Abstract
Purpose:
To compare macular thickness in children with functional amblyopia and those without amblyopia using optical coherence tomography (OCT).
Methods:
This case-control study was conducted on 93 children aged 3–10 years including 44 cases with unilateral amblyopia and 49 subjects without amblyopia. Amblyopic eyes were considered as the case group and their fellow eyes as internal controls; eyes of non-amblyopic children served as the external control. Macular thickness of all eyes were measured by optical coherence tomography in the center (foveola), 1 mm ring (fovea), and 3 and 6 mm rings and compared.
Results:
Although macular thickness was generally not different between the study groups, there was a significant difference in central macular thickness between eyes with moderate to severe amblyopia and the external controls (P = 0.037). Foveal thickness difference exceeding 10 microns between fellow eyes was detected in a larger number of amblyopic children as compared to non-amblyopic controls (P = 0.002). Mean foveal thickness was greater in boys (P = 0.037) but there was no significant difference in foveal thickness among various types of refractive errors.
Conclusion:
Although there was no significant relationship between macular thickness and amblyopia, foveolar thickness in eyes with moderate to severe amblyopia was significantly greater than the external controls. Further studies with more cases of moderate to severe amblyopia are recommended.
Keywords: Amblyopia, Macular Thickness, Optical Coherence Tomography
INTRODUCTION
Amblyopia is a unilateral or bilateral reduction of visual acuity usually caused by abnormal visual experience early in life during development of the visual system.[1] Common risk factors for amblyopia are strabismus, anisometropia, high refractive errors and visual deprivation.[1,2,3,4,5,6,7] “Idiopathic amblyopia” is used in the absence of apparent etiology.[1]
Treatment of amblyopia includes correcting refractive errors with glasses or contact lenses, patching or penalization of the fellow eye which depends on various factors such as the severity of amblyopia, amount and type of refractive error, eye alignment status and age at initiation of treatment and the degree of compliance.
Despite appropriate treatment for amblyopia, about 50% of children cannot achieve complete vision of 20/20.[1] It is possible that there is subclinical pathology in the retina, especially in the macula of eyes not responding well to conventional amblyopia treatment.[1]
Optical coherence tomography (OCT) provides high resolution images of the retina and its layers.[1,2,3,4,5,6,7] OCT has high repeatability and is not influenced by high refractive errors, age and sex of the subjects.[1,5]
Normal thickness of the foveola (center), fovea (1 mm) and macula in Chinese children 7–14 years of age has been reported to be 130 ± 17.4, 153.8 ± 17.6 and 176.7 ± 14.8 microns (μ), respectively using OCT.[1] The superior and inferior regions of the macula were thicker than the nasal and temporal regions (157–159 μ vs. 102–109 μ).[2]
Increased macular thickness and decreased foveal depression have been reported in amblyopic patients using OCT.[1,2,3,4,5,6,7] The reason can be related to lack of ganglion cells apoptosis which normally occurs shortly after birth in healthy infants.[5]
On ophthalmoscopic examination, foveal depression and reflex are absent in these eyes and slight discoloration (Wine coloration) of the fovea is observed,[1] however, these are not consistent findings in the amblyopic fundus.
Genetic factors, particularly in myopic cases are also reported to be the cause of increased thickness in the inner nuclear layer (INL), inner plexiform layer (IPL) and ganglion cell layer.[8] There is no general agreement on the relationship between amblyopia and increased macular thickness in all papers[1,2,3,4,5,6,7] and some authors believe that the increased macular thickness in amblyopic eyes is due to magnification because of high hyperopic refractive errors (> +5 diopters [D]) or a kind of artifact.[1,7]
The purpose of the present study was to compare macular thickness between amblyopic children and age and sex matched non-amblyopic controls.
METHODS
This case-control study was performed on 93 children aged 3–10 years who were referred to the eye clinic at Imam Hossein Medical Center, Tehran, Iran. The study was approved by the Ethic Committee at the Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences and written informed consent was obtained from all parents or legal guardians.
