Abstract
Objective
HAART largely decreases morbidity and mortality in chronic HIV-1-infected patients, but immune nonresponders (INRs) with full viral suppression still fail to reverse the immune deficiency. This study evaluated the safety and immunological responses of human umbilical cord mesenchymal stem cell (MSC) therapy in HIV-1-infected INRs.
Design and Methods
A total of 13 HIV-1-infected INRs were enrolled in this pilot prospectively open-labeled controlled clinical trial. Seven patients were administered three umbilical cord-MSC transfusions at 1-month interval during 12-months of follow-up, whereas six control patients were treated with saline in parallel. Immunological parameters were monitored in these patients throughout the trial.
Results
All patients tolerated the umbilical cord-MSC transfusions well throughout the trial. The umbilical cord-MSC transfusions preferentially increased circulating naive and central memory CD4 T-cell counts and restored HIV-1-specific IFN-γ and IL-2 production in the INRs. These enhancements in immune reconstitution were also associated with the reduction of systemic immune activation and inflammation in vivo.
Conclusions
umbilical cord-MSC transfusions are well tolerated and can efficiently improve host immune reconstitution in INRs, suggesting that such treatments may be used as a novel immunotherapeutic approach to reversing immune deficiency in HIV-1-infected INRs (ClinicalTrials.gov identifier: NCT01213186).
Keywords: clinical trial, HIV-1, immune reconstitution, mesenchymal stem cells, T cells
Introduction
Chronic HIV-1 infection is generally characterized by progressive CD4 T-cell depletion associated with increased immune activation and risk of opportunistic infections. HAART effectively suppresses viral replication, leading to a significant immune recovery and a dramatic reduction in the incidence of AIDS-defining events [1,2]. However, approximately 20% of individuals who exhibit stable viral suppression by HAART still fail to achieve sufficient immune reconstitution [3–5] and are considered immune nonresponders (INRs). These INRs often experience an increased risk of opportunistic infections [6,7] and shorter life expectancy compared with matched immune responders [8]. Therefore, efficiently treating these INR patients has become one of the most difficult challenges in the clinic.
The mechanism of immunopathogenesis in INRs remains unclear. It is generally believed that persistent viral replication, generalized immune overactivation and/or inflammation as well as the reduced thymic output [9], are the major factors leading to the failure of immune reconstitution. This paradoxical immune overactivation in the immune-deficient individuals generally includes elevated activation markers on immune cells [10], as well as elevated levels of serum proinflammatory cytokines [11], chemokines and bacterial products such as lipopolysaccharides (LPS) translocated from the gut [12]. Although these markers can be significantly reduced in these INR patients by HAART, they remain abnormal compared with healthy individuals [12], suggesting that immune reconstitution cannot be efficiently obtained by HAART alone in INR patients even with full viral suppression [10,13]. Indeed, no consensus has been reached regarding when or how to treat INR patients. Immune-based therapy such as interleukin (IL)-2 has been shown to increase CD4 T-cell counts but yielded no clinical benefit in a large randomized study [14]. IL-7 is also being tested, but its therapeutic effect is currently unclear [15,16]. Chloroquine treatment following HAART may decrease immune activation [17,18], but it was shown to significantly increase immune activation and viral load in HAART-naive HIV-1-infected patients [19]. Therefore, development of novel interventions to reduce immune overactivation/inflammation and enhance immune reconstitution in INRs is a high priority.
Mesenchymal stem cells (MSCs) can interact with multiple immune cells and suppress their activation, functions and release of proinflammatory cytokines in vitro and in vivo [20]. These properties have been used in clinical trials for graft-versus-host-disease [21] and appear effective in modulating the immune response in settings such as tissue injury, transplantation, autoimmunity and liver diseases [20]. Here, we postulate that MSC transfusions can potentially reduce HIV-1-induced immune overactivation and persistent inflammation and further enhance immune reconstitution in INR patients. We, therefore, conducted a pilot study specifically to assess the safety and efficacy of umbilical cord-MSC transfusions in INR patients. These results indicate that umbilical cord-MSC transfusions may provide a novel approach for attenuating aberrant activation of the immune system that can be used in combination with an efficient HAART for treating HIV-1-infected INRs.
Materials and methods
Patients and umbilical cord-mesenchymal stem cell transfusions
This prospective, open-labeled and controlled study was registered at ClinicalTrial.gov of the National Institutes of Health (NIH, Bethesda, Maryland, USA, registration number NCT01213186) and also authorized by the General Logistic Ministry of Health, China [registration number 2009(126)] and the Ethics Committee of Beijing 302 Hospital, Beijing, China. This study enrolled a total of 13 eligible HIV-1-infected adults who had CD4 T-cell counts less than 250 cells/µl and plasma HIV RNA loads less than 50 copies/ml for at least 6 month while receiving HAART for at least 12 months. The exclusion criteria included: a history of autoimmune disease, any malignancy, opportunistic infections and AIDS-defining tumors, pregnancy, and concomitant or previous treatment with interferons, anti-HIV vaccines, steroids or any other immunomodulators within the previous 12 months. Each patient provided written informed consent in accordance with the Institutional Review Board guidelines for the protection of humans. The 13 enrolled patients were randomized into the treated group with umbilical cord- MSC transfusions (n = 7) or control group receiving saline (n = 6) in parallel. The baseline clinical parameters were matched between the two groups (Table 1).
Table 1.
