Abstract
Background
The purpose of this study was to determine whether the use of optimized CT treatment planning offered better coverage of axillary level III (LIII)/supraclavicular (SC) targets than the empirically derived dose prescription that are commonly used.
Materials/Methods
Thirty-two consecutive breast cancer patients who underwent CT treatment planning of a SC field were evaluated. Each patient was categorized according to body mass index (BMI) classes: normal, overweight, or obese. The SC and LIII nodal beds were contoured, and four treatment plans for each patient were generated. Three of the plans used empiric dose prescriptions, and these were compared with a CT-optimized plan. Each plan was evaluated by two criteria: whether 98% of target volume receive >90% of prescribed dose and whether < 5% of the irradiated volume received 105% of prescribed dose.
Results
The mean depth of SC and LIII were 3.2 cm (range, 1.4–6.7 cm) and 3.1 (range, 1.7–5.8 cm). The depth of these targets varied according across BMI classes (p = 0.01). Among the four sets of plans, the CT-optimized plans were the most successful at achieving both of the dosimetry objectives for every BMI class (normal BMI, p = .003; overweight BMI, p < .0001; obese BMI, p < .001).
Conclusions
Across all BMI classes, routine radiation prescriptions did not optimally cover intended targets for every patient. Optimized CT-based treatment planning generated the most successful plans; therefore, we recommend the use of routine CT simulation and treatment planning of SC fields in breast cancer.
Keywords: Supraclavicular, Axillary, Lymph node, Treatment planning, Computed tomography
INTRODUCTION
Simulation using CT simulation is now widely available for radiation treatment planning to treat breast cancer. It is an important tool to help define the tumor target and normal tissue based on anatomical features of an individual patient. However, despite the availability of this technology, a common practice for delivering radiation to supraclavicular (SC) nodal bed during breast cancer treatment is to use 6MV photons empirically prescribed to the depth of maximum dose (Dmax), or 3 cm (1, 2). Indeed, a pattern of care analysis showed that in two thirds of breast cancer treatments to a SC field, the radiation dose was prescribed to a specific depth in 67.5% and to midplane in 17% of the patients (1). It is likely that empiric prescription depth points may not properly cover the target nodal basin in all patients. Previously, Bentel et al. (3) reported that the depth of the SC lymph nodes is related to the anterior–posterior diameter and that the nodes are deeper for those who are thicker or heavier. In their report, the depth ranged from 2.4 to 9.5 cm. With such a wide range, a better approach may be to use CT information to optimize treatment delivery to the target volume.
Supraclavicular radiation fields are typically used in patients who have undergone an axillary level I/II dissection and are found to have positive lymph nodes. For such patients, the areas of greatest risk for residual nodal disease are in the level III (LIII) axilla (the region superomedial to the pectoralis minor muscle) and the SC fossa. Information from CT can be used to identify and delineate these regions. Interestingly, little available research focuses on the techniques for properly covering the LIII nodal basin in radiation fields.
The purpose of this study was to compare different treatment planning techniques for treating the LIII and SC, using different choices of beam energy together with varying the calculation points. We examined whether higher energy photons or optimized CT treatment planning would offer better coverage of LIII and SC nodal basins than using 6-MV photon prescribed conventionally.
METHODS AND MATERIALS
This study was approved by the Institutional Review Board at the University of Texas M. D. Anderson Cancer Center. Thirty-two consecutive breast cancer patients treated with postmastectomy radiation that included a SC field were selected for the study. Each patient underwent CT-simulation for radiation treatment planning. Patients were immobilized in supine position with the ipsilateral shoulder abducted and head rotated slightly toward the contralateral side. The CT scan images were obtained with high-speed helical scanner at 2.5-mm to 3-mm slice thickness through the region of interest. The SC field was designed with the lower half of the beam blocked to match with the tangential fields inferiorly, with this border typically placed below the head of the clavicle. A 15-degree lateral gantry rotation was used to avoid treating the spinal cord. Customized blocks were used to shield the spinal cord and the ipsilateral humeral head. The treatment plans were generated using Philips Pinnacle treatment planning software (version 6.2). The regions of interest were identified initially with the assistance of the thoracic radiologist (J.J.E.). Medially, the SC fossa extended to the lateral edge of the trachea. Superiorly, this region extended to the level of the lower edge of the cricoid cartilage. Anteriorly, the SC nodal bed was bounded by the posterior border of the sternocleidomastoid muscle. The posterolateral border of SC nodal bed was defined by the anterior border of the anterior scalene muscle. The inferior border of the SC nodal bed was defined by the subclavian artery.