Forty-four participants had unilateral strabismic or anisometropic amblyopia with no anatomical disorder; their amblyopic eyes were considered as the case group and their fellow non-amblyopic eyes comprised the internal control group. Both eyes of 49 age and sex matched children without amblyopia served as the external control group.
Children aged >10 years, those with history of seizures, prematurity (birth weight < 1500 g or gestational age <34 weeks), mental retardation, high ametropia (>5 D), ocular anomalies and fundus lesions, nystagmus, glaucoma, organic lesions and history of intraocular surgery were excluded. A complete eye examination was performed including Tumbling E optotype visual acuity testing on a Snellen chart at 6 meter distance, evaluation of ocular duction and versions, alternate prism cover test for diagnosis and measurement of eye deviation, cycloplegic refraction 45 min after installing cyclopentolate 1% and tropicamide1%, one drop 5 min apart, funduscopy to rule out optic nerve and macular lesions and Titmus test to evaluate stereoacuity.
Any difference in spherical or cylindrical errors equal or greater than 1.5 D between fellow eyes was considered as anisometropia. Refractive errors < 1 D were considered as plano.
Amblyopia was classified into three groups according to severity, mild amblyopia (best corrected visual acuity [BCVA] of 20/30–20/40), moderate amblyopia (BCVA 20/40–20/100) and severe amblyopia (BCVA < 20/100). Spectral domain OCT (OCT 3000, version A 3.0; Carl Zeiss-Humphrey system, Dublin, CA, USA) of the macula was performed in both eyes of all children at least 3 times to obtain reasonable quality images. Thickness of the center (foveola), 1 mm circle (fovea), middle circle (3 mm in diameter), outer circle (6 mm in diameter, macula) and total macular volume were measured as primary outcomes of the study. The middle and outer circles were divided into superior, nasal, temporal and inferior zones to compare their thickness separately.
In this study, macular thickness of amblyopic eyes was compared to their non-amblyopic fellow eyes (internal controls) and both eyes of non-amblyopic age and sex matched children (external controls). Macular Difference > 20 micron between amblyopic eyes and their fellow eyes (internal controls) or between 2 eyes of external controls was considered as significant (10% of normal macular thickness). For quantitative data, t-test, Chi square test and Fisher exact test were used. The Mann Whitney test was employed for qualitative data and General Equation Estimation (GEE) analysis was performed for compensating the similarity effect of enrolling both eyes. P <0.05 were considered as statistically significant.
RESULTS
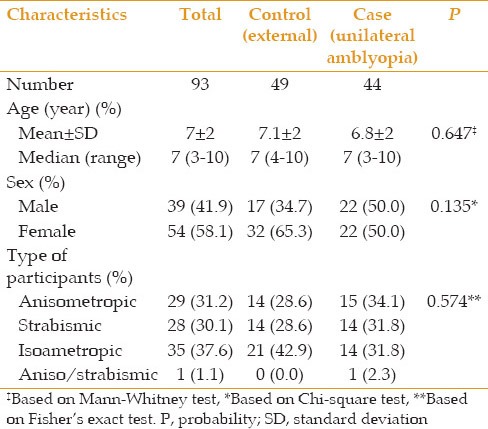
A total of 93 children aged 3–10 years with mean age of 7 ± 2 years including 54 (58.1%) female subjects were studied. Forty-four cases had unilateral amblyopia whose amblyopic eyes were considered as the case group (44 eyes) and their non-amblyopic fellow eyes as the internal control group (44 eyes). The 49 children without amblyopia served as the external control group (98 eyes). There was no significant difference in epidemiologic characteristics between the case and control groups [Table 1].
Table 1.
Demographic characteristics of children in the case and control groups
Mean BCVA was 0.28 ± 0.14, 0.05 ± 0.05 and 0.02 ± 0.05 logMAR in cases, the internal and external control groups, respectively [P = 0.001 for both comparisons, Table 2].
Table 2.