Baseline information on enrolled patients with HIV-1 infection.
| Cases | gender | Age (yr) |
Infection route |
Infection time (years) |
HAART time (months) |
HAART catergory |
CD4 T cells (cells/ml) | Baseline CD4/CD8 |
Baseline HIV-1 loads (copies/ml) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nadir | HAART onset | UC-MSC onset | ||||||||||
| UC-MSC | 1 | male | 26 | sex | 5 | 60 | 3TC+NVP+EFV | 38 | 38 | 100 | 0.13 | <50 |
| 2 | male | 41 | sex | 13 | 41 | ZDV+NVP+EFV | 13 | 13 | 46 | 0.04 | <50 | |
| 3 | male | 27 | sex | 6 | 12 | 3TC+ZDV+NVP | 18 | 18 | 152 | 0.18 | <50 | |
| 4 | female | 27 | blood | 6 | 24 | 3TC+ZDV+EFV | 14 | 14 | 224 | 0.41 | <50 | |
| 5 | male | 30 | sex | 7 | 15 | D4T+3TC+NVP | 120 | 151 | 118 | 0.14 | <50 | |
| 6 | male | 30 | sex | 3 | 19 | D4T+3TC+EFV | 35 | 64 | 136 | 0.22 | <50 | |
| 7 | female | 49 | blood | 14 | 38 | 3TC+ZDV+NVP | 100 | 190 | 223 | 0.39 | <50 | |
| Median | 30 | 6 | 24 | 35 | 38 | 136 | 0.14 | |||||
| Control | 8 | male | 40 | blood | 14 | 18 | 3TC+ZDV+NVP | 24 | 24 | 88 | 0.10 | <50 |
| 9 | female | 37 | blood | 7 | 15 | 3TC+ZDV+NVP | 95 | 110 | 164 | 0.21 | <50 | |
| 10 | male | 19 | blood | 13 | 33 | 3TC+ZDV+EFV | 35 | 109 | 210 | 0.25 | <50 | |
| 11 | male | 55 | sex | 6 | 37 | 3TC+ZDV+EFV | 21 | 21 | 199 | 0.21 | <50 | |
| 12 | male | 34 | sex | 5 | 16 | 3TC+ZDV+NVP | 228 | 228 | 241 | 0.13 | <50 | |
| 13 | male | 52 | blood | 14 | 40 | D4T+ZDV+NVP | 193 | 260 | 114 | 0.22 | <50 | |
| Median | 38 | 10 | 26 | 65 | 110 | 187 | 0.21 | |||||
| P value | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | ||||
3TC, lamivudine; D4T, stavudine; EFV, efavirenz; NVP, nevirapine; UC-MSC, umbilical cord mesenchymal stem cells; ZDV, zidovudine.
Maternal donors of fresh human umbilical cords after delivery also provided written consent. All of the donors were screened for the standard infectious agents including hepatitis B virus, hepatitis C virus, HIV and some other common infectious agents such as fungi, bacteria, mycoplasma and chlamydia. If tested positive for any of these infections, the umbilical cords from the donors were excluded from clinical usage. Umbilical cord-MSCs were prepared according to our previously described protocols [22]. In brief, the umbilical cord vessels were removed, and the mesenchymal tissue in Wharton’s jelly was diced into cubes, washed and finally seeded into a tissue culture flask. After 12–15 days of culture, the remnants of the cord fragments were removed, and the adherent cells were cultured generated and collected between the third and fourth passages. The collected umbilical cord-MSCs were resuspended and transfused intravenously (i.v.) into the patients at a dose of 0.5 × 106/kg body weight. Before use in transfusions, umbilical cord-MSCs were subjected to quality control tests, including evaluation of phenotypes, cytokine-producing profiles and the capacity for osteogenesis and adipogenesis. Additionally, umbilical cord-MSCs were tested for pathogens at every passage and prior to injection [22].
Safety and efficacy assessments
Umbilical cord-MSC or saline transfusions were administered three times to each individual on day 0, month 1 and month 2 (treatment period). The patients received follow-up for 12 months from the beginning of the study. During the treatment and follow-up period, all patients continued to receive HAART (unchanged regimen for each patient) (Supplemental Figure 1, http://links.lww.com/QAD/A320). The clinical safety in these enrolled individuals was assessed via an interim medical history and physical examination through the reporting of adverse events, such as the presence of fever, peripheral edema, rash, nausea and vomiting, as well as occurrence of opportunistic infections and tumors. All patients were regularly checked (month 3, 6, 9 and 12 during follow-up) for liver function, routine blood tests and virological and immunological parameters. Our primary aim was to evaluate the events related to safety as mentioned above and the changes from baseline in CD4 T-cell counts.
Antibodies and reagents
All antibodies were purchased from commercial sources as listed in Supplemental Table 1, http://links.lww.com/QAD/A320. Three PE-conjugated major histocompatibility complex class I pentamer complexes loaded with the HIV-1 gag p17 (77–85, SLYNTVATL; restricted by HLA-A0201), HIV-1 nef (RL9, RYLKDQQLL; restricted by HLA-A2402) or cytomegalovirus (CMV, pp65 495–503; NLVPMVATV, NV9; restricted by HLA-A0201) epitope (ProImmune, Oxford, UK) were used to stain for HIV-1-specific or CMV-specific T cells.
Flow cytometric analysis of immune parameters
CD3, CD4 and CD8 T-cell counts of fresh whole blood were performed by using BD Trucount Tubes (Cat No: 340334; BD Biosciences, San Jose, California, USA) on a FACSCalibur. The PBMCs were isolated and cryopreserved from an additional 10 ml of peripheral blood from these patients. At the end of the follow-up, PBMCs preserved at various time-points from the same patient were thawed simultaneously to perform more detailed immunological assays. The samples were immunostained and analyzed using a four-color FACSCalibur cytometer (BD Biosciences) and FlowJo software (SarsTree; Supplemental Table 13, http://links.lww.com/QAD/A320). In brief, the percentages of B cells (defined as CD19+ cells), myeloid dendritic cell (mDC, defined as Lin-1−CD11c+ HLA-DR+ cells) and plasmacytoid dendritic cell (pDC, defined as Lin-1−CD123+HLA-DR+ cells) subsets, regulatory T cells (defined as CD3+CD4+CD25+ CD127− cells), NK cells (defined as CD3−CD56+ cells), NK T cells (defined as CD3+CD56+) and γδT cells (defined as CD3+γδTCR+ cells) were detected. In addition, expression of PD-1 and B and T-lymphocyte-associated antigen (BTLA) (costimulatory molecules), CD38 and HLA-DR (activation markers), Ki67 (proliferation marker), CD31 (thymic newly-out-produced marker), CD57 (replicative senescence marker) and CD45RA and CD27 (memory markers; naive T cells, CD27+CD45RA+; central memory T cells, CD27+CD45RA−; effector memory T cells, CD27−CD45RA−; terminally differentiated effector T cells, CD27−CD45RA+) on T cells were comprehensively characterized using a combination of conjugated monoclonal antibodies. PD-1 and CD38 expression on HIV-1 and CMV-specific pentamer+ cells were also analyzed. The absolute numbers for each cell population were generated by multiplying the proportion of the subset by the absolute number of lymphocytes or T cells.