The superior border the axillary level III (LIII) nodal bed was defined as the most superior aspect of the pectoralis minor muscle. The inferior border was defined at the level of the insertion of the clavicle into the manubrium. Anteriorly, the LIII nodal bed was bounded by pectoralis major muscle. Posterior border of the LIII nodal bed was defined by subclavian-axillary artery. Laterally, the LII nodal bed extended to the medial aspect of the pectoralis minor muscle. Medially, the LIII nodal bed extended to the lateral border of the clavicle. For the purpose of this study, the LIII and SC nodal beds were considered to be a single target. The maximum depth of the LIII and SC nodal bed was measured vertically from the skin surface.
In total, 128 plans were generated for the study. For each patient, four treatment planning techniques for the SC field were evaluated. Conventional plans that used 6MV photons prescribed to 1.5 cm (Dmax, 6MV-1.5) and to 3 cm depth (6MV-3.0) were generated. Separate plans, one using 18MV photons prescribed to 3.3 cm (D max, 18MV) and one that combined 6MV and 18MV photons manually optimized to cover the target volume with 90% of the prescribed dose (CT opt), were created for comparison. For the CT opt, we also used individual calculation points for each patient to achieve the best coverage of the targets. Each plan was evaluated and scored using two criteria; one point was awarded for meeting each of the criteria. The first criterion was the ability to cover 98% of target volume with 90% or greater of the prescribed dose (V90). The second criterion was the avoidance of hot spots so that no more than 5% of the irradiated volume received more than 105% of the prescribed dose (V105). The irradiated volume was determined by the volume that received 90% or more of the prescribed dose. The combined score of 0, 1, and 2 were designated poor, good, and best scores, respectively.
We hypothesized that the improvement in accuracy of CT-guided treatment planning would vary with the body mass index (BMI). Therefore, we calculated BMI for each patient using the formula: weight (kg)/(height [m])2. The patients were divided into three groups: normal (BMI 18.5–24.9), overweight (BMI, 25.0–29.9), and obese (BMI ≥30.0). The patient’s BMI was correlated with the depth of SC and L III nodal beds using linear regression analysis. The score of evaluated treatment plans were correlated with the patient’s BMI using the Chi-square test. A p value ≤0.05 (two-sided) was considered significant. SPSS statistical software (version 11.5) was used for analysis.
RESULTS
Eight patients were classified as having a normal BMI, 14 as overweight, and 10 as obese. The mean BMI was 29.1 (range, 19.2–57.9). Figure 1 shows an example of the contours of SC and LIII nodal beds. The mean maximum depth of SC and LIII nodal beds were 3.2 cm (range, 1.4–6.7 cm) and 3.1 cm (range, 1.7–5.8 cm), respectively. Body mass index significantly correlated with the depth of SC and LIII using linear regression analysis with significant p value <0.0001 as shown in Fig. 2.
Fig. 1.
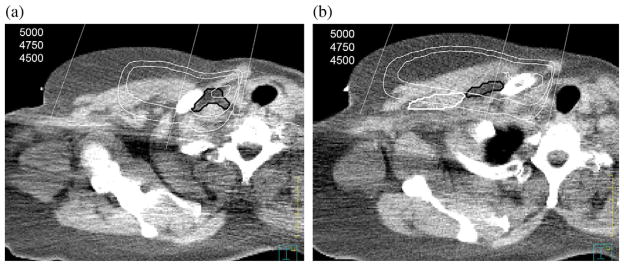
(a) Axial computed tomography (CT) from the CT simulation showing the supraclavicular nodal region outlined in black. The isodose line display is from the “CTopt” plan. (b) Axial CT from the CT simulation showing the axillary level III (LIII) nodal region outlined in black and pectoralis minor muscle was outlined in white. The isodose line display is from the “CTopt” plan.
Fig. 2.
