Summary
While both direct and indirect allorecognition are involved in allograft rejection, evidence to date suggests that tolerance is primarily dependent on indirect pathway-triggered CD4+CD25+ T cell-mediated immunoregulation. However, the precise influence of these two pathways on CD4+CD25+ T-cell function has not been addressed. In the current study, we have utilized an adoptive transfer model to assess selectively how the absence of either direct or indirect allorecognition affects CD4+CD25+ T-cell function. The effects of the loss of the direct pathway were assessed by transplanting skin grafts from minor histocompatibility mismatched B10.D2 (H-2d) donors onto Balb/c (H-2d) recipients, or by placing bone marrow chimeric DBA/2 (H-2d/H-2b) allografts onto C57BL/6 (H-2b) hosts. The requirement for indirect allorecognition was tested by grafting DBA/2 skin allografts onto either C57BL/6- or MHC-II-deficient C57BL/6 recipients. We report here that although CD4+CD25+ regulatory T cells can suppress both directly and indirectly generated alloresponses, immunoregulation is favored when indirect presentation is the sole mechanism of allorecognition. Hence, in the absence of indirect presentation, net CD4+CD25+ T cell-dependent immunoregulation is weak, and high ratios of CD4+CD25+ to CD4+CD25− T cells are required to ensure graft survival.
Keywords: allorecognition, immunoregulation, regulatory T cells, tolerance
Introduction
In transplantation, alloantigens can be recognized by recipient T cells through either of two pathways [1]. Direct allorecognition involves direct interaction between the T-cell receptor (TCR) expressed on recipient T cells and intact major histocompatibility complex (MHC) molecules present on the surface of donor antigen-presenting cells (APCs). On the other hand, in the indirect pathway of allorecognition, peptides derived from donor major and minor histocompatibility molecules are processed by recipient APCs and presented on self-MHC molecules to recipient T cells. It is clear that both direct and indirect allorecognition pathways are involved in graft rejection, with each pathway contributing differently to the magnitude and temporal pattern of the rejection response [2-4]. In contrast, data from various reports suggest that transferable tolerance mediated by regulatory T cells is a phenomenon almost exclusively dependent on indirectly triggered immunosuppressive alloresponses [5-9]. CD4+ CD25+Foxp3+ regulatory T cells (TRegs) have recently emerged as a principal regulatory T-cell subtype mediating immunological tolerance in both autoimmune [10-15] and alloimmune models [16-18]. Given the importance of TRegs in the induction of transplantation tolerance, it has been postulated that these regulatory T cells may also be restricted in their specificity toward indirect allorecognition [19,20]. This assumption is, however, at variance with experiments performed both in vitro and in vivo. For example, purified TRegs have been shown to be capable of suppressing T-cell proliferation in mixed lymphocyte cultures in which only direct allopresentation was available [21]. In addition, infusion of donor-type TRegs is capable of diminishing graft-versushost disease across MHC barriers in a system in which direct presentation is the primary pathway of allorecognition [22].
Most TRegs originate in the thymus, where regulatory T-cell precursors appear to be positively selected on the basis of high affinity for self-antigens expressed on thymic epithelium [23-25]. This favors the emergence of TRegs highly enriched in autospecific cells [26,27], and presumably with a lower capacity to interact effectively with intact foreign MHC molecules than CD4+CD25− T cells [26]. A TReg TCR repertoire heavily biased toward recognition of auto- rather than allo-MHC molecules could indeed explain why TRegs would require indirect allopresentation to induce allograft tolerance. According to this model, in absence of indirect allopresentation, TReg activity would be grossly impaired. Recent evidence indicates that TRegs can also arise in the periphery from ‘conventional’ T cells [28,29]. While the role of these ‘inducible’ TRegs in transplantation tolerance has not been completely elucidated, data available suggest that indirect allopresentation is critical for their generation and function [30].
