Abstract
An initial reflexive constriction of the pupil to stimulation – the light reflex – is primarily modulated by brightness, but is attenuated when participants are under threat of shock (e.g. "fear-inhibited light reflex").The present study assessed whether the light reflex is similarly attenuated when viewing emotional pictures. Participants viewed erotic, violent, and neutral scenes that were matched in brightness; as an additional control, scrambled versions identical in brightness were also presented. Compared to viewing neutral scenes, the light reflex was reliably modulated by hedonic content, with significant attenuation both when viewing unpleasant, as well as pleasant, pictures. No differences in the light reflex were found among scrambled versions. Modulation of the initial light reflex is therefore not confined to a context of fear, and also is not indicative of differences in brightness when viewing pictures of natural scenes.
Pupil diameter during visual perception is modulated by a number of different perceptual and psychological variables: The initial light reflex is an early-occurring, parasympathetically-mediated constriction of the pupil that occurs primarily in response to changes in brightness, but is also modulated during aversive anticipation. Using cues to signal threat of shock, the reflexive constriction to a flash of light presented during the anticipatory period is attenuated, compared to safe periods, eliciting a "fear-inhibited light reflex" (Bitsios, Szabadi, & Bradshaw, 1996, 2004). In a previous study (Bradley, Miccoli, Escrig, & Lang, 2008) we found greater late pupil dilation when viewing emotionally arousing, compared to neutral pictures, and follow-up analyses suggested that specific highly arousing contents, whether unpleasant (e.g., violence) or pleasant (e.g., erotica) may have modulated the amplitude of even the initial light reflex. Differences in brightness, however, could have mediated this effect. Thus, in the current study, we examined this issue more closely by presenting highly arousing pictures of erotica and violence that were matched exactly in brightness to each other, as well as to a set of neutral pictures. If the initial light reflex is generally attenuated during emotional picture viewing, it would suggest that modulation of this early pupillary reaction is not specific to aversive stimulation.
Pictures were presented in grayscale, and brightness was exactly matched for erotic, violent, and neutral content. Moreover, as an additional control for assessing effects of brightness on the initial light reflex, each picture was presented in both an intact and scrambled version. In the scrambled version, pixels were randomly shuffled such that brightness was identical to the intact version but no content (semantic or emotional) remained. If emotion modulates the initial light reflex, we expected that viewing emotional scenes -- whether erotic or violent - would elicit a smaller light reflex compared to when viewing neutral scenes, but that the light reflex for scrambled pictures, identical in brightness to the intact versions, would not vary as a function of original picture content.
Methods
Participants
Twenty-seven 18–21-year-old University of Florida General Psychology students (13 male, 82% Caucasian) signed a consent form and participated for course credit.
Materials and Design
Stimuli were 36 pictures1 selected from the International Affective Picture System (IAPS: Lang, Bradley, & Cuthbert, 2008), consisting of 12 erotic (mean pleasure/arousal= 6.6, 6.4), 12 neutral (mean pleasure/arousal=5.2, 3.6), and 12 violent (mean pleasure/arousal= 1.9, 6.4) pictures. All pictures portrayed people, were landscape in orientation and displayed in 256-bit grayscale, and across the three contents, contrast and figure-ground ratings were matched. The 36 pictures were divided into 12 sets of 3 pictures, with each set containing one erotic, one neutral, and one violent picture of identical brightness. Across the 12 sets, brightness naturally varied from low to high. A scrambled version of each picture was then created which randomly rearranged pixels to produce a same brightness version of the original intact picture. The final set of stimuli consisted of 36 intact and 36 scrambled pictures, for a total of 72 pictures.
Each picture was displayed for a 6 s free-viewing period, followed by a varying intertrial interval of 9–12 s that presented a fixation cross on a gray screen. Picture presentation was counterbalanced such that an intact and scrambled version of each hedonic content (e.g., erotic, neutral, violence) was presented within a block of 6 trials. Across participants, three presentation orders were constructed which counterbalanced whether a specific picture was presented early, middle or late in the series.