Enzyme-linked immunospot assays
Enzyme-linked immunospot (ELISpot) assays were performed precisely as outlined in the manufacturer’s protocol. Briefly, the 96-well plate was incubated with coating antibody overnight at 4–8°C. PBMCs were then added at a concentration of 200 000 cells/well (for IFN-γ assay) or 500 000 cells/well (for IL-2 assay) in the plates. HIV-1 gag-1, gag-2 and nef peptide pools and CMV pp65 peptide pool (NIH, AIDS Research and Reference Reagents Program) were added directly to the wells at a final concentration of 1 µg/ml. Phytohemagglutinin stimulation served as a positive control, and unstimulated cells were negative controls. After incubation for 16–18 h at 37°C, 5% CO2, the plates were washed, labeled with 1 µg/ml biotin-labeled anti-IFN-γ (Cat No. 3420–2H) or anti-IL-2 (Cat No, 3440–2H; MabTech, Nacka Strand, Sweden), and then developed by incubation with streptavidin-HRP, followed by the substrate solution. Responses were considered positive if the number of spots per well minus the background was at least 50 SFC/106 PBMCs with a background of fewer than 15 SFC/106 PBMCs. All HIV-1-specific responses ex vivo were evaluated at least in duplicate.
Luminex and ELISA
The plasma C-reactive protein (CRP), D-dimer, cystanin, LPS and total IgG were detected using ELISA. Other cytokines were detected using a Luminex Bio-Plex ProTm Human Cytokine Standard Group I 27-Plex (Cat No: 5021099, including IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, eotaxin, basic FGF, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, regulated upon activation normal T-cell expressed and secreted (RANTES), TNF-α and VEGF) and IFN-α2 on the Luminex 100 System (BioRad, Hercules, California, USA) according to the manufacturer’s protocol.
Statistical analysis
All data were analyzed using SPSS 13.0 for Windows software (SPSS Inc., Chicago, Illinois, USA). Multiple comparisons were made among the different groups using the Kruskal–Wallis test for nonparametric data. Comparisons between various individuals were performed using the Mann–Whitney U test. Comparisons between parameters in the same individual were performed using the Wilcoxon matched pairs t test. For all tests, two-sided P values less than 0.05 were considered to be significant.
Results
Safety and tolerability of umbilical cord-mesenchymal stem cell transfusions in HIV-1-infected patients
For patients receiving umbilical cord-MSC treatment in the study, no short-term clinical adverse effects, including pain, pruritus (skin rash), infection, coma or shock were observed. Only one patient developed a self-limiting fever (37–38°C) within 4 h after the umbilical cord-MSC transfusion. No HIV-1 load rebound or significant rises in liver function enzymes, granulocytes and red blood cells were observed throughout the study (Supplemental Table 2, http://links.lww.com/QAD/A320). In addition, no opportunistic infections or AIDS-defining tumors were observed in patients with umbilical cord-MSC treatment throughout the trial period. Antiretroviral regimens or compliance issues with antiretroviral drugs were not changed for these patients during our clinical trial. These observations indicate that umbilical cord-MSC transfusions were clinically and biologically well tolerated in HIV-1-infected patients.