In the current study, we have attempted to reconcile the somewhat contradictory experimental evidence on TReg specificity obtained in alloimmunity models. This has been performed by analyzing the capacity of effector and regulatory T-cell subpopulations to function in two different types of experimental situations: (i) models in which allopresentation takes place exclusively through MHC-matched APCs (indirect allorecognition) and (ii) a model in which allopresentation is solely mediated by donor-type MHC-mismatched APCs (direct allorecognition). Our results confirm previous reports [1,31,32] indicating that effector T-cell populations can utilize both direct and indirect allorecognition pathways to mediate allograft rejection. In addition, we provide evidence indicating that TReg activation in vivo can also occur through both direct and indirect pathways, although their immunoregulatory function is more pronounced in allograft responses driven via the indirect pathway. In fact, the absence of indirect allopresentation markedly disrupts the balance between effector and regulatory T-cell populations by disproportionally compromising the suppressive capacity of TRegs. Hence, our data suggest that in the absence of indirect allopresentation TRegs will only prevent graft rejection if present at very high numbers. Our data are consistent therefore with the notion that CD4+CD25+, unlike CD4+CD25−, T cells exhibit higher indirect than direct alloreactivity.
Material and methods
Animals
Wild-type C57BL/6 CD45.1 or CD45.2 (H-2b), Balb/c (H-2d), DBA/2 (H-2d), Balb/c, and C57BL/6 recombination activating gene-2-deficient mice (Rag−/−) were purchased from Taconic Farms (Germantown, New York, NY, USA). MHC class II-deficient (MHC-II−/−) C57BL/6 were also obtained from Taconic Farms, while MHC class II and Rag double-deficient C57BL/6 mice (Rag−/−/MHC-II−/−) were generated in our laboratory. Mice were maintained in pathogen-free conditions in the animal facility at Beth Israel Deaconess Medical Center (BIDMC) and used at 6–8 weeks of age. Animal experiments were approved by BIDMC Institutional Animal Care Committee.
Bone marrow chimeras
To generate bone marrow (BM) chimeras, DBA/2 mice were lethally irradiated with 1000 rad total body irradiation, and were then injected i.v. with 10 × 106 unfractionated allogeneic C57BL/6 BM cells. Earlier studies have shown that after allogeneic, but not syngeneic, BM transplantation and skin APCs, including Langerhans cells, are completely replaced by donor cells within a few weeks [33-35]. As a control group, irradiated DBA/2 mice were transplanted with DBA/2 BM cells. BM chimeras were used for transplant experiments 60 days after BM infusion. The replacement of recipient-type APC by BM-derived donor cells was confirmed in all cases by flow cytometry before performing transplant experiments.
Skin transplantation
Full thickness tail skin grafts from donor mice were grafted onto the dorsum of recipient mice. Skin grafts were sutured in place and covered with Vaseline gauze; a bolster dressing was applied for 7 days. Graft survival was followed by daily visual inspection, with rejection defined as complete loss of viable skin.
Cell purification and adoptive transfer experiments
CD4+CD25+ and CD4+CD25− T-cell sorting was achieved by using a MoFlo® High-Performance Cell Sorter (Cytomation; Fort Collins, CO, USA) after staining with fluorochrome-conjugated anti-CD25 and anti-CD4 (all from BD PharMingen; San Diego, CA, USA). CD4+CD25+ T cells were sorted based on the high CD25 fluorescence, based on the previous experiments from our laboratory indicating that >90% of these cells express Foxp3+. Purity of CD4+CD25− and CD4+CD25+ preparations was consistently >90%. CD4+CD25+ and/or CD4+CD25− T cells were adoptively transferred at various cell ratios into Rag−/− recipient mice i.v. 1 day before skin allograft transplantation. In this adoptive transfer system, the administration of CD4+CD25− T cells results in rapid skin allograft rejection, while CD4+CD25+ T cells do not induce rejection and prevent CD4+CD25− T cells from destroying the grafts [36]. For T-cell expansion experiments, 106 CD45.1-positive CD4+CD25− or CD4+CD25+ were adoptively transferred into Rag−/− CD45.2-positive or Rag−/−/MHC-II−/− CD45.2-positive recipient mice i.v. 1 day before DBA/2 skin allograft transplantation. Allograft recipients were sacrificed 14 days later, and the absolute numbers of CD45.1-positive T cells were quantified by flow cytometry in both spleen and draining lymph nodes.
