Abstract
Purpose
We conducted a systematic review and pooled analysis of small renal masses under active surveillance to identify progression risk and characteristics associated with metastases.
Materials and Methods
A MEDLINE search was performed to identify all clinical series reporting surveillance of localized renal masses. For studies reporting individual level data, clinical and radiographic characteristics of tumors without progression were compared to those progressing to metastases.
Results
18 series (880 patients, 936 masses) met screening criteria from which 18 patients progressing to metastasis were identified (mean 40.2 months). Six studies (259 patients, 284 masses) provided individual level data for pooled analysis. With a mean follow up of 33.5±22.6 months, mean initial tumor diameter was 2.3±1.3 cm and mean linear growth rate was 0.31±0.38 cm/year. 65 masses (23%) exhibited zero net growth under surveillance; of which none progressed to metastasis. Pooled analysis revealed increased age (75.1±9.1 vs. 66.6±12.3 years, p=0.03), initial tumor diameter (4.1±2.1 vs. 2.3±1.3 cm, p<0.0001), initial estimated tumor volume (66.3±100.0 vs. 15.1±60.3 cm3, p<0.0001), linear growth rate (0.8±0.65 vs. 0.3±0.4 cm/yr, p=0.0001), and volumetric growth rate (27.1±24.9 vs. 6.2±27.5 cm3/yr, p<0.0001) in the progression cohort.
Conclusions
A substantial proportion of small renal masses remain radiographically static following an initial period of active surveillance. Progression to metastases occurs in a small percentage of patients and is generally a late event. These results indicate that in patients with competing health risks, radiographic surveillance may be an acceptable initial approach with delayed intervention reserved for those exhibiting significant linear or volumetric growth.
Introduction
Few data evaluating the natural history of solid human malignancies under active surveillance (AS) are available and are limited to observational data from small cohort studies and limited prospective registries. Provocative data regarding the surveillance of localized prostate cancers1 and renal masses2, 3 in select patients have recently been presented, and despite the lack of level I evidence, these studies represent the most robust contemporary data regarding the natural history of untreated solid tumors.
Approximately 54,400 new cases of cancer of the kidney and renal pelvis are diagnosed annually in the United States, with the majority representing renal cell carcinomas (RCC)4. Due to the increased utilization of cross sectional abdominal imaging5, 6, the greatest increase in detection has been observed in small localized tumors (<4cm), and incidental detection of asymptomatic lesions now accounts for greater than 50% of all renal masses discovered7. A concurrent increase in median age at RCC diagnosis has also been observed, with the greatest increase in patients 70 to 90 years of age4. Radiographically stage I renal masses represent a heterogenous entity, with as many as 20% being benign8 and an estimated 20–25% being potentially aggressive9, 10.
Traditionally, clinical stage I renal masses have been treated with extirpation, most commonly by radical nephrectomy11. However, concerns that nephrectomy may predispose patients to the sequelae of chronic kidney disease12, 13 including increased cardiovascular risk and shortened overall survival14–16 has resulted in the increased utilization of nephron sparing procedures with the goal of preserving long term renal function without affecting cancer control17. Beyond excision, tumor ablation has been broadly applied, despite a lack of published endpoints, thereby confounding treatment decisions18. Whereas five year cancer specific survival of surgically treated stage I small renal masses (SRMs) remains in excess of 95%18, the formidability of RCC tumor biology has been questioned as no treatment data have been compared to lesions managed expectantly19. Moreover, there is a growing recognition that competing risks from co-morbidities may outweigh the benefit of intervention in elderly and infirmed patients20. While surgical excision is the empiric practice standard21, AS is now a recognized option in patients with limited life expectancy18. Here we report a systematic review and pooled analysis of published clinical series investigating the AS of SRMs to establish clinical and radiographic characteristics of lesions progressing to metastatic disease.