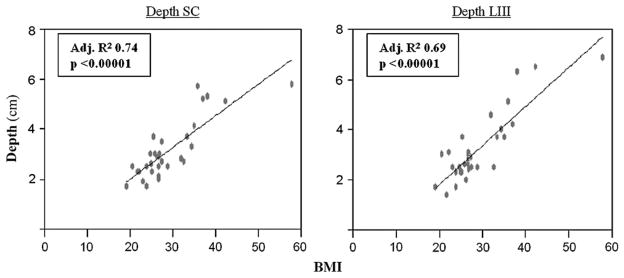
The relationship of body mass index (BMI) to depth of anatomical targets. This graph shows that BMI is significantly correlated with the depth of the supraclavicular (SC) and the axillary level III (LIII) nodal region using linear regression analysis (p <0.0001).
Table 1 shows the success rate of each plan in each of the BMI groups. Computed-tomography-optimized plans were the most successful among four plans for every BMI group (normal BMI, p = 0.003; overweight BMI, p < 0.0001; obese BMI, p < 0.001). For the normal and overweight BMI classes, the 6MV-1.5 plans yielded a score of 2 in 75% and 92.9% of the patients, respectively. In these groups of patients, 18MV plans would have led to undertreatment of the target superficially. Conversely, in obese BMI classes, the 18MV plans achieved a score of 2 in 80% of patients compared with 20% for the 6MV-1.5 plans (p = 0.023). The 6MV-3.0 plans provided a low rate of best scores in all BMI categories (50% normal, 64% overweight, 20% obese).
Table 1.
The success rates of various radiation plans according to body mass index groups.
| BMI group | Plan | Score (%)
|
|||
|---|---|---|---|---|---|
| Poor | Good | Best | p value* | ||
| Normal | 6MV-1.5 | 0 | 25 | 75 | |
| 6MV-3.0 | 0 | 50 | 50 | ||
| 18MV | 0 | 88 | 13 | ||
| CTopt | 0 | 0 | 100 | 0.003 | |
| Overweight | 6MV-1.5 | 0 | 7 | 93 | |
| 6MV-3.0 | 0 | 36 | 64 | ||
| 18MV | 0 | 79 | 21 | ||
| CTopt | 0 | 0 | 100 | <0.0001 | |
| Obese | 6MV-1.5 | 0 | 80 | 20 | |
| 6MV-3.0 | 30 | 50 | 20 | ||
| 18MV | 0 | 20 | 80 | ||
| CTopt | 0 | 0 | 100 | <0.001 | |
Abbreviation: BMI = body mass index.
CTopt vs. others.
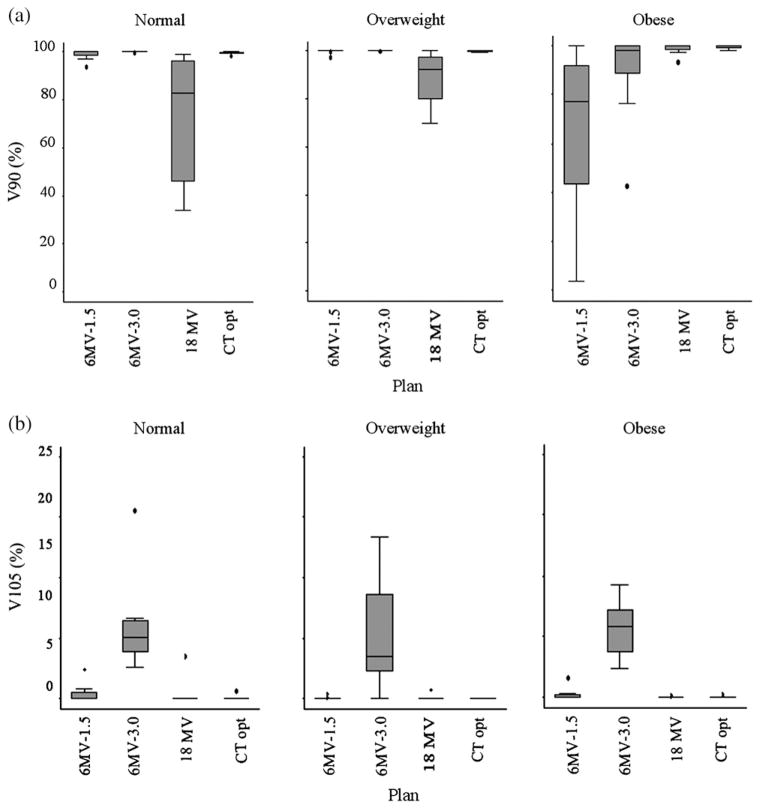
Overall, the 6MV-3.0 plan scored the worst of the various plan. This plan created a hot spot in most of the patients in each BMI group. Figure 3 shows stem-and-leaf plots that provide details of the coverage and dose homogeneity of each plan according to BMI group as shown.