Statistical analysis
Comparisons of the survival of transplanted allografts were performed by employing the log-rank in Kaplan–Meier survival analysis.
Results
TRegs powerfully prevent rejection of minor histocompatibility-mismatched skin grafts
To analyze the effect of direct presentation on the function of effector and regulatory T-cell populations, we adoptively transferred TRegs and/or CD4+CD25− T cells into Balb/c Rag−/− recipients of minor histocompatibility-mismatched, MHC-matched, skin grafts (B10.D2). Minor histocompatibility antigens require processing and presentation on self-MHC molecules to induce an immune response, and hence do not elicit direct alloreactivity. The co-transfer of CD4+CD25− T cells and TRegs at a ratio of 1:1 resulted in indefinite survival of B10.D2 grafts, but had a far less profound effect on the survival of C57BL/6 MHC-mismatched skin allografts (Fig. 1a). Our results indicate that net immunoregulatory effects in transplantation (i.e. balance of effector to regulatory T cells) are favored in the absence of direct allorecognition.
Figure 1.
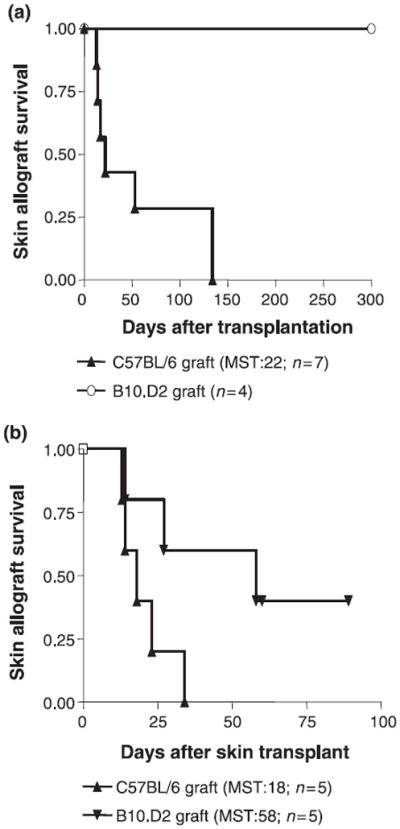
TRegs powerfully prevent the rejection of minor histocompatibility mismatched skin allografts. (a) 4 × 105 TRegs adoptively transferred into syngeneic Rag−/− Balb/c hosts together with 4 × 105 CD4+CD25− T cells show a greater capacity to delay the acute rejection of minor histocompatibility mismatched (B10.D2; H-2d) than that of MHC-mismatched (C57BL/6; H-2b) skin allografts (P < 0.0039). (b) The transfer of 4 × 105 CD4+CD25− T cells results in more rapid acute rejection of C57BL/6 than B10.D2 allografts (P < 0.0499). MST, median survival time in days.
The absence of donor APCs in skin allografts enhances TReg immunosuppressive function
To more clearly assess the impact of indirect allopresentation, in the absence of direct allopresentation, on the function of regulatory and effector T-cell populations, we utilized a model in which C57BL/6 Rag−/− recipients were transplanted with skin allografts harvested from BM chimeric DBA/2 donors, generated by reconstituting lethally irradiated DBA/2 (H-2d) mice with allogeneic recipient strain C57BL/6 (H-2b) BM. Control mice were generated by reconstituting lethally irradiated DBA-2 mice with syngeneic DBA/2 BM. C57BL/6 Rag−/− recipients receiving naïve syngeneic CD4+CD25− T-cell transfer acutely rejected skin allografts harvested from either C57BL/6-chimeric DBA/2 donors or control DBA/2-chimeric donors with similar tempo (Fig. 2), suggesting that the capacity of effector T cells to induce allograft rejection in this model is not markedly impaired in the absence of direct allopresentation. Similar results were observed after infusing CD4+CD25− T cells into recipients of grafts harvested from wild-type DBA/2 mice (data not shown). In contrast, the co-transfer of CD4+CD25+ TRegs together with CD4+CD25− T cells could only significantly prolong allograft survival when skin allografts were obtained from chimeric DBA/2 donors bearing C57BL/6 APCs, and not from wild-type DBA/2 mice or control hematopoietic chimeric DBA/2 donors bearing DBA/2 APCs (Fig. 2).