Materials and Methods
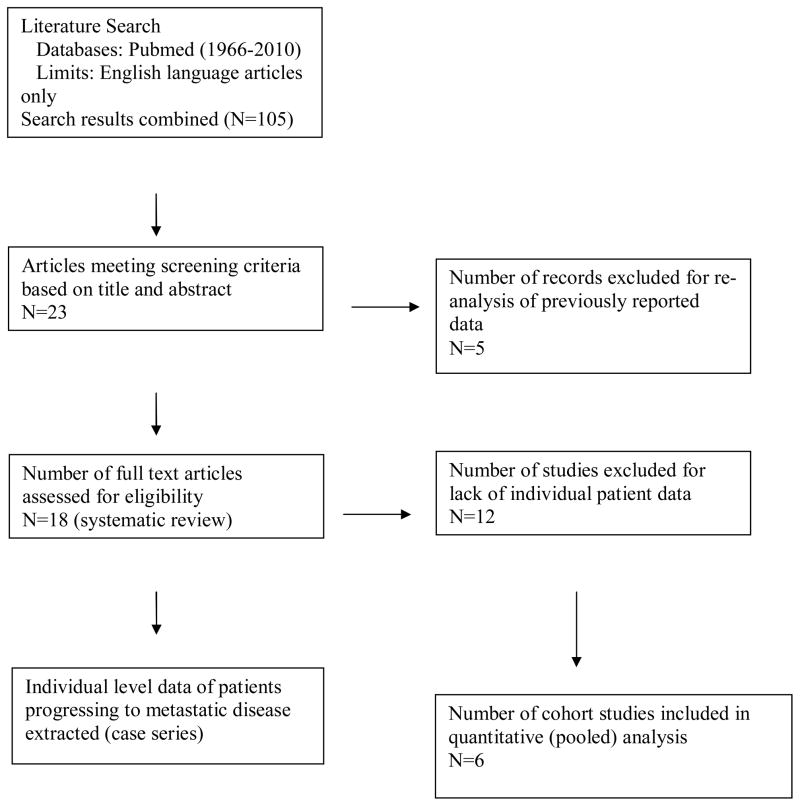
A MEDLINE search was conducted (1966–2010) using the National Center for Biotechnology Information database combining the following medical subject heading terms: “kidney, neoplasms, natural history, surveillance, ± delayed intervention” to identify all studies reporting on the observation of suspected renal malignancies. Included studies were limited to “English language”. Study titles and abstracts were reviewed to identify all series analyzing clinically localized lesions at the time of presentation based on standard radiographic staging protocols. Series investigating hereditary lesions, metastatic lesions at the time of initial presentation, and those that did not discriminate between localized and metastatic disease were excluded. Our initial search revealed 105 articles, of which 23 retrospective studies investigating the observation of localized solid renal tumors were identified (Figure 1). Five redundant series that were updated in subsequent publications were excluded22–26. 18 studies met final screening criteria3, 27–43, of which 5 reported individual level data30, 32, 33, 36, 41 which were pooled with unpublished individual level data from our previously reported institutional AS series3. From our systematic review, individual patient data for SRMs progressing to metastases (N+ or M+) were extracted as a case series for comparison purposes. Patients who progressed to metastasis within 6 months of surveillance were assumed to have undocumented or micrometastatic systemic disease at presentation and were not included in our progression cohort. In comparison to a traditional meta-analysis, only individual patient-level data were included in an effort to reduce the confounding effect of “ecologic fallacy”44, 45.
Figure 1.
Flow diagram of study selection
Each study was reviewed to extract patient and mass characteristics by a single investigator with the findings confirmed by the additional investigators. When available, indications for surveillance were categorized as absolute (patients who were not surgical candidates due to prohibitive competing health risks), relative (a potential need for renal replacement therapy following treatment), and elective (refuse surgical intervention despite acceptable or low operative and nephrologic risks)3. Extracted data included demographics, maximal tumor diameter (MTD; cm) and estimated tumor volume (ETV; cm3) at the time of presentation, MTD and ETV at the time of intervention or progression, duration of surveillance (months), calculated linear and volumetric tumor growth rates (cm/yr; cm3/yr), surgical pathology, and progression to metastatic disease. Tumor volume was calculated depending on the available dimensions reported on imaging data. If three dimensions were present an ellipsoid volume formula (0.5326xyz) was utilized. If two dimensions were present the formula 0.5326xy(x+y/2) was utilized. If only one dimension was reported, the formula for the volume of a sphere (0.5326×3) was employed41.
Mean estimates were calculated for patient age, MTD and ETV, linear and volumetric growth rates, and duration of observation. Characteristics from pooled cohort studies were compared between SRMs exhibiting zero growth and positive growth during a period of observation and SRMs managed with observation alone and those who progressed to delayed intervention. Masses that progressed to metastasis under a period of observation and those that did not were compared using aggregated case and cohort data. Comparisons were performed using Wilcoxon rank-sum tests, with nominal p-values of 0.05 used as the criteria for statistical significance. Where possible, we confirmed Wilcoxon rank-sum p-values and point estimates using mixed effects linear regressions including random intercepts for study and patient46. For post-baseline measures, we included individual level follow-up time as a covariate in the regressions. However, due to small sample sizes and few patients with multifocal tumors (mean 1.1 observations per patient), the models were often not estimable. Analyses were conducted using STATA version 10 (StataCorp, College Stage, Texas).
Results
Pooled analysis of small renal masses under observation
Our systematic literature review revealed 18 retrospective studies comprising 880 patients and 936 SRMs. Six studies with available individual level data (all ≤ level III evidence) met inclusion criteria for pooled analysis (259 patients, 284 SRMs). Five of the six identified studies were small (<50 patients)30, 32, 33, 36, 41, 43, while one series included 154 patients3. Pooled analysis of cohort studies (Table I) revealed a mean age of 66.6±12.3 years (median 69; range 35–88) in 233 patients, with indication for AS meeting elective (75.6%), relative (10.9%), and absolute (13.5%) criteria respectively (n=193). 85.4% of lesions were categorized as solid and 14.6% as cystic (n=226), and multi-focal disease was reported in 8.1% of patients. Mean MTD (n=284) and ETV (n=284) at the time of diagnosis were 2.3±1.3 cm (median 2; range 0.2–12) and 15.1±60.0 cm3 (median 4.3; range 0.004–903.7) respectively. In comparison, mean MTD (n=252) and ETV (n=284) at the conclusion of observation were 3.0±1.6 cm (median 2.7; range 0.9–15) and 29.5±109.3 cm3 (median 10.3; range 0.27–1765.1). With a mean duration of observation (n=284) of 33.5±22.6 months (median 27.5; range 5.3–156), calculated linear (n=251) and volumetric (n=284) growth rates were 0.31±0.38cm/yr (median 0.25; range −1.4–2.5) and 6.3±27.4 cm3/yr (median 1.6; range −20.0–430.7). Of patients with available pathologic data, 88% of lesions were malignant (n=117), and predominantly low grade (80.5%). Of benign lesions, oncocytoma (10.3%, n=12) and angiomyolipoma (1.7%, n=2) were most common. Of malignant lesions, predominant histologic subtypes included clear cell (64.1%, n=75) and papillary (17.1%, n=20) disease, while mixed or unclassifiable (4.3%, n=5), chromophobe (1.7%, n=2) and collecting duct (0.9%, n=1) carcinomas were rare.