Fig. 3.
(a) A stem-and-leaf plot showing the V90 coverage for each plan in each body mass index (BMI) group. The criterion to achieve a score of 1 was when V90 cover more than 98% of the target. (b) A stem-and-leaf plot showing the V105 coverage for each plan in each BMI group. The criterion to achieve a score of 1 was when V105 cover less than 5% the target.
DISCUSSION
In this study, we found that radiation of SC fields using prescriptions of radiation dose to empiric depths often leads to suboptimal coverage of targeted volumes, unnecessary degrees of dose inhomogeneity, or both. Specifically, a routine prescription of 6MV to Dmax provided the adequate coverage only in some patients in the normal and overweight BMI groups and was optimal in only 20% of the obese patients. Alternatively, the routine prescription of 6MV to a fixed depth of 3 cm generated “hot spots” in the irradiated volume in most of the patients. Finally, the plan using 18MV photons to Dmax led to an underdosage of superficial target regions for patients who had normal or overweight BMI, but it gave acceptable coverage for obese patients.
Our conclusion from these data is that the best way to prescribe radiation dose to SC fields used in breast cancer treatment is to use CT simulation, delineate the SC/LIII as a target, and generate an optimized treatment plan for each individual patient. We found that this method gave the best combination of target coverage and homogeneity. To achieve this success, we used not only a combination of 6MV and 18MV photon beams but also individualized calculation points.
The anatomical location of the supraclavicular and infraclavicular nodes has been well described in the literature, and SC fields have been treated routinely by assuming that the radiation coverage will be acceptable if this region is treated with 6MV photons with the dose prescribed to a specific depth, most commonly 3 cm. However, the anatomical locations of the supraclavicular and axillary nodal beds vary from patient to patient, and the use of a standard depth does not take into account this difference. We found that the mean maximum depth of supraclavicular and axillary level III nodal beds was 3.2 cm (range, 1.4–6.7 cm) and 3.1 cm (range, 1.7–5.8 cm), respectively. These findings were comparable with the previous reports that showed the mean depth of SC nodes was 3.9–6 cm (range, 2.1–8.3 cm) (4–8), and the mean depth of the axillary level III nodal bed was 3.6–6.7 cm (range, 1.9–7.4 cm). This variation in the depth of both nodal beds suggested the need for customized radiation treatment rather than the use of routine radiation prescription. Our study is the first to show a significant linear relationship between the BMI and the maximum depth of the SC and LIII nodal beds with a p value of <0.001. Patients with higher BMI tend, logically, to have deeper nodal beds. This finding is consistent with those reported by Bentel et al. (3) who used A/P diameter as a surrogate of the size of the patients.
Recently, CT simulation has increasingly been used in treatment planning for breast cancer patients. However, only a few studies have been done to improve treatment planning or radiation coverage for this region (4, 8–10). Madu et al. (4) reported the improvement of the SC/L III nodal beds coverage by using conformal optimized plans for individual patients. They used CT simulation for localization of the target and different gantry angles, as well as individualized normalization points, to achieve coverage of 90% of the target. Cavey et al. (8) reported the superior target volume coverage of three-dimensional conformal radiation therapy (3D CRT) or intensity-modulated radiation therapy (IMRT) plans over conventional plans for the SC field. For the 3D CRT or IMRT plans, they used opposing AP/PA fields angled 10–15 degrees to avoid the spinal cord and used heavier weighting of the anterior field for 2:1 or greater. This study showed that using 6MV photons and routine prescription to the depth of 3 and 5 cm not only produced significantly inferior target volume coverage (V90-107) to IMRT (p = 0.55 and p = 0.014, respectively) but also produced significantly greater dose heterogeneity (D95-5) (p = 0.031 and p = 0.043, respectively). Our study differs from the report by Cavey et al. (8) in that we used anterior–posterior approach for the treatment planning and used individualized calculation point as well as combination of 6MV and 18MV photons. Our approach can achieve a good coverage of the targets while avoiding excess radiation dose to surrounding structures.