Figure 2.

In the absence of donor APCs, the capacity of TRegs to prevent the rejection of MHC-mismatched skin allografts is increased. Skin allografts harvested from irradiated DBA/2 mice reconstituted with either DBA/2 (DBA/2-DBA/2 chimera) or C57BL/6 (C57BL/6-DBA/ 2 chimera) BM, and placed onto Rag−/− C57BL/6 recipients, are similarly rejected after the transfer of 4 × 105 syngeneic CD4+CD25− T cells. In contrast, while the co-transfer of 4 × 105 TRegs together with 4 × 105 CD4+CD25− T cells delays the rejection of C57BL/6-DBA/ 2 chimeric grafts, it has virtually no effect on the survival of DBA/2-DBA/2 chimeric skin allografts (DBA/2-DBA/2 versus C57BL/6-DBA/2 chimeras, P < 0.045). MST, median survival time in days.
Indirect alloantigen presentation is required for efficient TReg function
To further establish the requirement of indirect allopresentation for TReg function, we performed additional adoptive transfer experiments using Rag−/− or Rag−/−/MHC-II−/− double-deficient C57BL/6 mice as recipients of wild-type MHC-mismatched DBA/2 skin allografts. In this strain combination involving Rag−/−/MHC-II−/−) recipients, the activation of CD4+ alloreactive T cells is solely triggered by direct interaction with DBA/2 donor APCs migrating from the skin allograft. CD4+CD25− T cells rapidly rejected skin allografts after being transferred into Rag−/−/MHC-II−/− recipients, indicating that direct allopresentation is sufficient to induce a productive cytopathic alloimmune response (Fig. 3a). In contrast, TRegs co-transferred together with CD4+CD25− T cells into Rag−/−/MHC-II−/− recipients exhibited weaker graft-protecting properties than when transferred into conventional Rag−/− recipients expressing self-MHC-II molecules (Fig. 3b). Although TRegs could indeed suppress effector T cells and induce universal indefinite graft survival in the Rag−/−/MHC-II−/−) hosts, this result required the administration of a very high (4:1) CD4+CD25+ to CD4+CD25− T-cell ratio (MST>90 days, n = 4, data not shown). These data are consistent with a very low precursor frequency of directly alloreactive T cells among the TReg subpopulation.
Figure 3.
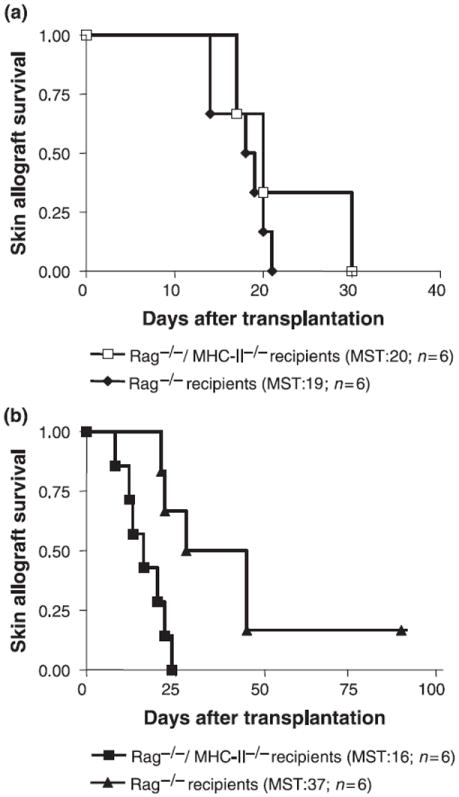
TRegs are less efficient at suppressing the cytopathic alloimmune responses in hosts lacking class II self-MHC molecules. (a) Adoptive transfer of 2 × 105 C57BL/6 CD4+CD25− T cells results in rapid rejection of DBA/2 grafts placed onto Rag−/− C57BL/6 recipients regardless of the presence or absence of recipient MHC molecules. (b) The absence of MHC-II in Rag−/− /MHC-II−/− C57BL/6 recipients markedly diminishes the immunoregulatory function of 4 × 105 TRegs transferred together with 2 × 105 CD4+CD25− T cells (Rag−/− recipients versus Rag−/−/ MHC-II−/− hosts, P < 0.0064).