Table I.
Retrospective cohort studies of small renal masses (SRMs) managed with active surveillance (AS) with available individual level data for pooled analysis
| Study | N patients (No. SRMs) |
Age (yrs) mean median range |
Reason for AS No. (%) |
Imaging Charact eristics No. (%) |
Initial MTD (cm) mean median range |
Initial ETV (cm3) mean median range |
Final MTD (cm) mean median range |
Final ETV (cm3) mean median range |
Linear GR (cm/yr) mean median range |
Volume tric GR (cm3/yr) mean median range |
Follow Up (months) mean median range |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fujimoto et al.32 | 6 (6) | 59.7 57 47–70 |
A - 3(50) R - 0 E - 3(50) |
S - 6(100) | 2.47 2.4 1.7–3.4 |
9.7 6.9 2.6–20.9 |
3.78 3.8 2.8–4.7 |
31.4 28.5 11.7–55.3 |
0.57 0.58 0.39–0.74 |
9.7 8.6 4.1–16.2 |
29 20.3 9.7–71 |
| Bosniak et al.30 | 37 (40) | 65.1 65.5 42–84 |
M - 9(24) | 1.73 1.8 0.2–3.5 |
4.6 2.9 0.004–22.8 |
3.11 3 1.2–7 |
24.3 14.4 0.9–182.7 |
0.4 0.36 0–1.1 |
5.26 3.1 0–42.1 |
43.9 39 21–102 |
|
| Volpe et al.41 | 29 (32) | N/A 71* 27–84 |
S - 25(78) C - 7(22) |
2.48 2.4 0.9–3.9 |
10.06 7.01 0.39–31.59 |
18.2 13.7 0.3–63.7 |
0.1‡ | 3.8 1.2 0–33.8 |
35.3 27.5 5.3–143 |
||
| Kato et al.33 | 18 (18) | 55.1 56.5 37–71 |
E – 18(100) | 1.98 2 0.8–3.4 |
6.1 4.3 0.3–20.9 |
2.81 2.8 1.4–4.4 |
14.7 11.7 1.5–45.4 |
0.42 0.28 .08–1.6 |
4.4 3.0 0.3–20.6 |
27 22.5 12–63 |
|
| Matsuzaki et al.36 | 15 (15) | 67 72 44–87 |
A – 5(33) E – 10(67) |
S - 15(100) | 2.2 2 1–3.9 |
8.9 4.3 0.5–31.6 |
2.4 2.2 1–4.2 |
10.6 5.7 0.5–39.6 |
0.06 0 −0.09–0.28 |
0.67 0 −0.77–7.3 |
38 37 8–91 |
| Crispen et al.3 | 154† (173) | 69 71 35–88 |
A – 18(12) R – 21(13) E – 115(75) |
S - 147(85) C - 26(15) M – 12(8) |
2.45 2 0.4–12 |
20 4.18 0.3–904 |
3.1 2.5 0.9–15 |
37.1 8.2 0.4–1765.1 |
0.29 0.15 −1.4–2.47 |
17 3 −20–431 |
31 24 12–156 |
| Totals | 259 (284) | 66.6 69 35–88 |
A – 26 (13.5) R – 21 (10.9) E – 146 (75.6) |
S – 193 (85.4) C – 33 (14.6) M – 21 (8.1) |
2.3 2 0.2–12 |
15.1 4.3 0.004–903.7 |
3.0 2.7 0.9–15 |
29.5 10.3 0.27–1765.1 |
0.31 0.25 −1.4–2.5 |
6.3 1.6 −20–430.7 |
33.5 27.5 5.3–156 |
Mean linear tumor diameter – MTD, Estimated tumor volume – ETV, Absolute – A, Relative – R, Elective – E, Solid – S, Cystic – C, Multifocal – M, GR – growth rate
median value
includes 3 patients who progressed to metastases
Sub-cohort analyses
Small renal masses with positive versus zero net growth
65 SRMs (22.9%) demonstrated zero net growth while under a period of surveillance. On pooled analysis, there was no difference in initial MTD between lesions exhibiting positive (N=219) and zero (N=65) growth (2.3±1.3 cm, [median 2; range 0.2–12] vs. 2.5±1.3 cm [median 2.1; range 1–9]; p=0.21). Mean linear growth rate in the positive growth group (N=206) was 0.45±0.41 cm/yr (median 0.34; range 0.02–2.7). Pathologic malignancy rates were comparable between groups (88.2% vs. 92.3%, p=1.0), but importantly, of the lesions that exhibited zero growth over time, none progressed to metastases.