Irradiation of the SC fossa is associated with the risks of injury to normal tissue. For selected patients with positive lymph nodes found on axillary dissection, these risks are warranted to avoid the risk posed by having persistent disease within this area. However, when treatments are given, it is critical that every effort is made to ensure that the target volumes are adequately covered and the dose inhomogeneity within the treatment field is minimized. The data from our study support the use of CT-based treatment planning because this best meets the requirements for appropriate target coverage and dose homogeneity.
CONCLUSIONS
Routine radiation prescriptions did not optimally cover the SC and LIII nodal beds for patients categorized by BMI. Optimized CT-based treatment planning generated the most successful plans for proper target coverage with only small hot spots; therefore, we recommend the use of routine CT-simulation and treatment planning of SC fields in breast cancer.
Acknowledgments
Supported in part by National Cancer Institute grants CA16672 and T32CA77050 and the Arlette and William Coleman Foundation.
Footnotes
Note—An online CME test for this article can be taken at http://asro.astro.org under Continuing Education.
This work was presented at the 48th Annual Meeting of the American Society of Therapeutic Radiology and Oncology (ASTRO), Philadelphia, PA 2006.
Conflict of interest: none.
References
- 1.Pierce LJ, Moughan J, White J, et al. 1998–1999 patterns of care study process survey of national practice patterns using breast-conserving surgery and radiotherapy in the management of stage I–II breast cancer. Int J Radiat Oncol Biol Phys. 2005;62:183–192. doi: 10.1016/j.ijrobp.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 2.White J, Moughan J, Pierce LJ, et al. Status of postmastectomy radiotherapy in the United States: A patterns of care study. Int J Radiat Oncol Biol Phys. 2004;60:77–85. doi: 10.1016/j.ijrobp.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 3.Bentel GC, Marks LB, Hardenbergh P, et al. Variability of the depth of supraclavicular and axillary lymph nodes in patients with breast cancer: Is a posterior axillary boost field necessary? Int J Radiat Oncol Biol Phys. 2000;47:755–758. doi: 10.1016/s0360-3016(00)00485-5. [DOI] [PubMed] [Google Scholar]
- 4.Madu CN, Quint DJ, Normolle DP, et al. Definition of the supraclavicular and infraclavicular nodes: implications for three-dimensional CT-based conformal radiation therapy. Radiology. 2001;221:333–339. doi: 10.1148/radiol.2212010247. [DOI] [PubMed] [Google Scholar]
- 5.Dijkema IM, Hofman P, Raaijmakers CP, et al. Loco-regional conformal radiotherapy of the breast: delineation of the regional lymph node clinical target volumes in treatment position. Radiother Oncol. 2004;71:287–295. doi: 10.1016/j.radonc.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Qatarneh SM, Kircuta IC, Brahme E, et al. Three-dimensional atlas of lymph node topography based on the visible human data set. Anat Rec B New Anat. 2006;289:98–111. doi: 10.1002/ar.b.20102. [DOI] [PubMed] [Google Scholar]
- 7.Goodman RL, Grann A, Saracco P, et al. The relationship between radiation fields and regional lymph nodes in carcinoma of the breast. Int J Radiat Oncol Biol Phys. 2001;50:99–105. doi: 10.1016/s0360-3016(00)01581-9. [DOI] [PubMed] [Google Scholar]
- 8.Cavey ML, Bayouth JE, Endres EJ, et al. Dosimetric comparison of conventional and forward-planned intensity-modulated techniques for comprehensive locoregional irradiation of post-mastectomy left breast cancers. Med Dosim. 2005;30:107–116. doi: 10.1016/j.meddos.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Jephcott CR, Tyldesley S, Swift CL. Regional radiotherapy to axilla and supraclavicular fossa for adjuvant breast treatment: A comparison of four techniques. Int J Radiat Oncol Biol Phys. 2004;60:103–110. doi: 10.1016/j.ijrobp.2004.02.057. [DOI] [PubMed] [Google Scholar]
- 10.Krueger EA, Fraas BA, McShan DL, et al. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:1023–1037. doi: 10.1016/s0360-3016(03)00183-4. [DOI] [PubMed] [Google Scholar]