Apparatus
Pictures were presented using an IBM-compatible computer running Presentation software (Neurobehavioral Systems, San Francisco, CA). Pictures were displayed on a 19-inch monitor located in the experimental room, at a distance of 30 inches (76.2 cm) from where the participant was seated, subtending 8.9 × 9.1 degrees of visual angle.
Pupil diameter was continuously sampled at 60 Hz from 2-s before picture onset to 250 ms before the end of the inter-trial interval using an ASL model D6 desk mounted remote eye tracker (Applied Science Laboratories, Bedford, MA). This system consists of a video camera and an infrared light source, which is focused on the participant’s right eye. Face recognition is used to track head movements and keep the pupil in focus. The recording video camera was located in front of the participant, situated just below the stimulus presentation monitor.
Procedure
After arriving at the laboratory, each participant signed a consent form and was seated in an upright chair in a small, sound-attenuated, dimly lit room. A calibration procedure was conducted in which the participant was instructed to sequentially look at 9 dots that appeared one at a time on the screen. Several scrambled images of varying brightness were presented in order to ensure reliable pupil discrimination at different levels of picture brightness. The participant was then instructed to view each picture for the entire time that it was on the screen and to look at the fixation cross at all other times.
Data Reduction
Pupil diameter was converted offline from arbitrary units to millimeters and linear interpolation was used to estimate pupil size for samples in which the pupil was occluded due to blinking. For each trial, pupil diameter during a one-second baseline prior to picture viewing was subtracted from each of the following pupil samples. Five participants were removed from the final analysis due to unsuccessful pupil discrimination on more than 15% of trials.
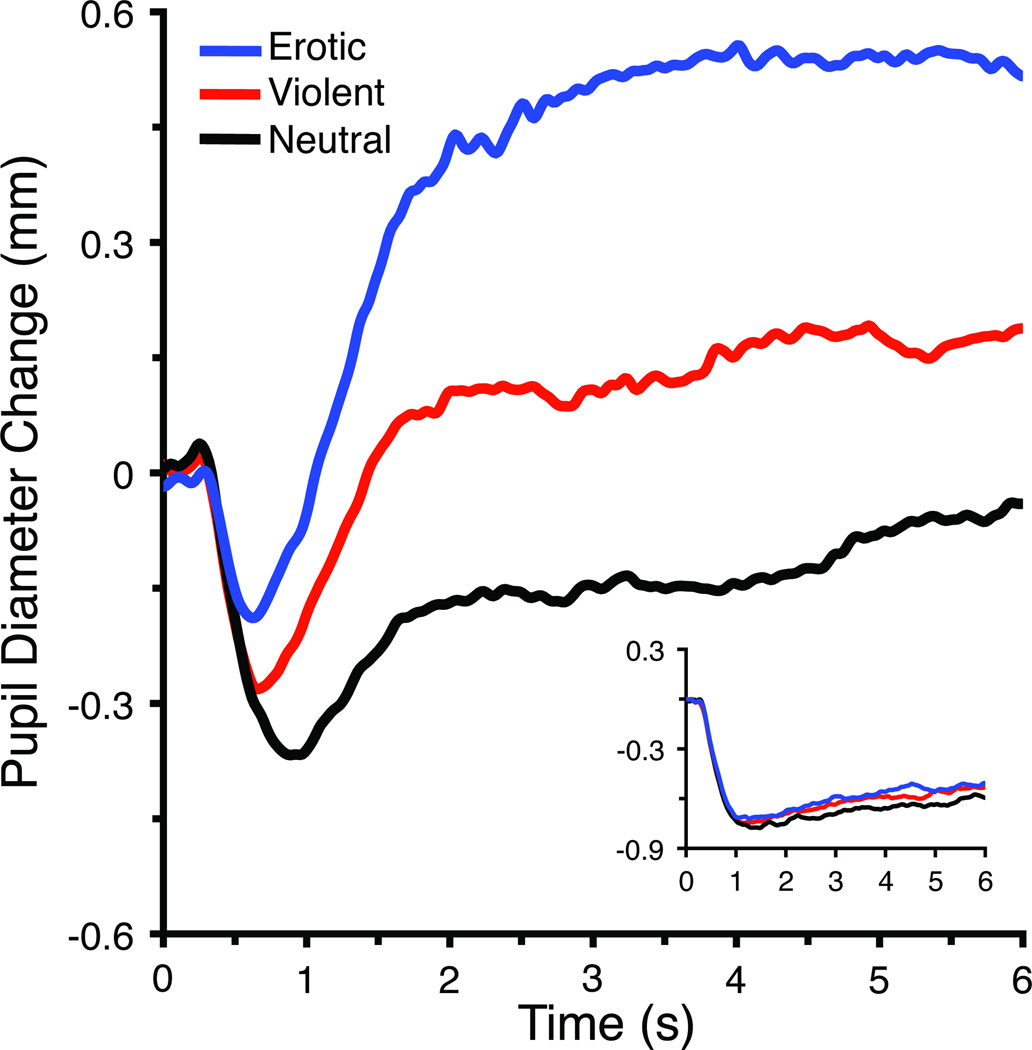
Based on the average waveform, the initial light reflex was calculated as the mean pupil change from 0.5 to 1.3 s after picture onset (Figure 1a). Trials in which pupil discrimination was less than 50% accurate in this time window were counted as missing (3.2% of total trials), resulting in an average of 11.3 to 12.0 trials remaining for each condition2. Late pupil diameter was calculated as the mean pupil diameter change from 2 to 6 seconds following picture onset (Figure 1b) and included the same trials as used in the analysis of the initial light reflex.
Figure 1.