Small renal masses progressing to intervention compared to those continuing active surveillance
Of the 284 SRMs from pooled cohort study data, 129 (45.4%) underwent delayed intervention after a mean period 30.5±21.8 months (median 24, range 6.4–143), while 155 were managed strictly with surveillance. Reason for progression to therapy (n=85) included patient preference (57.2%), improved medical condition (7.1%), tumor growth (35.7%), or other (1.2%). Comparison of patients continuing surveillance and those who progressed to intervention revealed similar initial MTD (2.4±1.2cm [median 2.1; range 0.8–9] vs. 2.2±1.3 [median 2; range 0.2–12]; p=0.26) and initial ETV (14.3±35.3cm3 [median 4.6; 0.27–381.3] vs. 16.0±80.3 [median 4.3; range 0.004–903.7]; p=0.30). However, significant differences were observed in the mean linear (0.24±0.35cm/yr [median 0.17; range −0.64–2.7] vs. 0.38±0.39 [median 0.31; range −1.4–1.6]; p<0.001) and volumetric (4.8±12.3 cm3/yr [median 1.1; range −20.0 −102.8] vs. 8.5±38.4 [median 3.1; range −15.2–430.7]; p<0.001) growth rates. Difference in mean growth rate also reached statistical significance using linear mixed effects modeling (p=0.04).
Small renal masses progressing to metastases under periods of observation compared with those who did not progress
18 patients progressing to metastatic disease (case series) were identified from systematic review of 880 patients with SRMs under surveillance3, 27, 29, 31, 35, 38–40, 43, 47 (Table II). Indications for AS (n=13) were absolute in 61.5% and elective in 38.5%. All lesions that progressed had a positive growth rate over time. Pathologic confirmation of diagnosis was made in 9 cases (50%); three with percutaneous biopsy3, 38, 43, five at the time of surgical exploration3, 27, 39, and one unknown47. Of the 11 patients with available information, 8 (72.7%) were diagnosed with distant visceral or bony disease with or without positive lymphadenopathy, and 3 patients were diagnosed with pathologic lymph node involvement only (27.3%). Histologic subtype was predominantly clear cell (66.7%)3, 27, 35, 39, 43, 47 and papillary (22.2%)27, 38, with one lesion exhibiting mixed clear cell and papillary features (11.1%)3.
Table II.
Clinical and radiographic characteristics of documented small renal masses progressing to metastasis under a period of active surveillance (AS) (case series)
| Age (years) | Sex | Indication for AS | Size at diagnosis: MTD (cm)/ETV (cm3) | Pathologic stage; Histologic sub-type; Fuhrman grade | Size at metastasis: MTD (cm)/ETV (cm3) | Time to Metastasis (months) | Growth rate: change in MTD (cm/yr)/ change in ETV (cm3/yr) | Site of Metastasis | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Lamb et al.35 | |||||||||
| 79 | elective | Clear cell; FG 3 | 132 | Alive at 136 months | |||||
| Sowery et al.40 | |||||||||
| 74 | M | CHR | 8.8/ 363 | 10.7/ 653 | 68 | 0.33/ 50.9 | nodal, liver | Managed with angioembolizationMortality at 70 months | |
| Wong et al.43 | |||||||||
| 78 | F | CHR | 2.5×2/ 6 | Clear cell; FG 2 | 5.9×5.4/ 95.8 | 15 | 2.7/ 71.8 | nodal, pulmonary | Mortality at 20 months |
| Fernando et al.31 | |||||||||
| F | 20 | Pulmonary | Mortality at 22 months | ||||||
| Siu et al.39 | |||||||||
| F | CHR | 3/ 14.4 | Clear cell | 6/ 115 | 78 | 0.46/ 15.5 | nodal | Grossly positive lymph nodes at attempted partial nephrectomy, which was aborted. Patient ultimately died from locally advanced disease | |
| Abou Youssif et al.27 | |||||||||
| elective | 2.7/ 10.5 | 5.8/ 103.9 | 40 | 0.94/ 28.3 | Spine | ||||
| elective | 2.7/ 10.5 | pT3aN0Mx; Clear cell; FG 3 | 4.5/ 48.5 | 29 | 0.75/ 15.8 | Pulmonary | nephrectomy at 26 months, mortality at 35 months | ||
| elective | 4.5×3.5/ 33.5 | pT1bNXMx; Papillary | 4.8×4.1/ 46.6 | 37 | 0.1/ 4.8 | Pancreas, nodal | nephrectomy at 13 months, developed metastasis at 37 months from diagnosis | ||
| Biesland et al.29 | |||||||||
| 81 | CHR | 6.2/ 126.9 | Mortality at 9 months from diagnosis | ||||||
| 82 | CHR | 6.7/ 160.2 | |||||||
| Crispen et al.3† | |||||||||
| 84 | M | CHR | 3×2.5/ 11 | 8×4/ 102.1 | 54 | 1.1/ 20.2 | Pulmonary | Hospice, mortality | |
| 70 | F | CHR | 3.2×3.1/ 16.6 | pT1bN2MX; Mixed clear cell and papillary; FG 4 | 4.8×4.3/ 51.1 | 63 | 0.3/ 6.6 | nodal | Partial nephrectomy and lymph node dissection revealed pT1bN2 disease. No adjuvant therapy, deceased |
| 54 | M | CHR | 2.7 | pT3a, N2, MX; Clear cell; FG 2 | 6.5×5.5.×4.2/ 80 | 38 | nodal | Alive, no adjuvant therapy | |
| Rosales et al.38 | |||||||||
| 2/ 4.3 | Papillary | 4.1/ 36.7 | 22* | 1.2/18 | |||||