BCVA and refraction in the case and control groups
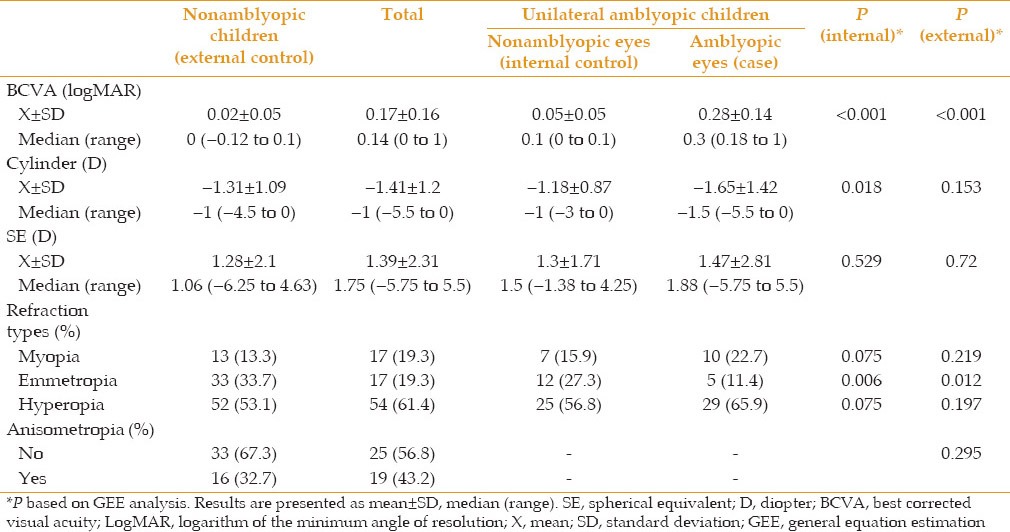
Although there was no significant difference among the refractive errors of these groups, the difference in cylindrical error was significant between amblyopic their fellow eyes (P = 0.018) and emmetropia was less frequent in amblyopic eyes as compared to the internal and external control groups [P = 0.012, Table 2].
Significant differences were observed among BCVA in amblyopic eyes, and internal and external controls under different types of anisometropic, isoametropic and strabismic amblyopia (P = 0.001 in all three groups), but unexpectedly, BCVA of internal control eyes was less than the external controls [P = 0.001, Figure 1].
Figure 1.
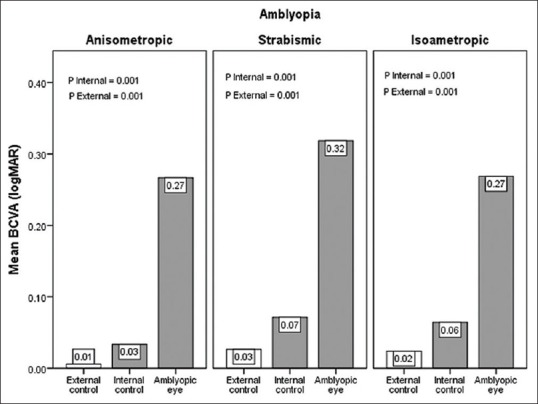
Mean best corrected visual acuity of amblyopic eyes, internal and external control groups in anisometropic, strabismic and isometropic amblyopia. BCVA, best corrected visual acuity; logMAR, logarithm of the minimum angle of resolution.
Amblyopic and nonamblyopic eyes showed similar macular thickness in the center, 1, 3 and 6 mm rings and their superior, inferior, nasal and temporal areas [Table 3 and Figure 2]. However, moderate and severe amblyopic eyes showed greater thickness (229 ± 36 vs. 198 ± 31 μ, P = 0.037) as compared to the external control group in central thickness but were comparable in other areas [Figure 3]. There was a significant difference in macular volume between amblyopic eyes and their fellow eyes in anisometropic children (P = 0.042), but other types of amblyopia showed no difference [Figure 4].
Table 3.