Change (mm) in pupil diameter from a 1 s baseline preceding picture onset when viewing erotic, neutral, or violent scenes. a) The initial light reflex was averaged in a window from 0.5 to 1.3 s following picture onset, and b) late pupil diameter was averaged in a window from 2 to 6 s post picture onset. Inset: For scrambled pictures, the light reflex did not differ as a function of original picture content.
Data were analyzed using a Content (3: erotic, neutral, violent) X Version (2: intact, scrambled) repeated measures analysis of variance for each of the dependent variables (initial light reflex, late pupil diameter). Greenhouse-Geisser was used to correct degrees of freedom for sphericity. Means and standard deviations for all comparisons are displayed in Table 1.
Table 1.
Means (standard errors) of the change in pupil diameter (mm) when viewing intact and scrambled versions of erotic, neutral, and violent scenes for the initial light reflex (.5 to 1.3 s) and late pupil diameter (2 – 6 s).
| Hedonic Content |
Intact Pictures | Scrambled Versions | ||
|---|---|---|---|---|
| Initial Light Reflex |
Late Pupil Diameter |
Initial Light Reflex | Late Pupil Diameter |
|
| Erotic | −.07 (.04) | .52 (.07) | −.61 (.04) | −.57 (.07) |
| Neutral | −.32 (.04) | −.12 (.06) | −.64 (.05) | −.67 (.06) |
| Violent | −.20 (.06) | .15 (.08) | −.63 (.05) | −.60 (.05) |
Results
Light Reflex
Figure 1 illustrates the change in pupil diameter during free-viewing of natural scenes, which indicates that the initial light reflex was clearly attenuated when viewing intact erotic and violent, compared to neutral, scenes. Supporting this were significant main effects of Content, F(2,20) = 23.5, p < .001, Version, F(1,21) = 179.9, p < .001, and their interaction F(2,20) = 13.4, p < .001. Follow-up analyses indicated a significant effect of hedonic content when viewing intact, F(2,20) = 35.1, p < .001, but not scrambled, pictures. For intact pictures, viewing either erotic or violent scenes elicited significantly less early pupil constriction, compared to neutral scenes, F(1,21) = 107.7, p < .001, and F(1,21) = 14.5, p < .005, respectively, and erotic scenes prompted less constriction than violent scenes, F(1, 21) = 15.4, p < .001.
Early modulation of the initial light reflex by emotional content was not due to differences in brightness, since the light reflex did not differ among scrambled versions of the same pictures, which were identical in brightness (F < 1; see inset, Figure 1). Overall, pupil constriction was larger when viewing scrambled, compared to intact, pictures (all F’s (1, 21) > 58, p < .001).