| 3.1/ 15.9 | 3.8/ 29.2 | 22* | 0.4/7.4 | ||||||
| 5.1/ 70.7 | 6.1/ 120.9 | 22* | 0.6/27.9 | ||||||
| 7.2/ 198.8 | 8.7/ 350.7 | 22* | 0.83/84.4 | ||||||
| Jewett et al.46 | |||||||||
| 74 | F | Elective | 2.7×2.4×2.4/8.3 | Clear cell; FG 2 | 3.1×3×2.7/13.4 | 12 | 0.4/5.1 | Pulmonary | Alive on tyrosine kinase inhibitors |
| Totals (N=18) | |||||||||
| 75.1 | 4.1/ 66.4 | 5.9/ 132.1 | 40.2 | 0.8/ 27.1 | |||||
Maximum linear tumor diameter - MTD, Estimated tumor volume - ETV, Competing health risk – CHR, Fuhrman grade - FG
mean time to metastasis in this cohort was 22 months
patients included in cohort study pooled analysis
Comparing SRMs that progressed to metastatic disease (n=18) and those that did not (n=281), the duration of observation was similar between groups (40.2±31.2 vs. 33.3±22.6 months; p=0.47), but there was a significant difference in mean patient age (75.1±9.1 vs. 66.6±12.3 years; p=0.03). Trends in SRMs progressing to metastases (Table III) included larger tumors at diagnosis [initial MTD (4.1±2.1 vs. 2.3±1.3cm; p<0.001) and ETV (66.4±100.0 vs. 15.1±60.3 cm3; p<0.001)] as well as at the conclusion of observation [final MTD (5.9±2.1 vs. 3.0±1.6cm; p=<0.001) and ETV (132.1±170.9 vs. 29.0±109.7 cm3; p<0.001)]. Significant differences in both mean linear (0.80±0.7 vs. 0.30±0.4 cm/year; p<0.001) and volumetric growth rate (27.1±24.9 vs. 6.2±27.5 cm3/year; p<0.001) were also observed. Differences in patient age (p=0.03), initial MTD (p<0.001) and ETV (p=0.01), and volumetric growth rate (p=0.01) were confirmed using linear mixed effects models. Unadjusted point estimates were compared with adjusted estimates using fixed-effects components of the linear mixed effects models with only modest differences observed when model convergence was achieved.
Table III.
Comparison of clinical and cross sectional imaging characteristics in patients who did not progress to metastasis (pooled cohort series data) and patients that demonstrated evidence of progression (case series data) while under periods of observation
| Characteristic | Non-Progressors | Progressors | P Value | ||
|---|---|---|---|---|---|
| N | Mean + SD (median; range) | N | Mean + SD (median; range) | ||
| Age (years) | 230 | 66.6±12.3 (69; 35–88) | 9 | 75.1±9.1 (78; 54–84) | 0.03 |
| Initial MTD (cm) | 281 | 2.3±1.3 (2; 0.2–12) | 16 | 4.3±2.1 (3.1; 2–8.8) | <0.001 |
| Initial ETV (cm3) | 281 | 15.1±60.3 (4.3; 0.004–903.7) | 16 | 66.3±100.0 (15.2; 4.3–363) | <0.001 |
| Final MTD (cm) | 249 | 3.0±1.6 (2.7; 0.9–15) | 14 | 5.9±2.1 (5.9; 3.1–10.7) | <0.001 |
| Final ETV (cm3) | 281 | 29.0±109.8 (10.3; 0.3–1765.1) | 14 | 132.1±170.9 (87.9; 13.4–653) | <0.001 |
| Linear growth rate (cm/yr) | 249 | 0.4±0.3 (0.25; −1.4–2.47) | 13 | 0.80±0.7 (0.65; 0.1–2.72) | <0.001 |
| Volumetric growth rate (cm3/yr) | 281 | 6.2±27.5 (1.6; −20.0–430.7) | 14 | 27.1±24.9 (19.1; 4.8–84.4) | <0.001 |
| Time under AS (months) | 281 | 33.3±22.6 (27; 5.3–156) | 17 | 40.2±31.2 (29; 9–132) | 0.47 |
Discussion
Retrospective cohort studies demonstrating that localized renal tumors exhibit slow radiographic growth and a low metastatic potential30 have led to increased interest in expectant management as an alternative to surgery or ablation in select candidates. This has been supported by recent data suggesting that 20% of SRMs are benign10, 48 and only a relatively small subset of localized renal masses exhibit potentially aggressive features49. As tumor size increases, there appears to be a greater probability of malignant pathology, high grade disease, clear cell histology,50, 51 and the presence of synchronous metastases52.
Although a clear association between tumor size and risk of metastasis has not been determined, most reports utilize a 3cm threshold that has been extrapolated from clinical data in patients with von Hippel-Lindau syndrome53. Substantiating this threshold, Nguyen et al. estimated the risk of synchronous metastases to be <5% for tumors ≤3cm in 24,000 patients using SEER data52. Although there is emerging anecdotal data suggesting that cT1b-2 tumors may be judiciously observed for short periods in select patients with significant medical co-morbidity26, the biology of these lesions must be distinguished from the infrequent case of a localized mass with aggressive malignant potential that develops metachronous disease during a period of AS. Supporting the notion that all metastatic tumors start off as SRMs, 5 of 15 (33%) patients progressing ultimately to metastatic disease were <3cm at diagnosis; although all lesions were ≥3cm at the time metastatic disease was detectable.
