Abstract
Gene therapy has a growing research potential particularly in the field of ophthalmic and retinal diseases owing to three main characteristics of the eye; accessibility in terms of injections and surgical interventions, its immune-privileged status facilitating the accommodation to the antigenicity of a viral vector, and tight blood-ocular barriers which save other organs from unwanted contamination. Gene therapy has tremendous potential for different ocular diseases. In fact, the perspective of gene therapy in the field of eye research does not confine to exclusive monogenic ophthalmic problems and it has the potential to include gene based pharmacotherapies for non-monogenic problems such as age related macular disease and diabetic retinopathy. The present article has focused on how gene transfer into the eye has been developed and used to treat retinal disorders with no available therapy at present.
Keywords: Adenovirus, Gene Transfer, Genetic Vector, Hereditary Retinal Diseases
INTRODUCTION
In medicine, the term “magic bullet” is often used to describe the perfect, most precise treatment for a medical condition. Such idealism has hardly been achieved. We have sought the “magic bullet” solution for cancer and for autoimmune disorders but in most cases, without a result. In spite of these setbacks, the trial demonstrated that gene therapy is the ultimate form of medical treatment and in fact constitutes “the magic bullet” is far from hyperbole. Gene therapy is fundamental for it is centered on disease etiology at the DNA level. Thus implications of gene therapy are enormous and research activity in this area is rapidly expanding. Although the majority of research has been in the field of cancer and hereditary immunodeficiencies, significant work has taken place in ophthalmology, where human trials are underway to address several hereditary retinal disorders and age-related macular degeneration. In the end, ophthalmology may prove to be the poster child for the success of gene therapy.
WHAT IS GENE THERAPY?
Gene therapy refers to a technique of introducing foreign DNA constructs into host cells in order to treat a medical condition. The most basic version of this therapy involves monogeneic disorders such as cystic fibrosis. By introducing a wild-type copy of the CF gene into a patient with the mutant gene, one can compensate for the mutation by giving cells the ability to produce wild-type protein. Gene therapy can also be used to modify host immunologic cells to enhance their recognition of specific infections or tumor markers on cancer cells. The final form of gene therapy is to turn the host cell machinery into a “bio-factory”. In this situation, the gene that is introduced expresses a therapeutic protein that would have otherwise been given exogenously to the patient.
The basic requirements of gene therapy are: (1) Cloned copy of the wild-type gene linked to a particular disorder. (2) A promoter system that assures transcription of the gene in the targeted tissue. (3) A vector: This is usually a virus stripped of its replicative and virulence factors. The vector carries the DNA construct into the cell. (4) A host with a gene mutation or condition who may benefit from receiving the DNA construct into his/her cells.
The eye is particularly suited to gene therapy. Because of its immune-privileged status, the eye can accommodate more readily the antigenicity of a viral vector than other organs. Because of the eye's tight barriers relative to the rest of the body, systemic contamination is unlikely. There are also many monogeneic disorders that are exclusively ophthalmic and thus by treating the eye, one can treat the entire disease. Finally, because of its location, the eye is easily accessible for treatment and can receive a gene through a simple intravitreal injection or through a common operation such as vitrectomy.
THE HISTORY OF GENE THERAPY
In 1990, French Anderson launched gene therapy in humans by treating a 4-year-old girl suffering from severe combined immunodeficiency (SCID) with the wild-type form of the ADA gene.[1] The success of that initial treatment was short-lived but the field received significant media coverage. Additional trials were launched, including one in 2000 in which patients with the X-linked form of SCID were treated.[2] Unfortunately, two of the patients in that trial developed T-cell leukemia because of insertional mutagenesis;[3] and with this, the enthusiasm over gene therapy temporarily waned because of concerns for its potential hazards. The trial however demonstrated that gene therapy is a realistic mode of treatment and as of 2012, 8 of the 9 patients from one arm of the trial have been reported to be well and alive.[4]
Another major landmark in the chronicle of gene therapy is the 2006 Rosenberg study involving several patients with end-stage metastatic melanoma.[5] Immune cells from these patients were transfected with the gene encoding a cell-surface protein that would help focus the attack against a tumor-specific antigen on the melanoma cells. Two of 15 patients had effective control of their tumor cells and outlived the 3–6 months that was predicted for such patients.
As of June 2012, there have been over 1800 gene therapy clinical trials. These have taken place in over 31 countries and covered a variety of medical specialties. The three most common areas of research have been in oncology, cardiology and monogeneic diseases. Ocular diseases account for 1.3% of all gene therapy clinical trials but the most profound successes in gene therapy may ultimately emerge out of ophthalmology.[6]
HISTORY OF GENE THERAPY FOR RETINAL DISEASES
The first demonstration of retinal gene transfer was in a mouse model of retinitis pigmentosa (RP).[7] Bennett and Maguire were able to rescue mice with a defect in the phosphodiesterase gene by introducing the wild-type version of the gene on an adenovirus vector. The vector was delivered via subretinal injection. This form of delivery proved effective and became the standard for subsequent experiments and trials.
Bennet and Maguire continued this work by using the same techniques to rescue Briard dogs with Leber's Congenital Amaurosis (LCA).[8] LCA is a progressive autosomal recessive disease marked by loss of photoreceptors, declining visual fields and flat electroretinography (ERG) tracings. Most patients are profoundly blind by the second decade of life. The underlying defect is in the RPE65 gene, encoding an isomerohydrolase that is expressed in RPE cells and responsible for generation of 11-cis retinal. Without a functioning RPE65, the RPE cell cannot deliver Vitamin A to the photoreceptors.