Central, 1, 3, 6 mm rings macular thickness and total macular volume in cases and controls
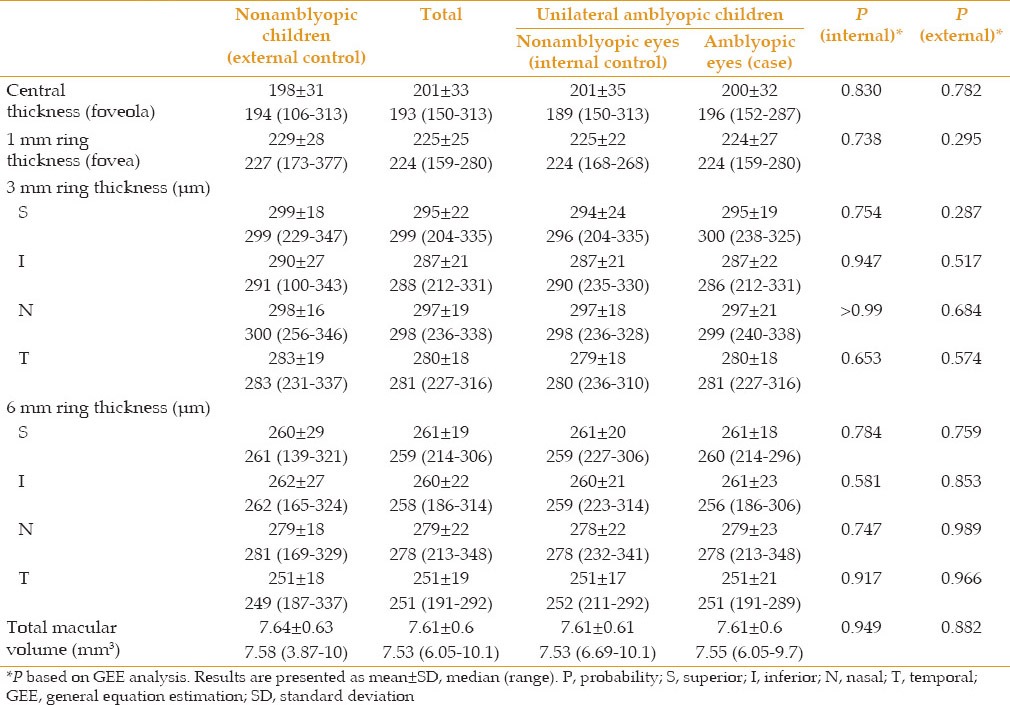
Figure 2.
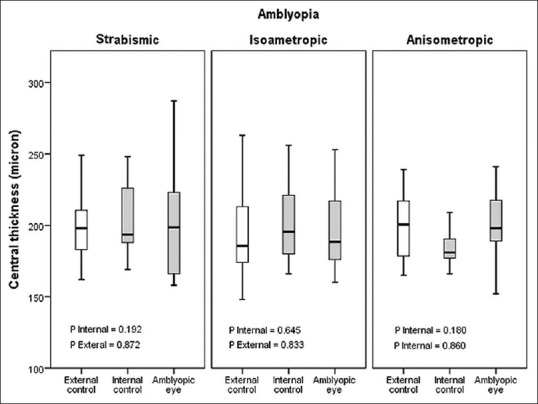
Central thickness (foveola) in strabismic, isometropic and anisometropic amblyopia.
Figure 3.
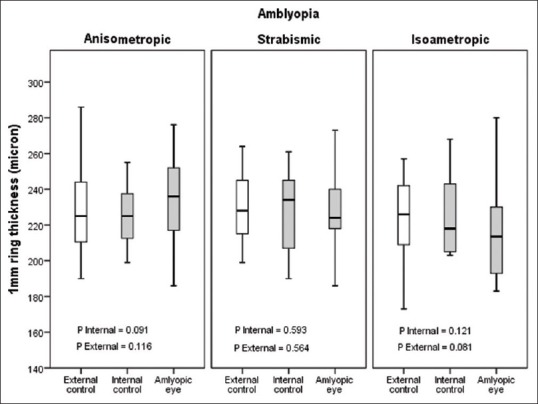
One mm ring thickness (fovea) in strabismic, isometropic and anisometropic amblyopia.