Picture analysis
To further determine whether the initial light reflex is reliably modulated by emotion during picture viewing, a hierarchical regression was conducted using each intact picture as the unit of analysis, first assessing effects of brightness and then effects of emotionality. As expected, light reflex amplitude was highly influenced by brightness, F(1, 34) = 106.6, p < .001, R2 =.75, with pictures higher in brightness prompting larger light reflexes than lower brightness pictures. After removing effects due to brightness, however, ratings of emotionality (i.e. arousal; Lang et al., 2008) continued to account for significant variance in the amplitude of the initial light reflex, F(2,33) = 11.3, p < .001, R2 = .06.
Late Pupil Diameter
Pupil diameter later in the viewing interval was also modulated by hedonic content, with larger changes when participants viewed emotional, compared to neutral, scenes (Figure 1b). Significant main effects were obtained for Content, F(2,20) = 55.3, p< .001, Version, F(1,21) = 169.1, p < .001, and their interaction, F(2,20) = 28.7, p < .001. Follow-up analyses indicated a significant effect of picture content for intact pictures only, F(2,20) = 72.0, p<.001, in which viewing pictures of erotica or violence prompted significantly larger increases in late pupil diameter, compared to neutral scenes, F (1,21) = 200.6, p < .001, and F (1, 21) = 19.1, p < .001, respectively, and erotic scenes elicited larger changes than violent scenes, F(1,21) = 49.3, p < .001. Overall, late pupil diameter was smaller when participants viewed scrambled, compared to intact, versions (all F’s (1,21) > 70, p < .001), but did not differ among scrambled versions of each content.
Discussion
During picture viewing, emotional modulation of pupil diameter is apparent as early as the initial light reflex. Although this reflex is strongly affected by brightness, initial pupil constriction was nonetheless significantly attenuated for emotional, compared to neutral images, in both the subject and picture analyses. When the same pictures were scrambled to produce versions that were identical in brightness to the intact pictures but lacking any content, there was no difference in the amplitude of the initial light reflex or late pupil diameter as a function of original picture content, confirming that the differences found when viewing intact pictures did not simply reflect subtle differences in brightness.
Previous studies have reported that simply anticipating an aversive event, such as the possible presentation of an electric shock, attenuates initial pupil constriction (i.e., "fear-inhibited" light reflex; Bitsios et al., 1996, 2004). In these studies, early pupil constriction was attenuated under threat, compared to safety, and, importantly, did not show the same attenuation when participants anticipated the presentation of a weak acoustic stimulus. On the other hand, effects of highly pleasant stimulation on early constriction were not tested, and here, we found greatly attenuated light reflexes when pictures of erotica were presented. At the least, the current data suggest that modulation of the initial light reflex is not solely confined to a context of fear.
Although changes in pupil diameter are determined by both sympathetic and parasympathetic influences on the sphincter and dilator muscles of the iris (Steinhauer, Siegle, Condray, & Pless, 2004), initial pupil constriction is thought to be mediated by parasympathetic projections from the Edinger-Westphal nucleus to the sphincter muscle. Consistent with this, administration of dapriprazole (a peripheral sympathetic blockade) does not alter the fear-inhibited light reflex (Giakoumaki, Hourdaki, Grinakis, Theou, & Bitsios, 2005).
One interpretation is that variations in pupil diameter during affective picture viewing might be related to locus coeruleus activity. In animals, phasic firing of neurons in the locus coeruleus is associated with increases in pupil size, and both locus coeruleus activity and pupil diameter increase when rare or unexpected stimuli occur (Rajkowski, Kubiak, & Aston-Jones, 1994), consistent with modulation by motivationally or behaviorally relevant stimuli. Because the locus coeruleus has inhibitory connections to the Edinger-Westphal nucleus and excitatory projections to preganglionic sympathetic neurons, it could attenuate the parasympathetically mediated light reflex as well as contribute to later sympathetically mediated changes in pupil diameter (Szabadi, 2012). Future studies utilizing additional pharmacological blockades of appropriate receptors in the dilator and sphincter muscles (e.g. Steinhauer, et al., 2004) would be useful in further understanding how the pupil is modulated by emotion.