Umbilical cord-mesenchymal stem cell transfusions induce a significant increase of CD4 T cells in immune nonresponders
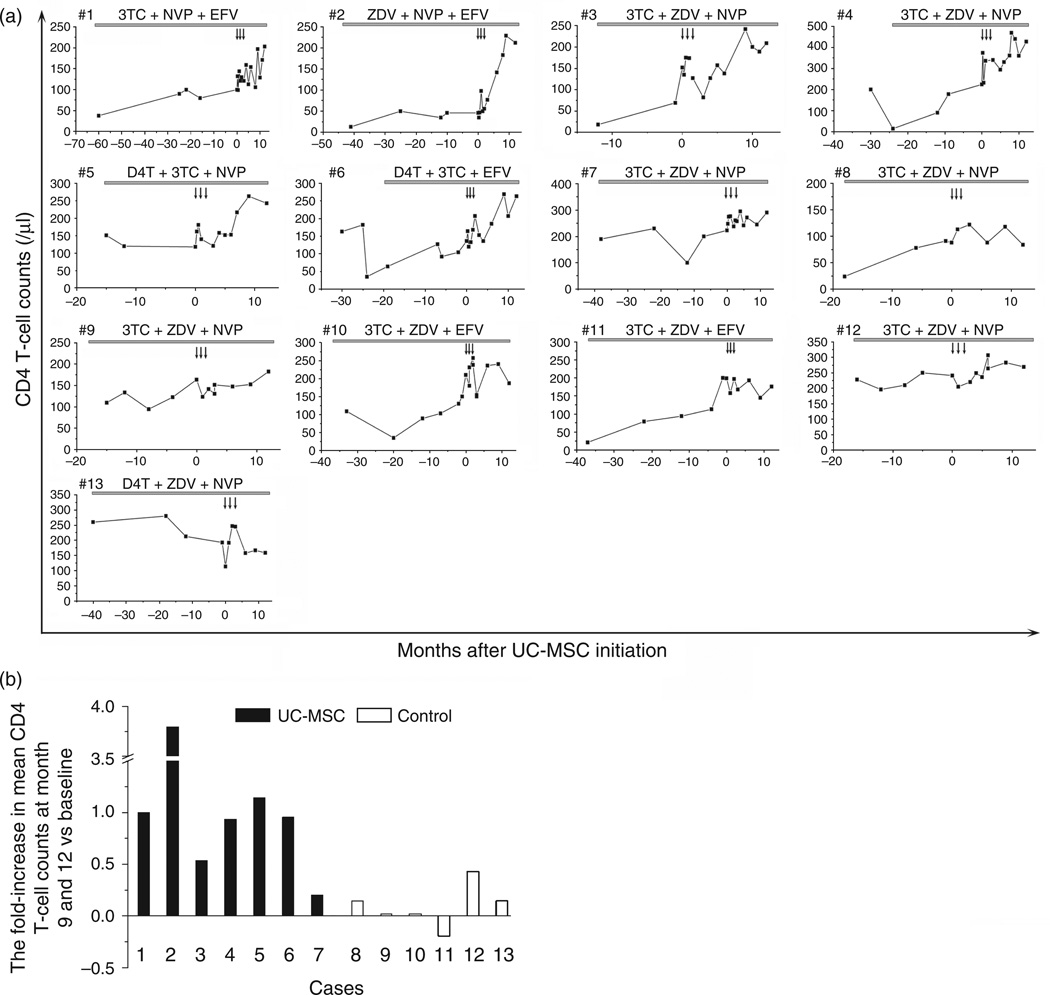
Through a comprehensive analysis of CD4 T-cell counts, we found that long-term HAART (>12 months) only slightly increased or maintained CD4 T-cell counts at low levels in the 13 INR patients. After umbilical cord-MSC transfusions, six patients (Pt1–Pt6) displayed a sharp increase in CD4 T-cell counts (Fig. 1a). Further analysis indicated that these six patients had a more than 50% increase of CD4 T-cell counts at months 9 and 12 after starting umbilical cord-MSC therapy compared with baseline values. By contrast, no control patients displayed such an increase in CD4 T-cell counts during the observation period (Fig. 1a and b). Thus, the umbilical cord-MSC treatment significantly increased CD4 T-cell counts and CD4/CD8 ratio after 6 month of treatment compared with the individual baseline data as well as with controls (Supplemental Figure 2B and 2D, http://links.lww.com/QAD/A320). Meanwhile, no significant alterations in counts of CD3 and CD8 T cells (Supplemental Figure 2A and 2C, http://links.lww.com/QAD/A320), CD19+ B cells, CD3−CD56+ NK cells, CD3+CD56+NK T cells, Lin-1−HLA-DR+CD11c+ mDCs, Lin-1−HLA-DR+CD123+ pDCs and γδT cells (Supplemental Figure 3, http://links.lww.-com/QAD/A320) were observed in the treatment and control patients throughout the study period.
Fig. 1. Longitudinal changes of peripheral CD4 T-cell counts in 13 enrolled patients before and after treatment.
(a) CD4 T-cell counts. (b) Fold-increase in CD4 T-cell counts relative to baseline value at months 9 and 12. umbilical cord-mesenchymal stem cell (MSC) transfusions were initiated at month 0. Arrows in panel (a) indicate time-points of umbilical cord-MSC transfusions.
Naive and central memory CD4 T-cell populations are preferentially increased in patients with umbilical cord-mesenchymal stem cell treatment
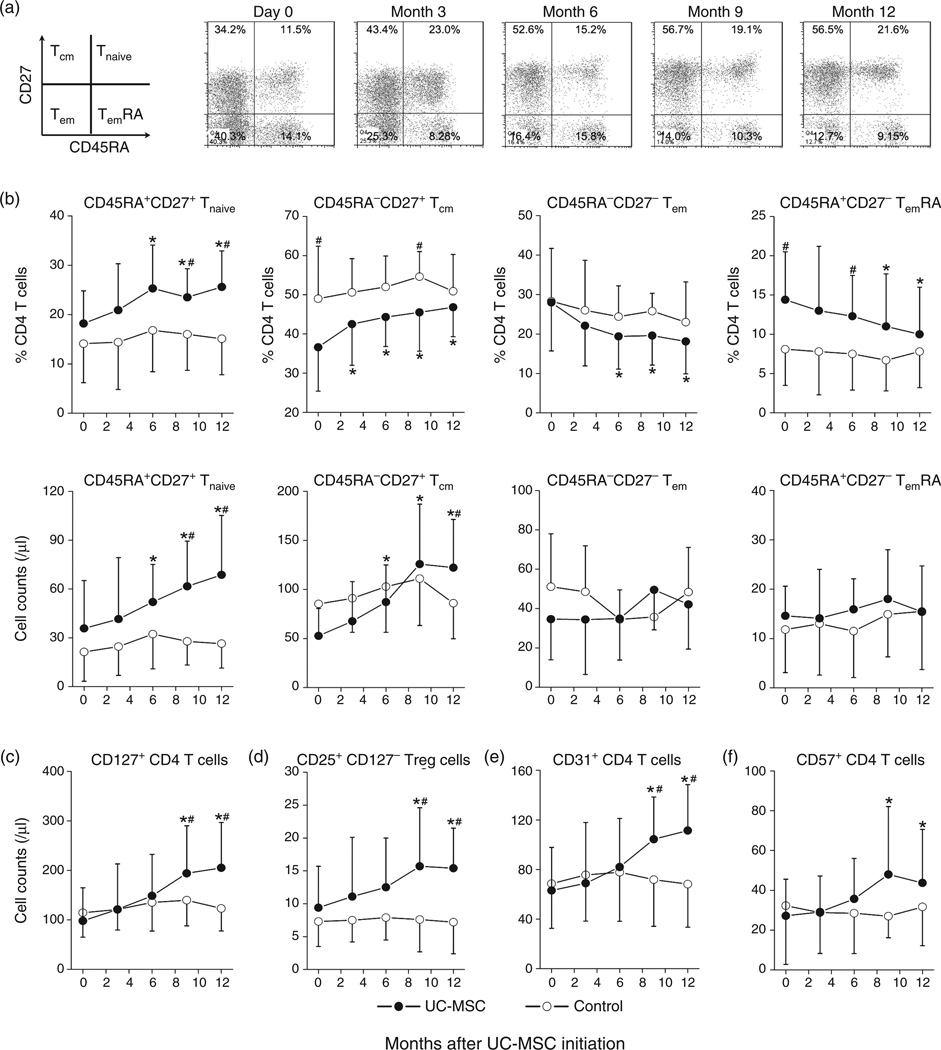
We further analyzed changes in naive (Tnaive), central memory (Tcm), effector memory (Tem) and terminally differentiated effector (TemRA) T-cell subsets using CD45RA and CD27 markers and found that the percentages of Tnaive and Tcm subsets were gradually increased, whereas the Tem and TemRA subsets were gradually decreased by umbilical cord-MSC treatment (Fig. 2a). The pooled data also confirmed these observations (Fig. 2b, top). Accordingly, umbilical cord-MSC therapy preferentially expanded CD4 Tnaive and Tcm cell counts but not Tem and TemRA subsets as compared with baseline (P < 0.05 at months 6, 9 and 12) and control patients (P < 0.05 at months 9 and 12; Fig. 2b, bottom).