Figure 4.
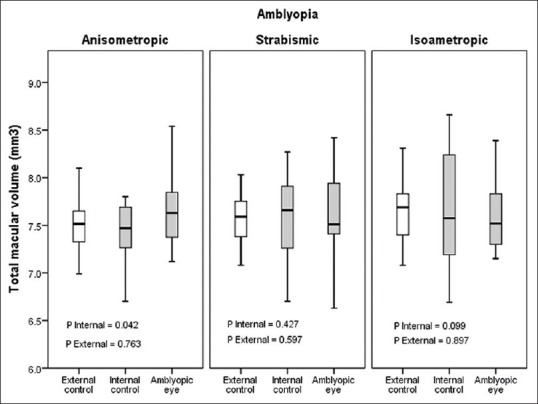
Total macular volume in anisometropic, strabismic and isometropic amblyopia.
The superior and temporal zones of the 3 mm ring, and the nasal and temporal zones of the 6 mm ring had the most and least thickness in each ring, respectively. Central (foveolar) and foveal (1 mm ring) thickness in girls was less than boys in all amblyopic, internal and external groups (P = 0.037 for fovea and P = 0.033 for foveola), but macular volume was not different between the two genders.
There was no difference between foveal, foveolar thickness and macular volume with different types of refractive errors. Although foveal thickness in myopes was the lowest, its difference was not significant (223 μ for myopia, 227 μ for emmetropia and 228 μ for hyperopia). No case of macular difference exceeding 20 μ was observed between amblyopic and non-amblyopic eyes.
Fundus examination of amblyopic and non-amblyopic eyes showed no remarkable difference and foveal discoloration was not clinically significant.
A 10–year-old boy showed reduced foveal depression on OCT which was more marked in his right eye than the left one. Refraction was plano-1.75 * 180 in both eyes with mild amblyopia of the right eye (BCVA: Right eye = 20/30, left eye = 20/20). Foveolar and foveal thickness was 280 and 300 μ in the right and 263 and 297 μ in the left eye, respectively. No clear explanation was found for the lack of central foveal depression in this patient.
DISCUSSION
In the present study, we did not detect any significant difference in macular thickness between amblyopic and non-amblyopic eyes of children aged 3-10 years.
In the study by Liu et al on 15 seven to fourteen-year-old children with refractory amblyopia, mean central and foveal thicknesses measured by OCT was 201.5 ± 17.9 and 226.9 ± 11.4 μ, respectively, comparable to our study. Liu compared the results of his study with the findings of Liu et al on normal Chinese children who had normal thickness of 153.8 ± 17.6 and 176.7 ± 14.8 μ in central and foveal areas, respectively and reported a significant difference between amblyopic and normal eyes (P = 0.000).[1] Methodologically, comparing OCT thickness values of amblyopic children from one study with that of non-amblyopic children from another study as a control group has some limitations. It would have been more accurate if subjects of both groups were simultaneously selected from the same patient population as in our study.
Kee et al studied 26 amblyopic and 42 non-amblyopic children with mean age of 8 years by comparing their findings with results from other studies.[2] They did not find any significant difference between foveal thickness in amblyopic and non-amblyopic eyes which is similar to our results (P = 0.5), however their reported foveal thickness (157.4 μ in non-amblyopic and 158.8 μ in amblyopic cases) was less than ours which could be due to differences in race, examiners and devices.