Modulation of pupil diameter was largest when participants viewed erotic scenes, which is consistent with studies finding the largest late positive potential (Schupp, et al., 2004) and electrodermal reactions (e.g., Lang, Bradley, & Cuthbert, 1997) when young adults view pictures of erotica, compared to other contents rated as emotionally arousing. An additional finding is that scrambled pictures in general elicited a larger initial constriction than intact pictures, which probably reflects the higher spatial frequency in scrambled versions, which is also known to increase initial constriction (Link et al., 2006).
Summary
Viewing emotionally arousing pictures prompted modulation of pupil diameter beginning with the initial light reflex and persisting throughout the viewing interval, suggesting both rapid and sustained effects of emotional arousal on pupil size during free-viewing of affective pictures. These findings cannot be explained by subtle differences in picture brightness, as scrambled versions of the same pictures, identical in brightness to the intact scenes, did not elicit any reliable differences in pupil diameter. Future studies are needed to determine the relative contribution of activity in the sympathetic and parasympathetic branches of the autonomic nervous system to emotional modulation of pupil diameter during picture viewing. Most broadly, these data suggest that modulation of the initial light reflex is not confined to a context of fear, and is not indicative of a failure to control brightness when presenting pictures of natural scenes.
Acknowledgments
This work was supported in part by NIMH grants MH098078 and MH094386 to Peter J. Lang.
Footnotes
Preliminary results from this research study were presented at the 51st annual meeting of the Society for Psychophysiological Research in Boston, MA in 2011.
The IAPS pictures (Lang et al., 2008) used in this study were: Erotic: 4597, 4643, 4647, 4658, 4668, 4676, 4687, 4689, 4690, 4693, 4697, 4698; Neutral: 2026, 2101, 2191, 2211, 2308, 2359, 2372, 2411, 2441, 2513, 2595, 2850; Violence: 3030, 3059, 3064, 3068, 3103, 3131, 3150, 3225, 3530, 6560, 9163, 9433.
Because some trials were not included in the analysis, it is possible that brightness could vary among trials (pictures) retained in the final analysis. To confirm that these findings do not reflect subtle differences in brightness resulting from missing trials, a secondary analysis was conducted using only those trials in which all data was available for all conditions at each brightness level. Results were identical to those reported here.
References
- Bitsios P, Szabadi E, Bradshaw CM. The inhibition of the pupillary light reflex by threat of an electric shock: a potential laboratory model of human anxiety. Journal of psychopharmacology. 1996;10:279–287. doi: 10.1177/026988119601000404. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Szabadi E, Bradshaw CM. The fear-inhibited light reflex: importance of the anticipation of an aversive event. International Journal of Psychophysiology. 2004;53:87–95. doi: 10.1016/j.ijpsycho.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giakoumaki SG, Hourdaki E, Grinakis V, Theou K, Bitsios P. Effects of peripheral sympathetic blockade with dapiprazole on the fear-inhibited light reflex. Journal of Psychopharmacology. 2005;19:139–148. doi: 10.1177/0269881105048994. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: affect, activation, and action. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting. Mahwah, NJ: Erlbaum; 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- Link B, Jünemann A, Rix R, Sembritzki O, Brenning A, Korth M, Horn F. Pupillographic measurements with pattern stimulation: the pupil’s response in normal subjects and first measurements in glaucoma patients. Investigative ophthalmology & visual science. 2006;47:4947–4955. doi: 10.1167/iovs.06-0021. [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Research Bulletin. 1994;35:607–616. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Schupp H, Cuthbert B, Bradley M, Hillman C, Hamm A, Lang P. Brain processes in emotional perception: Motivated attention. Cognition & Emotion. 2004;18:593–611. [Google Scholar]
- Steinhauer S, Siegle G, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International journal of psychophysiology. 2004;53:77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Szabadi E. Modulation of physiological reflexes by pain: role of the locus coeruleus. Frontiers in Integrative Neuroscience. 2012;94:1–15. doi: 10.3389/fnint.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]