Fig. 2. Preferential increases of specific CD4 T-cell subsets in INR patients undergoing umbilical cord mesenchymal stem cell therapy.
(a) Representative dot plots show the alteration of memory CD4 T-cell populations throughout the study in an INR patient receiving umbilical cord-mesenchymal stem cell (MSC) transfusions. Numbers within the plots indicate the percentage of each T-cell population: naive (Tnaive, CD45RA+CD27+), central memory (Tcm; CD45RA−CD27+), effector memory (Tem, CD45RACD27−), terminally differentiated effector (TemRA; CD45RA+CD27−). (b) Pooled data of percentages and absolute counts of memory CD4 T-cell subsets in treatment and control patients. (c–f) Pooled data of absolute counts of CD127+ CD4 T cells (c), CD25+CD127− regulatory CD4 T cells (d), CD31+ CD4 T cells or recent thymic emigrants (e) and CD57+ senescent CD4 T cells (f) over time in umbilical cord-MSC-treated patients (dots) and control patients (circles). Means ± SD for each cohort are shown. *P < 0.05 vs. baseline data, Wilcoxon paired t test. #P < 0.05 vs. control; Mann–Whitney U test. umbilical cord-MSC transfusions were initiated at month 0.
We also investigated the dynamics of CD127 (IL-7Rα) expression on expanded CD4 T cells throughout the study, as the decrease of CD127 expression is closely associated with T-cell apoptosis and disease progression in HIV-1 infection [23,24]. Umbilical cord-MSC-treated patients were found to exhibit a two-fold increase in circulating CD127+ CD4 T cells at months 9 and 12 after starting umbilical cord-MSC transfusion therapy (both P < 0.01 vs. baseline, Fig. 2c). Additionally, the counts of CD4+CD25+CD127− regulatory T cells, CD31+ CD4 T cells (new thymic emigrants) and CD57+ CD4 T cells were also significantly increased at months 9 and 12 in umbilical cord-MSC-treated patients compared with baseline data and control patients (Fig. 2d and e). In contrast, control patients exhibited no significant alterations of these T-cell subsets throughout the study (Fig. 2d–f).
Umbilical cord-mesenchymal stem cell therapy increases T-cell function in immune nonresponder patients
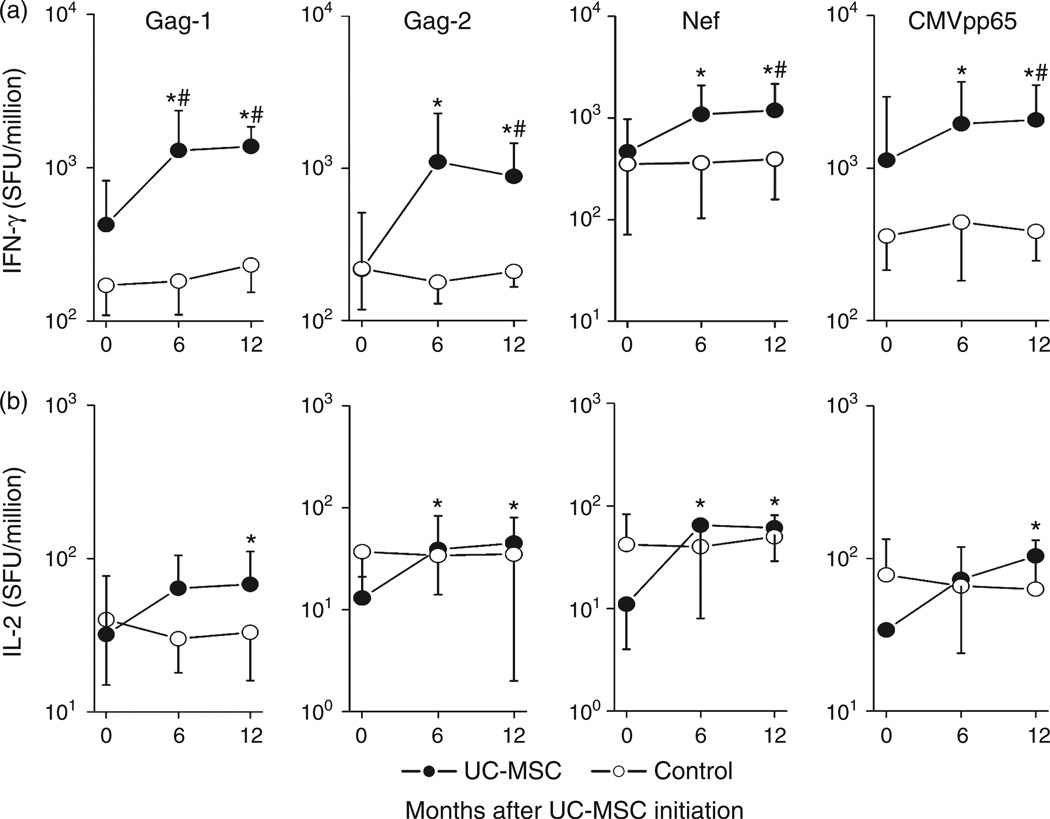
We also evaluated IFN-γ and IL-2 production of CD4 T cells in response to HIV-1 antigen stimulation in vitro using ELISpot assays. As shown in Fig. 3a and b, in response to peptide pools of HIV-1 gag-1, gag-2 and CMV pp65, IFN-γ and IL-2 spot-forming cells (SFCs) were significantly increased in patient PBMCs at month 6 and/or 12 after umbilical cord-MSC transfusions. In contrast, these SFCs were not increased in control patient PBMCs. Notably, the numbers of IFN-γ SFCs were significantly higher for umbilical cord-MSC-treated patients than for control patients at the 6–12 month time-points in response to the peptide pools (Fig. 3a).