Wang et al studied 14 subjects with hyperopic anisometropic amblyopia and reported no significant differences in peripapillary retinal nerve fiber layer, central macular thickness, and macular volume between amblyopic eyes and fellow eyes of the participants.[9] Similarly, Dickmann et al reported no significant difference between retinal nerve fiber layer thickness, macular thickness, and foveal volume in amblyopic versus fellow eyes in patients with unilateral amblyopia.[10] However, Pang et al reported that amblyopic children with unilateral high myopia tend to have a thicker fovea and thinner inner and outer macular ring in the amblyopic eye as compared to their normal fellow eye.[11]
Silva et al studied 19 amblyopic children up to the age of 18 including 15 strabismic and 4 anisometropic subjects and found that foveal thickness was significantly greater in the amblyopic eye; by contrast, significantly reduced thickness was found in the inner nasal, inner inferior and outer inferior macular areas of the amblyopic eye. Macular volume and retinal thickness in other macular areas were reduced in amblyopic eyes but not significantly.[12]
In a study on 14 children with unilateral amblyopia and strabismus, Altintas et al showed no significant difference between macular thickness and volume in amblyopic and non-amblyopic children which is similar to our results.[3]
Aguirre et al studied 192 eyes of children aged 4–10 years including 68 subjects with normal vision and 124 ametropic amblyopic eyes at the time of diagnosis (66 mild and 58 severe). The 3 mm macular ring was analyzed by OCT and divided into 4 areas (superior, inferior, temporal and nasal). They reported that all retinal areas were thicker in amblyopic eyes as compared to normal eyes (P < 0.05 in the upper and nasal), especially in mild amblyopia. Differences were greater in females (up to 5.9% thicker). The inferior area in hyperopic eyes proved to be thicker, with no differences with age.[13]
Although Yoon et al found no difference between macular thickness of 31 hyperopic and anisometropic amblyopic children (252.5 μ) with non-amblyopic ones (249.7 μ), retinal thickness was significantly thicker in amblyopic eyes (P = 0.019).[4]
In the current study, superior and temporal zones of the 3 mm ring and nasal and temporal zones of the 6 mm ring had the most and least thickness in each ring, respectively, whereas in the study by Varma et al, the superior and inferior zones had the most and nasal and temporal zones had the least thickness.[14] According to Liu et al, retinal thickness in the nasal zone was less than the temporal zone.[1]
We observed a significant difference in central thickness of eyes with moderate to severe amblyopia and external controls in our study. Due to the small number of such children (6 cases, 14%), this finding should be interpreted with caution and needs to be tested with larger sample size.
The present study shows lower mean BCVA in all kinds of amblyopic eyes as compared to both internal and external controls. Unexpectedly, BCVA of eyes in the internal control group (normal fellow eyes of amblyopic children) was significantly (P = 0.001) lower than normal external control eyes. This difference suggests that fellow eyes of children with unilateral amblyopia cannot be considered as completely as normal. It may also necessitate enrolling non-amblyopic children as a control group in similar studies.
In this study, there was no relationship between refractive status (myopia, hyperopia and emmetropia) with macular thickness and volume, which may have been due to the exclusion of cases with high refractive errors. Mean foveal thickness in myopic eyes was less than others in our study which may be due to retinal thinning in these children.
Myopia and family history of high myopia affects evolution of the eye. It may cause macular dysplasia and impairment of apoptosis in the inner plexiform, inner nuclear and ganglion cell layers of the macula resulting in amblyopia poorly responsive to treatment.[1] This phenomenon was not seen in our study probably due to exclusion of high myopic refractive errors (>5 D).
Our study had some advantages such as having both internal and external control groups simultaneously, focusing on a similar population and exclusion of high refractive errors. Disadvantages of our study were the limited number of cases with moderate to severe amblyopia and mixed amblyopia (anisometropic and strabismic). One should also take into account that in certain eyes with low vision attributed to amblyopia mild macular hypoplasia actually causes reduced VA and therefore this group of eyes may falsely reduce mean OCT thickness values.