Linear growth rate on sequential imaging studies is currently the most common metric for assessing malignant potential, and is based on the assumption that the tumor is spherical and growth occurs uniformly in all directions54. However, linear growth may not fully reflect the overall change in tumor volume, which may be a more accurate method of quantifying biologic growth55. Similar to previous reports2, our pooled analysis yielded a cumulative linear growth rate of 0.31 cm/year and a volumetric growth rate of 6.3 cm3/year. Theses data confirm that a substantial proportion of carefully selected SRMs under AS exhibit slow growth kinetics, with the caveat that these observations are limited by a lack of consistent pathologic data. While intuitive that lesions undergoing definitive treatment grew at faster rates then those remaining on surveillance (0.38 cm/year vs. 0.24 cm/year, p<0.001), these data are also confounded by lack of standardized criteria for intervention among contemporary AS protocols. Furthermore, a large proportion of patients (57%) proceeded to intervention despite the absence of pre-determined clinical or radiographic triggers.
The most important endpoint is progression to metastasis. While the proportion of lesions that progressed is small (2%), no discernible clinical or demographic predictors of tumor growth or metastasis were identified. Lesions that progressed demonstrated a rapid linear growth rate (mean 0.8cm/yr), and were predominantly high grade, clear cell histology, and ≥cT1b at the time of diagnosis, perhaps reflecting poor initial case selection or substantive competing risks56, 57. Importantly, a subset of tumors under surveillance exhibited no measurable growth over time (23%). When comparing lesions with zero and positive net growth, MTD at diagnosis was similar between groups, but of all the lesions exhibiting zero growth over time, there were no documented cases of metastatic progression. While the rate of malignancy in zero growth lesions appears similar to those of growing lesions at the time of surgical extirpation24 and biopsy proven benign lesions can grow at similar rates as malignant lesions39, 58, a positive growth rate may be the most accurate available predictor of potential for disease progression among readily available metrics. There has been one reported case of a 73 year old male with a 2.4cm renal mass progressing to bony metastases at 5 months with no increase in tumor size47. It is unclear from available data if this represents true clinical progression or occult undiagnosed systemic disease at the time of presentation and for this reason was not included in our progression cohort. This example also highlights the influence of patient selection with associated biases on our study’s findings. Inclusion of patients lost to follow up for prolonged periods and exclusion of patients with detectable systemic disease within 6 months inadvertently selects for less aggressive lesions with a more indolent course. While metastasis was generally a late event (>3 years), prospective data evaluation designed to minimize selection bias is necessary prior to the widespread integration of active surveillance into community practice.
One emerging clinical strategy is to initially observe select patients and intervene when specified size or growth rate criteria are met. To date there are no consistent AS protocol entrance criteria, and prospective comparisons are fraught with accrual challenges. Current recommendations suggest imaging with a consistent modality at defined intervals (3 to 6 months) which increase as stability of the lesion is demonstrated18. Patient age, indication for AS, radiation exposure, and risk of secondary malignancy must also be considered, and a transition to renal ultrasound or magnetic resonance imaging may be appropriate in select patients with stable growth kinetics. Importantly, patients must provide informed consent concerning the “calculated risks” of AS and understand that treatment may be necessary in the unlikely event their tumor exhibits rapid growth kinetics, a new or distant lesion, or clinical symptoms3, 18, 55. In an effort to match treatment to biology, we anticipate that renal mass biopsy will play a more prominent role in identifying suitable candidates for AS in the future. Percutaneous biopsy appears to be diagnostic in more than 80% with minimal risk of negative sequelae;59 although tumor undergrading60 and descriptive histology (for example, “oncocytic lesion”) remain problematic61. Biomarkers are required and ultimately may enhance the role of biopsy in management decisions on an individualized basis47, 62.
Conclusions
Our pooled analysis of carefully selected SRMs under AS demonstrates the indolent biology of localized tumors during an initial course of observation. 23% of these tumors exhibited zero radiographic growth, of which none metastasized. Eighteen lesions progressed to metastases with growth rates more than double (0.8cm/year) non-progressors (0.3cm/year), but these were generally late events (mean time to metastases 40.2 months). Of available characteristics, linear growth rate appears to be the most useful predictor of metastatic potential, but growth parameters and triggers for intervention must be more fully defined. Prospective randomized surveillance data are necessary but not forthcoming. In the absence of level I data, AS for localized solid renal masses should only be considered as an alternative to definitive therapy in select patients with limited life expectancy, competing health risks precluding surgery, or significant potential for requiring renal replacement therapy. Patients and clinicians must accept the calculated risks of surveillance of solid renal tumors and consider all treatment trade-off decisions. These provocative data may provide insight not only into the inherent biology of localized renal tumors, but perhaps other solid human malignancies.
Acknowledgments
“This publication was supported in party by grant number P30 CA006927 from the National Cancer Institute. Its contents are solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Additional funds were provided by Fox Chase Cancer via institutional support of the Kidney Cancer Keystone Program.”