Fig. 3. Effects of umbilical cord-mesenchymal stem cell therapy on HIV-1-specific T-cell functions.
Pooled data of the counts of IFN-γ (a) and IL-2 (b) SFCs per million peripheral blood mononuclear cells upon stimulation with HIV-1 gag1, gag2, nef and CMVpp65 peptide pools in seven umbilical cord-mesenchymal stem cell (MSC)-treated patients (dots) and six control patients (circles). *P < 0.05 vs. baseline data in treated patients, Wilcoxon paired t test. #P < 0.05 vs. control, Mann-Whitney U test. umbilical cord-MSC transfusions were initiated at month 0.
Effects of umbilical cord-mesenchymal stem cell therapy on expression and immune activation of coinhibitory molecules
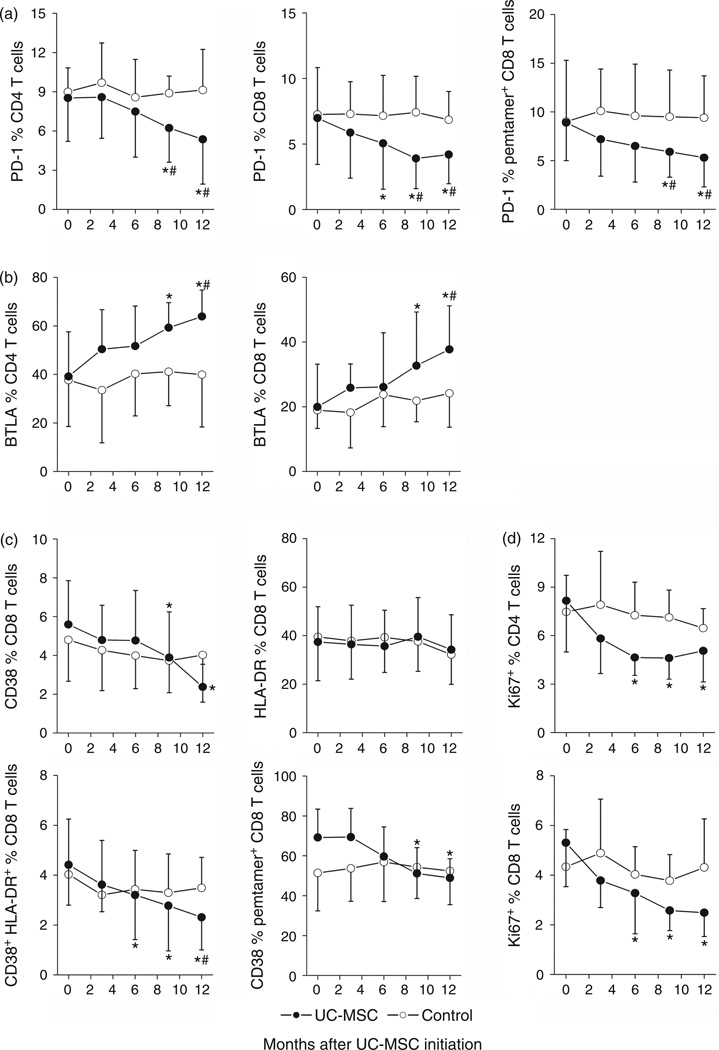
The upregulation of programmed cell death 1 (PD-1) [25] and the downregulation of BTLA [26] have been associated with HIV-specific T-cell exhaustion and T-cell immune overactivation in HIV-1 infection, respectively. Significantly decreased PD-1 expression on total CD4, CD8 T cells and on HIV-1-specific pentamer+ CD8 T cells at months 6, 9 and 12 (P < 0.05, Fig. 4a), and significantly increased BTLA expression levels on total CD4 and CD8 T cells were at months 9 and 12 (P < 0.05, Fig. 4b) were found after starting umbilical cord-MSC transfusions as compared with baseline and control patients. No significant change in PD-1 and BTLA expression was observed in control patients.
Fig. 4. Effects of umbilical cord mesenchymal stem cell therapy on co-inhibitory molecules and activation markers on CD8 T cells.
umbilical cord-mesenchymal stem cell (MSC) transfusions significantly downregulated PD-1 expression (a), upregulated BTLA expression (b), and reduced CD38 and HLA-DR (c) and Ki67 (d) expression on total CD4 and CD8 T cells as well as HIV-1-specific pentamer+ CD8 T cells in umbilical cord-MSC-treated patients (dots) and control patients (circles). Means ± SD for each cohort are shown. *P < 0.05 vs. baseline data, Wilcoxon paired t test. #P < 0.05 vs. control, Mann–Whitney U test. Umbilical cord-MSC transfusions were initiated at month 0.
Residual immune overactivation still persists in HIV-1-infected INRs despite control of viral load under HAART [10,11]. We, therefore, evaluated the effect of umbilical cord-MSC transfusions on CD8 T-cell activation markers such as CD38 and HLA-DR (Fig. 4c). Significant decrease in the percentages of CD38+ and CD38+HLA-DR+ CD8 T cells as well as HIV-1-specific CD38+pentamer+ CD8 T cells were observed at months 6, 9 and 12 after the start of umbilical cord-MSC treatment. Percentages of these activated CD8 T cells did not change significantly in control patients (Fig. 4c). Consistent with reduced immune activation, the Ki67 expression on CD4 and CD8 T cells, which also reflect immune overactivation during HIV-1 infection, was also significantly reduced by the umbilical cord-MSC transfusions (Fig. 4d). These data suggest that umbilical cord-MSC treatment may rectify the biased T-cell expression of coinhibitory molecules and significantly reduces the residual immune activation levels of T cells in INR patients.