In the canine model, dogs that received a subretinal rescue with the LCA wild-type gene were compared to those that received placebo. The treated dogs developed brisk pupillary response, recovered ERG's and improved ambulation through an obstacle course. Similar positive observations were made in a mouse model of LCA.
All in all, over 60 mice and dogs received the wild-type RPE 65 construct. One of the interesting findings that emerged from the animal studies was that younger animals were more efficiently rescued from their disease than older animals. This makes perfectly logical sense since LCA is a progressive degeneration of the photoreceptors. Rescue early in the disease process means preservation of still-intact photoreceptors and perhaps a more enduring beneficial effect. The animal studies also demonstrated that the gene was stably expressed, in one dog, for over a decade.
Phase I studies in 2008–2009 established safety and to some extent, efficacy of gene therapy in children with LCA.[9] Twelve patients were enrolled in this dose-escalation study in which an adenoviral vector was used to deliver the RPE65 gene subretinally and then treated eyes were compared to untreated eyes.
On average, each eye that received treatment became three-times more sensitive to light as compared to the untreated eye, and all 12 patients reported improved vision in dim light. Pupillary light reflex also improved in treated versus untreated eyes to the extent that an afferent pupillary defect was produced. Three of the subjects had substantial visual improvement; but the trend seen in animal studies toward better rescue associated with younger age was not seen in this small group of younger human subjects. Visual field testing demonstrated an increase in the size of the visual field compared to baseline and the field size roughly corresponded to the anatomic area covered by the subretinal injection. One of the most interesting results of the study was based on the subjective ability of patients walking through a dimly lit obstacle course. Patients using the treated eye were much better able to navigate their way through the course than when using the untreated eye.
The success of the Phase I study inspired a phase III study which is now well underway. This study is placebo-controlled and comprises of 8 patients receiving active treatment and 8 subjects in a placebo group. On average, patients in the Phase III trial will be younger than those in the Phase I/II study with one patient as young as 3 years of age. There is hope to have the wild-type RPE65 gene inserted and expressed in the host cells before significant photoreceptor degeneration has taken place.
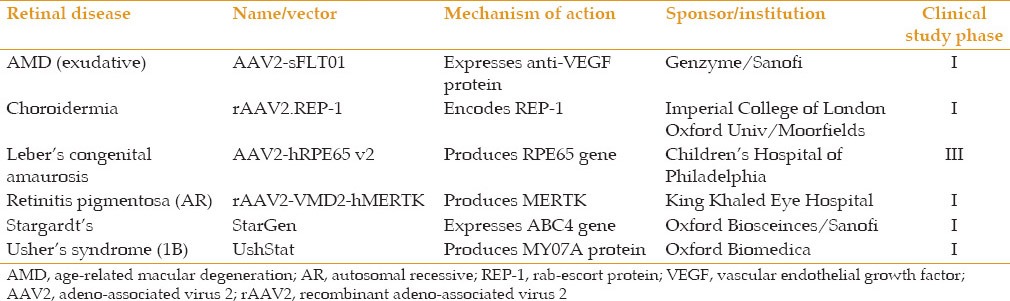
Gene therapy in ophthalmology has moved beyond the treatment of LCA. Phase I studies are underway to treat neovascular AMD, choroideremia, retinitis pigmentosa, Stargardt's disease and Ushers syndrome [Table 1]. Most of these trials are based on the same biotechnical concept noted in the LCA studies, notably a gene chimera delivered on a viral vector to the subretinal space. The exception is the Genzyme-sponsored study involving AAV2-sflt01 chimera, which is injected intravitreally instead of subretinally. This form of delivery is based on the fact that the protein product of the gene is essentially VEGF-trap which has already shown efficacy following intravitreal injection and does not need to be introduced into the subretinal space.
Table 1.
Select clinical trials of gene therapy for retinal diseases[10]
THE FUTURE
In the eye, gene therapy may become the overall test case for this unique therapeutic approach. In spite of significant scientific and clinical advances, there are several areas of exploration that may improve the overall success of gene therapy.
Vector development has become an important field by itself: it is called vectorology. By modifying the viral genome, one may be able to alter surface proteins so that they bind to specific cells. Such specificity would enable a virus to carry its gene depot to, for example, cone cells in order to treat an inherited form of cone dystrophy. The same respect for specificity would carry through to the gene cassette by having the promoter upstream of the gene in question work only in a specific type of cell.
Although gene therapy as it pertains to retinal disorders is in its infancy, an approved treatment for LCA is in the offing. Positive results have also been reported in the trial concerning choroideremia. It will be a groundbreaking achievement to have therapy 1-day that could potentially eradicate blindness in diseases that today can be treated with only compassionate words.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Anderson WF, Blaese RM, Culver K. The ADA human gene therapy clinical protocol: Points to consider response with clinical protocol, July 6, 1990. Hum Gene Ther. 1990;1:331–362. doi: 10.1089/hum.1990.1.3-331. [DOI] [PubMed] [Google Scholar]
- 2.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 3.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 4.Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363:355–64. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 – An update. J Gene Med. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 7.Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, et al. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- 8.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 9.Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: A phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. Erratum in: Lancet 2010;375:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant JS, Duker JS, Reichel E. Review of Ophthalmology. Avenue of the Americas. NY: Johnson Publishing LLC; 2012. Gene therapy for retinal disease; p. 89. [Google Scholar]


