric N. Verteporfin therapy and intravitreal bevacizumab combined and alone in choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2007;114:1179–1185. doi: 10.1016/j.ophtha.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser PK, Boyer DS, Garcia R, Hao Y, Hughes MS, et al. Registry of Visudyne AMD Therapy Writing Committee. Verteporfin photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2009;116:747–755. doi: 10.1016/j.ophtha.2008.12.057. 755.e1. [DOI] [PubMed] [Google Scholar]
- 17.Lim JY, Lee SY, Kim JG, Lee JY, Chung H, Yoon YH. Intravitreal bevacizumab alone versus in combination with photodynamic therapy for the treatment of neovascular maculopathy in patients aged 50 years or older: 1-year results of a prospective clinical study. Acta Ophthalmol. 2012;90:61–67. doi: 10.1111/j.1755-3768.2009.01841.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahn JK, Moon HJ. Changes in aqueous vascular endothelial growth factor and pigment epithelium-derived factor after ranibizumab alone or combined with verteporfin for exudative age-related macular degeneration. Am J Ophthalmol. 2009;148:718–724.e1. doi: 10.1016/j.ajo.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadieh H, Taei R, Soheilian M, Riazi-Esfahani M, Karkhaneh R, Lashay A, et al. Single-session photodynamic therapy combined with intravitreal bevacizumab and triamcinolone for neovascular age-related macular degeneration. BMC Ophthalmol. 2007;7:10. doi: 10.1186/1471-2415-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yip PP, Woo CF, Tang HH, Ho CK. Triple therapy for neovascular age-related macular degeneration using single-session photodynamic therapy combined with intravitreal bevacizumab and triamcinolone. Br J Ophthalmol. 2009;93:754–758. doi: 10.1136/bjo.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakri SJ, Couch SM, McCannel CA, Edwards AO. Same-day triple therapy with photodynamic therapy, intravitreal dexamethasone, and bevacizumab in wet age-related macular degeneration. Retina. 2009;29:573–578. doi: 10.1097/IAE.0b013e3181a46a8a. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs KD, Quirk MT, Kinoshita T, Gautam S, Ceron OM, Murtha TJ, et al. A retrospective analysis of triple combination therapy with intravitreal bevacizumab, posterior sub-tenon's triamcinolone acetonide, and low-fluence verteporfin photodynamic therapy in patients with neovascular age-related macular degeneration. Retina. 2011;31:446–452. doi: 10.1097/IAE.0b013e3181f6391f. [DOI] [PubMed] [Google Scholar]
- 23.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III.The longitudinal study of cataract study group. Arch Ophthalmol. 1993;111:831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 25.Tatar O, Shinoda K, Kaiserling E, Claes C, Eckardt C, Eckert T, et al. Implications of bevacizumab on vascular endothelial growth factor and endostatin in human choroidal neovascularisation. Br J Ophthalmol. 2009;93:159–165. doi: 10.1136/bjo.2008.138594. [DOI] [PubMed] [Google Scholar]
- 26.Fingar VH. Vascular effects of photodynamic therapy. J Clin Laser Med Surg. 1996;14:323–328. doi: 10.1089/clm.1996.14.323. [DOI] [PubMed] [Google Scholar]
- 27.Costa RA, Farah ME, Cardillo JA, Calucci D, Williams GA. Immediate indocyanine green angiography and optical coherence tomography evaluation after photodynamic therapy for subfoveal choroidal neovascularization. Retina. 2003;23:159–165. doi: 10.1097/00006982-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Michels S, Schmidt-Erfurth U. Sequence of early vascular events after photodynamic therapy. Invest Ophthalmol Vis Sci. 2003;44:2147–2154. doi: 10.1167/iovs.02-0604. [DOI] [PubMed] [Google Scholar]
- 29.Schlötzer-Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt-Erfurth U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol. 2002;240:748–757. doi: 10.1007/s00417-002-0517-4. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Erfurth U, Laqua H, Schlötzer-Schrehard U, Viestenz A, Naumann GO. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol. 2002;120:835–844. [PubMed] [Google Scholar]