Effects of umbilical cord-mesenchymal stem cell transfusions on systematic inflammation
We investigated the impact of umbilical cord-MSC transfusions on the systemic inflammatory components such as plasma inflammatory proteins, cytokines and chemokines, which are usually elevated due to long-term immune activation during chronic HIV-1 infection [12]. Umbilical cord-MSC treatment was found to have significantly decreased plasma CRP levels and LPS levels at months 6 and 12 after initiating therapy as compared with the control group (P < 0.05). Meanwhile, levels of plasma D-dimer, cystatin and total IgG, which have been identified as markers of immune overactivation and inflammation in HIV-1-infected patients [11,27], were maintained at stable levels throughout the study in these INR patients (Supplemental Figure 4A, http://links.lww.com/QAD/A320).
Umbilical cord-MSC therapy also significantly reduced plasma levels of proinflammatory cytokines IFN-α2 (month 6), TNF-α (month 6), IL-1ra (month 6 and 12), IL-12 p70 (month 6 and 12), IL-6 (month 6 and 12), IFN-γ (month 6), IL-9 (month 6) (Supplemental Figure 4B, http://links.lww.com/QAD/A320), as well as chemokines MIP-1β (month 6 and 12), IP-10 (month 6 and 12), IL-8 (month 6 and 12), MCP-1 (month 6 and 12) and RANTES (month 6 and 12) (Supplemental Figure 4C, http://links.lww.com/QAD/A320) and growth factors G-CSF (month 6 and 12), PDGF-BB (month 6) and VEGF (month 6 and 12) levels (Supplemental Figure 4D, http://links.lww.com/QAD/A320) in these INR patients. No significant changes of the aforementioned markers were observed in the control patients throughout the study.
Discussion
In HAART-treated HIV-1-infected patients, long-term full viral suppression is not always associated with immune reconstitution and reversion of immune overactivation [10–13], suggesting that the development of complementary approaches to temper immune overactivation may enhance immune reconstitution. This prospective study demonstrates, for the first time, that intravenous umbilical cord-MSC transfusions are well tolerated and have the potential to reduce inflammation and immune overactivation, as well as to restore functional T cells in these INR patients.
The present study provides the following lines of evidences to support the notion that umbilical cord-MSC transfusions can inhibit the systematic immune overactivation and/or inflammation in INR patients: the umbilical cord-MSC treatment significantly reduced the proportion of total activated CD8 T cells expressing CD38, HLA-DR or Ki67 in the INR patients; the expression of BTLA on T cells, a marker associated with T-cell immune overactivation in HIV-1 infection [26], was significantly restored in patients treated with umbilical cord-MSC; the systemic inflammatory indicators, such as high levels of proinflammatory cytokines (IFN-α2, IL-12p70, IL-6, IL-1ra and TNF-α), chemokines (MIP-1β, IP-10, IL-8, RANTES and MCP-1) and growth factors (G-CSF and VEGF), were decreased by umbilical cord-MSC therapy in these INR patients. Thus, umbilical cord-MSC treatment may efficiently resolve the difficult-to-treat challenges such as persistent immune overactivation and/or inflammation in INR patients.
The mechanisms of umbilical cord-MSC treatment decreasing immune overactivation and/or inflammation are unclear, but it may involve increased regulatory T cells and reduced microbial translocation. As the depletion or inactivation of regulatory T cells may result in immune overactivation during chronic HIV-1 infection [28,29], their increase by umbilical cord-MSC therapy is likely to reduce immune overactivation in the INR patients. This finding is similar to a previous report in lupus patients who also showed elevated circulating regulatory T cells after bone marrow MSC treatment [30]. In addition, chronic HIV-1 infection often increases gut mucosal permeability and microbial translocation from the gut, as reflected by the increased LPS levels in plasma [31]. Notably, the umbilical cord-MSC transfusions also significantly decreased PD-1 expression and plasma LPS levels in the INR patients, suggesting that it may improve the gut mucosa permeability through reducing hyperactivation and microbial translocation [32]. In addition, MSC-derived IL-7 [22] may also be involved in the immune reconstitution [15,16]. Future studies should address whether umbilical cord-MSC can repair the damaged mucosa and further reduce immune overactivation or selectively induce the apoptosis of activated T cells [33] in treated patients.
An important outcome of the reduction of immune overactivation achieved by umbilical cord-MSC transfusions herein is a significant increase of circulating CD4 T-cell counts in INR patients. Importantly, the increase of CD4 T-cell counts is also likely aided by the restoration of a thymopoietic pathway of T-cell regeneration in INR patients treated with umbilical cord-MSCs. Our findings support this concept in that the umbilical cord-MSC treatment preferentially increased naive and central memory CD4 T-cell counts as well as CD127+ early memory T cells, which are usually functionally exhausted during HIV-1 infection [34], and significantly expanded recent thymic emigrants CD31+ CD4 T cells [35]. These observations, in line with other studies in patients with multiple sclerosis receiving autologous stem cell transplantation [36] and an animal model accepting hematopoietic stem cells [37], suggest that increased thymopoiesis after stem cell transplantation can correct the preexisting deficiency and normalize naive T-cell homeostasis. These changes may also represent a restoration in the regenerative potential of the immune system during umbilical cord-MSC therapy.
Interestingly, the CD4 T cells expanded in the INR patients by umbilical cord-MSC transfusion displayed functional improvement in HIV-1-specific IFN-γ and IL-2 production in vitro. This functional recovery of T cells is possibly associated with PD-1 downregulation, as the upregulation of PD-1 has been demonstrated to contribute to HIV-1-specific T-cell exhaustion [38–40]. Future studies should define the expression profiles of other coinhibitory molecules on T cells such as CTLA-4 and Tim-3, as well as their influences on T-cell functions after umbilical cord-MSC transfusion.