References
- 1.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 2.Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175(2):425–31. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 3.Crispen PL, Viterbo R, Boorjian SA, Greenberg RE, Chen DY, Uzzo RG. Natural history, growth kinetics, and outcomes of untreated clinically localized renal tumors under active surveillance. Cancer. 2009;115(13):2844–52. doi: 10.1002/cncr.24338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 5.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281(17):1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 6.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–4. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 7.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51(2):203–5. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 8.Kutikov A, Fossett LK, Ramchandani P, Tomaszewski JE, Siegelman ES, Banner MP, et al. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006;68(4):737–40. doi: 10.1016/j.urology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Crispen PL, Boorjian SA, Lohse CM, Sebo TS, Cheville JC, Blute ML, et al. Outcomes following partial nephrectomy by tumor size. J Urol. 2008;180(5):1912–7. doi: 10.1016/j.juro.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 10.Remzi M, Ozsoy M, Klingler HC, Susani M, Waldert M, Seitz C, et al. Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J Urol. 2006;176(3):896–9. doi: 10.1016/j.juro.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67(2):254–9. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735–40. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59(6):816–20. doi: 10.1016/s0090-4295(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 15.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181(1):55–61. doi: 10.1016/j.juro.2008.09.017. discussion 61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179(2):468–71. doi: 10.1016/j.juro.2007.09.077. discussion 72–3. [DOI] [PubMed] [Google Scholar]
- 17.Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR, Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178(1):41–6. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–9. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma--a meta-analysis and review. J Urol. 2008;179(4):1227–33. doi: 10.1016/j.juro.2007.11.047. discussion 33–4. [DOI] [PubMed] [Google Scholar]
- 20.Kutikov A, Egleston BL, Wong YN, Uzzo RG. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol. 2010;28(2):311–7. doi: 10.1200/JCO.2009.22.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heuer R, Gill IS, Guazzoni G, Kirkali Z, Marberger M, Richie JP, et al. A critical analysis of the actual role of minimally invasive surgery and active surveillance for kidney cancer. Eur Urol. 2010;57(2):223–32. doi: 10.1016/j.eururo.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Crispen PL, Viterbo R, Fox EB, Greenberg RE, Chen DY, Uzzo RG. Delayed intervention of sporadic renal masses undergoing active surveillance. Cancer. 2008;112(5):1051–7. doi: 10.1002/cncr.23268. [DOI] [PubMed] [Google Scholar]
- 23.Crispen PL, Wong YN, Greenberg RE, Chen DY, Uzzo RG. Predicting growth of solid renal masses under active surveillance. Urol Oncol. 2008;26(5):555–9. doi: 10.1016/j.urolonc.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol. 2007;177(3):849–53. doi: 10.1016/j.juro.2006.10.073. discussion 53–4. [DOI] [PubMed] [Google Scholar]
- 25.Rendon RA, Stanietzky N, Panzarella T, Robinette M, Klotz LH, Thurston W, et al. The natural history of small renal masses. J Urol. 2000;164(4):1143–7. [PubMed] [Google Scholar]
- 26.Mues AC, Haramis G, Badani K, Gupta M, Benson MC, McKiernan JM, et al. Active Surveillance for Larger (cT1bN0M0 and cT2N0M0) Renal Cortical Neoplasms. Urology. 2010;76(3):620–3. doi: 10.1016/j.urology.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Abou Youssif T, Kassouf W, Steinberg J, Aprikian AG, Laplante MP, Tanguay S. Active surveillance for selected patients with renal masses: updated results with long-term follow-up. Cancer. 2007;110(5):1010–4. doi: 10.1002/cncr.22871. [DOI] [PubMed] [Google Scholar]
- 28.Abouassaly R, Lane BR, Novick AC. Active surveillance of renal masses in elderly patients. J Urol. 2008;180(2):505–8. doi: 10.1016/j.juro.2008.04.033. discussion 08–9. [DOI] [PubMed] [Google Scholar]
- 29.Beisland C, Hjelle KM, Reisaeter LA, Bostad L. Observation should be considered as an alternative in management of renal masses in older and comorbid patients. Eur Urol. 2009;55(6):1419–27. doi: 10.1016/j.eururo.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Bosniak MA, Birnbaum BA, Krinsky GA, Waisman J. Small renal parenchymal neoplasms: further observations on growth. Radiology. 1995;197(3):589–97. doi: 10.1148/radiology.197.3.7480724. [DOI] [PubMed] [Google Scholar]
- 31.Fernando HS, Duvuru S, Hawkyard SJ. Conservative management of renal masses in the elderly: our experience. Int Urol Nephrol. 2007;39(1):203–7. doi: 10.1007/s11255-006-9119-0. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto N, Sugita A, Terasawa Y, Kato M. Observations on the growth rate of renal cell carcinoma. Int J Urol. 1995;2(2):71–6. doi: 10.1111/j.1442-2042.1995.tb00427.x. [DOI] [PubMed] [Google Scholar]
- 33.Kato M, Suzuki T, Suzuki Y, Terasawa Y, Sasano H, Arai Y. Natural history of small renal cell carcinoma: evaluation of growth rate, histological grade, cell proliferation and apoptosis. J Urol. 2004;172(3):863–6. doi: 10.1097/01.ju.0000136315.80057.99. [DOI] [PubMed] [Google Scholar]
- 34.Kouba E, Smith A, McRackan D, Wallen EM, Pruthi RS. Watchful waiting for solid renal masses: insight into the natural history and results of delayed intervention. J Urol. 2007;177(2):466–70. doi: 10.1016/j.juro.2006.09.064. discussion 70. [DOI] [PubMed] [Google Scholar]