- 31.Parodi MB, Da Pozzo S, Ravalico G. Angiographic features after photodynamic therapy for choroidal neovascularisation in age related macular degeneration and pathological myopia. Br J Ophthalmol. 2003;87:177–183. doi: 10.1136/bjo.87.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flower RW, von Kerczek C, Zhu L, Ernest A, Eggleton C, Topoleski LD. Theoretical investigation of the role of choriocapillaris blood flow in treatment of subfoveal choroidal neovascularization associated with age-related macular degeneration. Am J Ophthalmol. 2001;132:85–93. doi: 10.1016/s0002-9394(01)00872-8. [DOI] [PubMed] [Google Scholar]
- 33.Piermarocchi S, Sartore M, Lo Giudice G, Maritan V, Midena E, Segato T. Combination of photodynamic therapy and intraocular triamcinolone for exudative age-related macular degeneration and long-term chorioretinal macular atrophy. Arch Ophthalmol. 2008;126:1367–1374. doi: 10.1001/archopht.126.10.1367. [DOI] [PubMed] [Google Scholar]
- 34.Hatta Y, Ishikawa K, Nishihara H, Ozawa S, Ito Y, Terasaki H. Effect of photodynamic therapy alone or combined with posterior subtenon triamcinolone acetonide or intravitreal bevacizumab on choroidal hypofluorescence by indocyanine green angiography. Retina. 2010;30:495–502. doi: 10.1097/IAE.0b013e3181bcedbe. [DOI] [PubMed] [Google Scholar]
- 35.Iriyama A, Obata R, Inoue Y, Takahashi H, Tamaki Y, Yanagi Y. Effect of posterior juxtascleral triamcinolone acetonide on the efficacy and choriocapillaris hypoperfusion of photodynamic therapy. Graefes Arch Clin Exp Ophthalmol. 2008;246:339–344. doi: 10.1007/s00417-007-0667-5. [DOI] [PubMed] [Google Scholar]
- 36.Luttrull JK, Spink CJ. Prolongation of choroidal hypofluorescence following combined verteporfin photodynamic therapy and intravitreal triamcinolone acetonide injection. Retina. 2007;27:688–692. doi: 10.1097/IAE.0b013e318030e999. [DOI] [PubMed] [Google Scholar]
- 37.Sivaprasad S, Patra S, DaCosta J, Adewoyin T, Shona O, Pearce E, et al. A pilot study on the combination treatment of reduced-fluence photodynamic therapy, intravitreal ranibizumab, intravitreal dexamethasone and oral minocycline for neovascular age-related macular degeneration. Ophthalmologica. 2011;225:200–206. doi: 10.1159/000322363. [DOI] [PubMed] [Google Scholar]
- 38.Mataix J, Palacios E, Carmen DM, Garcia-Pous M, Navea A. Combined ranibizumab and photodynamic therapy to treat exudative age-related macular degeneration: An option for improving treatment efficiency. Retina. 2010;30:1190–1196. doi: 10.1097/IAE.0b013e3181d2f172. [DOI] [PubMed] [Google Scholar]
- 39.Spielberg L, Leys A. Treatment of neovascular age-related macular degeneration with a variable ranibizumab dosing regimen and one-time reduced-fluence photodynamic therapy: The TORPEDO trial at 2 years. Graefes Arch Clin Exp Ophthalmol. 2010;248:943–956. doi: 10.1007/s00417-009-1256-6. [DOI] [PubMed] [Google Scholar]
- 40.Ahmadieh H, Taei R, Riazi-Esfahani M, Piri N, Homayouni M, Daftarian N, et al. Intravitreal bevacizumab versus combined intravitreal bevacizumab and triamcinolone for neovascular age-related macular degeneration: Six-month results of a randomized clinical trial. Retina. 2011;31:1819–1826. doi: 10.1097/IAE.0b013e31820d58f2. [DOI] [PubMed] [Google Scholar]
- 41.Shima C, Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Tano Y. One-year results of combined photodynamic therapy and intravitreal bevacizumab injection for retinal pigment epithelial detachment secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009;247:899–906. doi: 10.1007/s00417-009-1067-9. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser PK, Boyer DS, Cruess AF, Slakter JS, Pilz S, Weisberger A, et al. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: Twelve-month results of the DENALI study. Ophthalmology. 2012;119:1001–1010. doi: 10.1016/j.ophtha.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Gupta B, Jyothi S, Sivaprasad S. Current treatment options for retinal angiomatous proliferans (RAP) Br J Ophthalmol. 2010;94:672–677. doi: 10.1136/bjo.2009.166975. [DOI] [PubMed] [Google Scholar]