In conclusion, although there was no relationship between macular thickness and amblyopia, central (foveolar) thickness was significantly increased in moderate to severe amblyopia as compared to normal controls. Due to the limited number of such cases in the present study, conducting a survey including more subjects with moderate to severe amblyopia is recommended in order to verify the difference and also evaluate the efficiency of OCT as a diagnostic tool.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Liu H, Zhong L, Zhou X, Jin QZ. Macular abnormality observed by OCT in children with amblyopia failing to achieve normal visual acuity after long-term treatment. J Pediatr Ophthalmol Strabismus. 2010;47:17–23. doi: 10.3928/01913913-20091019-06. [DOI] [PubMed] [Google Scholar]
- 2.Kee SY, Lee SY, Lee YC. Thicknesses of the fovea and retinal nerve fiber layer in amblyopic and normal eyes in children. Korean J Ophthalmol. 2006;20:177–181. doi: 10.3341/kjo.2006.20.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altintas O, Yüksel N, Ozkan B, Caglar Y. Thickness of the retinal nerve fiber layer, macular thickness, and macular volume in patients with strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2005;42:216–221. doi: 10.3928/01913913-20050701-03. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SW, Park WH, Baek SH, Kong SM. Thicknesses of macular retinal layer and peripapillary retinal nerve fiber layer in patients with hyperopic anisometropic amblyopia. Korean J Ophthalmol. 2005;19:62–67. doi: 10.3341/kjo.2005.19.1.62. [DOI] [PubMed] [Google Scholar]
- 5.Yen MY, Cheng CY, Wang AG. Retinal nerve fiber layer thickness in unilateral amblyopia. Invest Ophthalmol Vis Sci. 2004;45:2224–2230. doi: 10.1167/iovs.03-0297. [DOI] [PubMed] [Google Scholar]
- 6.Bozkurt B, Irkeç M, Orhan M, Karaagaoglu E. Thickness of the retinal nerve fiber layer in patients with anisometropic and strabismic amblyopia. Strabismus. 2003;11:1–7. doi: 10.1076/stra.11.1.1.14091. [DOI] [PubMed] [Google Scholar]
- 7.Repka MX, Goldenberg-Cohen N, Edwards AR. Retinal nerve fiber layer thickness in amblyopic eyes. Am J Ophthalmol. 2006;142:247–251. doi: 10.1016/j.ajo.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Park KA, Park DY, Oh SY. Analysis of spectral-domain optical coherence tomography measurements in amblyopia: A pilot study. Br J Ophthalmol. 2011;95:1700–1706. doi: 10.1136/bjo.2010.192765. [DOI] [PubMed] [Google Scholar]
- 9.Wang BZ, Taranath D. A comparison between the amblyopic eye and normal fellow eye ocular architecture in children with hyperopic anisometropic amblyopia. J AAPOS. 2012;16:428–430. doi: 10.1016/j.jaapos.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Dickmann A, Petroni S, Perrotta V, Parrilla R, Aliberti S, Salerni A, et al. Measurement of retinal nerve fiber layer thickness, macular thickness, and foveal volume in amblyopic eyes using spectral-domain optical coherence tomography. J AAPOS. 2012;16:86–88. doi: 10.1016/j.jaapos.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Pang Y, Goodfellow GW, Allison C, Block S, Frantz KA. A prospective study of macular thickness in amblyopic children with unilateral high myopia. Invest Ophthalmol Vis Sci. 2011;52:2444–2449. doi: 10.1167/iovs.10-5550. [DOI] [PubMed] [Google Scholar]
- 12.Silva F, Alves S, Pina S, Azevedo A, Pêgo P, Santos MJ, et al. Comparison of macular thickness and volume in amblyopic children using time domain optical coherence tomography. Oftalmologia. 2012;36:231–236. [Google Scholar]
- 13.Aguirre F, Mengual E, Hueso JR, Moya M. Comparison of normal and amblyopic retinas by optical coherence tomography in children. Eur J Ophthalmol. 2010;20:410–418. doi: 10.1177/112067211002000223. [DOI] [PubMed] [Google Scholar]
- 14.Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fiber layer thickness in normal latinos. Invest Ophthalmol Vis Sci. 2003;44:3369–3373. doi: 10.1167/iovs.02-0975. [DOI] [PubMed] [Google Scholar]