- 35.Lamb GW, Bromwich EJ, Vasey P, Aitchison M. Management of renal masses in patients medically unsuitable for nephrectomy--natural history, complications, and outcome. Urology. 2004;64(5):909–13. doi: 10.1016/j.urology.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzaki M, Kawano Y, Morikawa H, Shiga Y, Murata H, Komatsu H. Conservative management of small renal tumors. Hinyokika Kiyo. 2007;53(4):207–11. [PubMed] [Google Scholar]
- 37.Oda T, Miyao N, Takahashi A, Yanase M, Masumori N, Itoh N, et al. Growth rates of primary and metastatic lesions of renal cell carcinoma. Int J Urol. 2001;8(9):473–7. doi: 10.1046/j.1442-2042.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- 38.Rosales JC, Haramis G, Moreno J, Badani K, Benson MC, McKiernan J, et al. Active surveillance for renal cortical neoplasms. J Urol. 2010;183(5):1698–702. doi: 10.1016/j.juro.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 39.Siu W, Hafez KS, Johnston WK, 3rd, Wolf JS., Jr Growth rates of renal cell carcinoma and oncocytoma under surveillance are similar. Urol Oncol. 2007;25(2):115–9. doi: 10.1016/j.urolonc.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Sowery RD, Siemens DR. Growth characteristics of renal cortical tumors in patients managed by watchful waiting. Can J Urol. 2004;11(5):2407–10. [PubMed] [Google Scholar]
- 41.Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer. 2004;100(4):738–45. doi: 10.1002/cncr.20025. [DOI] [PubMed] [Google Scholar]
- 42.Wehle MJ, Thiel DD, Petrou SP, Young PR, Frank I, Karsteadt N. Conservative management of incidental contrast-enhancing renal masses as safe alternative to invasive therapy. Urology. 2004;64(1):49–52. doi: 10.1016/j.urology.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Wong JA, Rendon RA. Progression to metastatic disease from a small renal cell carcinoma prospectively followed with an active surveillance protocol. Can Urol Assoc J. 2007;1(2):120–2. doi: 10.5489/cuaj.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgenstern H. Uses of ecologic analysis in epidemiologic research. Am J Public Health. 1982;72(12):1336–44. doi: 10.2105/ajph.72.12.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–5. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 47.Jewett MA, Finelli A, Morash C, Chin JL, Siemens R, Tanguay S, et al. Active Surveillance of Small Renal Masses: A Prospective Multi-Center Canadian Uro-Oncology Group Trial: Abstract No. 896. J Urol. 2009;181(4 supplement):320. [Google Scholar]
- 48.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170(6 Pt 1):2217–20. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 49.Lane BR, Babineau D, Kattan MW, Novick AC, Gill IS, Zhou M, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178(2):429–34. doi: 10.1016/j.juro.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 50.Rothman J, Egleston B, Wong YN, Iffrig K, Lebovitch S, Uzzo RG. Histopathological characteristics of localized renal cell carcinoma correlate with tumor size: a SEER analysis. J Urol. 2009;181(1):29–33. doi: 10.1016/j.juro.2008.09.009. discussion 33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson RH, Kurta JM, Kaag M, Tickoo SK, Kundu S, Katz D, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol. 2009;181(5):2033–6. doi: 10.1016/j.juro.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen MM, Gill IS. Effect of renal cancer size on the prevalence of metastasis at diagnosis and mortality. J Urol. 2009;181(3):1020–7. doi: 10.1016/j.juro.2008.11.023. discussion 27. [DOI] [PubMed] [Google Scholar]
- 53.Duffey BG, Choyke PL, Glenn G, Grubb RL, Venzon D, Linehan WM, et al. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol. 2004;172(1):63–5. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 54.Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol. 1997;65(4):284–97. doi: 10.1002/(sici)1096-9098(199708)65:4<284::aid-jso11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 55.Mues AC, Landman J. Small renal masses: current concepts regarding the natural history and reflections on the American Urological Association guidelines. Curr Opin Urol. 2010;20(2):105–10. doi: 10.1097/MOU.0b013e32833625f8. [DOI] [PubMed] [Google Scholar]
- 56.Thompson RH, Hill JR, Babayev Y, Cronin A, Kaag M, Kundu S, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009;182(1):41–5. doi: 10.1016/j.juro.2009.02.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kunkle DA, Crispen PL, Li T, Uzzo RG. Tumor size predicts synchronous metastatic renal cell carcinoma: implications for surveillance of small renal masses. J Urol. 2007;177(5):1692–6. doi: 10.1016/j.juro.2007.01.029. discussion 97. [DOI] [PubMed] [Google Scholar]
- 58.Neuzillet Y, Lechevallier E, Andre M, Daniel L, Nahon O, Coulange C. Follow-up of renal oncocytoma diagnosed by percutaneous tumor biopsy. Urology. 2005;66(6):1181–5. doi: 10.1016/j.urology.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy--a renaissance? J Urol. 2008;179(1):20–7. doi: 10.1016/j.juro.2007.08.124. [DOI] [PubMed] [Google Scholar]
- 60.Dechet CB, Zincke H, Sebo TJ, King BF, LeRoy AJ, Farrow GM, et al. Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J Urol. 2003;169(1):71–4. doi: 10.1016/S0022-5347(05)64038-4. [DOI] [PubMed] [Google Scholar]
- 61.Crispen PL, Blute ML. Do percutaneous renal tumor biopsies at initial presentation affect treatment strategies? Eur Urol. 2009;55(2):307–9. doi: 10.1016/j.eururo.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 62.Uzzo RG. Renal masses--to treat or not to treat? If that is the question are contemporary biomarkers the answer? J Urol. 2008;180(2):433–4. doi: 10.1016/j.juro.2008.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]