Finally, the study shows that umbilical cord-MSC transfusions are well tolerated and feasible in HIV-1-infected patients. No significant side effects or complications were found throughout the trial. Plasma HIV-1 RNA levels were not noted to rebound throughout the follow-up period. These findings, therefore, demonstrate that umbilical cord-MSC transfusions can be safely used in the clinic for enhancing immune reconstitution in INR patients. It should be noted that the localization of the infused umbilical cord-MSC and the histological alterations of lymph nodes and gut mucosa in the studied patients were not analyzed here, since this exploratory trial was primarily aimed towards testing the safety and initial efficacy of umbilical cord-MSC transfusions in HIV-1-infected patients. Notably, the enhanced immune reconstitution in these patients with three times of MSC infusions could be obtained and still maintained throughout a more than 150-week follow-up check period (unpublished data). Meanwhile, we did not find that there was a significantly transitory increase in peripheral CD4 T-cell counts within 1–2 weeks since the onset of each MSC infusion. More important, umbilical cord-MSCs were found to be with a potential to produce IL-7 and TGF-β in vitro and in vivo [33] and preferentially expand CD4 T-cell response in the recipients. Therefore, our findings may support the notion that the observed immune reconstitution is mainly dependent on the immune modulation of the infused MSCs during repeated infusions of MSCs. However, whether the MSC transfusions in treated patients induce an allogenetic effect or production of associated antibodies in vivo remains unclear in the present study, Thus, future study is needed to examine these important issues.
In conclusion, umbilical cord-MSC treatment is safe and can significantly decrease systemic immune overactivation and improve immune reconstitution in INR patients, thus providing a promising novel therapeutic approach for INR patients in combination with HAART. Future large-scale randomized controlled studies should focus not only on perfecting the umbilical cord-MSC treatment regimen, including dose and treatment intervals and times, but also on uncovering the relevant mechanisms of host immune reconstitution for the disease.
Supplementary Material
Acknowledgements
Z.Z. designed, performed research, analyzed data, wrote article; J.F., X.X., J.Z. performed research; J.F., S.W. collected samples; R.X. UC-MSC preparation and quality control; M.Z.,W.N., X.W., T.L. were responsible for clinical supervision of patients; L.S. designed animal experiments and wrote article; F.S.W. coordinated, designed, and performed research, wrote article.
We thank Wang SS, Zhou CB, Yuan Y, Du ML and Wu X for excellent technical support and all patients, study-site staff, and all participating consultants for their contributions, which made this study possible.
This work was supported by the National Grand Program on Key Infectious Disease (2013ZX10001002–001–002, 2012ZX10001006–003–003, 2012ZX10001003–004–008), National Science Fund for Outstanding Young Scholars (81222024) and the National Key Basic Research Program of China (2012CB519005, 2009CB522507).
Footnotes
Conflicts of interest
The authors have no financial conflict of interest.
References
- 1.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 3.Vo TT, Ledergerber B, Keiser O, Hirschel B, Furrer H, Battegay M, et al. Durability and outcome of initial antiretroviral treatments received during 2000–2005 by patients in the Swiss HIV Cohort Study. J Infect Dis. 2008;197:1685–1694. doi: 10.1086/588141. [DOI] [PubMed] [Google Scholar]
- 4.Zhang F, Dou Z, Yu L, Xu J, Jiao JH, Wang N, et al. The effect of highly active antiretroviral therapy on mortality among HIV-infected former plasma donors in China. Clin Infect Dis. 2008;47:825–833. doi: 10.1086/590945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–794. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit C, Geskus R, Walker S, Sabin C, Coutinho R, Porter K, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 8.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T, Wu N, Dai Y, Qiu Z, Han Y, Xie J, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis. 2011;53:944–951. doi: 10.1093/cid/cir552. [DOI] [PubMed] [Google Scholar]
- 10.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 11.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appay V. Sauce D Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 13.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 14.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55:291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray SM, Down CM, Boulware DR, Stauffer WM, Cavert WP, Schacker TW, et al. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol. 2010;84:12082–12086. doi: 10.1128/JVI.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, ART-treated, immunological non-responders. Blood. 2011;118:3263–3272. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 19.Paton NI, Goodall RL, Dunn DT, Franzen S, Collaco-Moraes Y, Gazzard BG, et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uccelli A, Moretta L. Pistoia V Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 21.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(Suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 23.Bai F, Bellistri GM, Tincati C, Savoldi A, Pandolfo A, Bini T, et al. Reduced CD127 expression on peripheral CD4+ T cells impairs immunological recovery in course of suppressive highly active antiretroviral therapy. AIDS. 2010;24:2590–2593. doi: 10.1097/QAD.0b013e32833f9d64. [DOI] [PubMed] [Google Scholar]
- 24.Zhang SY, Zhang Z, Fu JL, Kang FB, Xu XS, Nie WM, et al. Progressive CD127 down-regulation correlates with increased apoptosis of CD8 T cells during chronic HIV-1 infection. Eur J Immunol. 2009;39:1425–1434. doi: 10.1002/eji.200839059. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann DE. Walker BD Programmed death-1 as a factor in immune exhaustion and activation in HIV infection. Curr Opin HIV AIDS. 2008;3:362–367. doi: 10.1097/COH.0b013e3282f9ae8b. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Xu X, Lu J, Zhang S, Gu L, Fu J, et al. B and T lymphocyte attenuator down-regulation by HIV-1 depends on type I interferon and contributes to T-cell hyperactivation. J Infect Dis. 2011;203:1668–1678. doi: 10.1093/infdis/jir165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xing S, Fu J, Zhang Z, Gao Y, Jiao Y, Kang F, et al. Increased turnover of FoxP3high regulatory T cells is associated with hyperactivation and disease progression of chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2010;54:455–462. doi: 10.1097/QAI.0b013e3181e453b9. [DOI] [PubMed] [Google Scholar]
- 29.Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, Daucher M, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 32.Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, et al. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest. 2012;122:1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akiyama K, Chen C, Wang DD, Xu X, Qu C, Yamaza T, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douek DC, Picker LJ. Koup RA T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 35.Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201:805–816. doi: 10.1084/jem.20041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanchot C, Le Campion A, Martin B, Leaument S, Dautigny N. Lucas B Conversion of naive T cells to a memory-like phenotype in lymphopenic hosts is not related to a homeostatic mechanism that fills the peripheral naive T cell pool. J Immunol. 2002;168:5042–5046. doi: 10.4049/jimmunol.168.10.5042. [DOI] [PubMed] [Google Scholar]
- 38.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 39.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.