TRegs and CD4+CD25− T cells expand similarly in MHC-II−/− hosts under lymphopenic conditions
The lack of strong TReg-mediated immunoregulation in MHC-II−/− hosts could be potentially due to a differential effect of MHC-II on the homeostatic proliferation of TRegs and CD4+CD25− T cells. To test this hypothesis, we directly compared the expansion of these two T-cell sub-populations after adoptive transfer into Rag−/− and Rag−/−/MHC-II−/− skin allograft recipients. The absence of MHC-II resulted in a marked decrease in the number of transferred T cells being recovered from the spleen or draining lymph nodes 14 days after adoptive transfer (Fig. 4). This reduction was, however, similar to both TRegs and CD4+CD25− T cells (Fig. 4). Hence, self-MHC-II molecules are equally required for the homeostatic expansion and survival of both regulatory and effector T-cell populations.
Figure 4.

Absence of class II self-MHC molecules similarly compromises the expansion and/or survival of TRegs and CD4+CD25− T cells. 1 × 106 CD45.1+ TRegs or CD45.1+ CD4+CD25− T cells were transferred into CD45.2+ Rag−/− syngeneic recipients bearing DBA/2 skin allografts, and 14 days later the number of CD4+ CD45.1+ cells recovered from either spleen or draining lymph nodes was quantified by flow cytometry. In Rag−/− recipients genetically lacking MHC-II, the expansion of both TRegs and CD4+CD25− T cells was similarly decreased. The results shown here correspond to 1 experiment representative of 3.
Discussion
Several recent reports from both animal [5-9,20] and human studies [37-39] suggest that in transplantation effective immunoregulation is primarily triggered by indirect alloantigen presentation. CD4+CD25+Foxp3+ TRegs, which are required for tolerance induction under various circumstances, appear to exhibit a repertoire of TCR specificities biased toward high-affinity recognition of self-MHC molecules [23,24,26,27,40]. Hence, it has been proposed that TReg stimulation in transplantation may only occur in response to indirectly presented alloantigens [19,20]. Similar conclusions have been drawn from experimental transplantation models in which TRegs arise in the periphery from conventional T cells after engagement with alloantigen-loaded host dendritic cells [30]. Nonetheless, whether the in vivo potency of TRegs varies with mode direct versus indirect of allopresentation has not been directly proven.
In the current study, we have utilized a variety of genetically modified mice, in an adoptive transfer model of the allograft response, to dissect the specific contributions of direct and indirect allopresentation to TReg function in transplantation selectively. A very high frequency of effector, but not regulatory, T cells cross-react with foreign MHC molecules in vitro [26,41-43]. Thus, given that the outcome of the transplant, rejection versus tolerance, resides in a balance between the contingent of immunoregulatory and alloaggressive T cells [19,44], it can be hypothesized that the absence of direct allopresentation would selectively impact on alloreactive effector T cells, diminishing their relative numbers and thereby allowing the pool of regulatory T cells to gain control of the alloimmune response.
Our first set of experiments involved the use grafts harvested from MHC-matched minor histocompatibility mismatched mice, a model in which antidonor immune responses are elicited by self-MHC molecules presenting minor histocompatibility antigens (indirect pathway). Allogeneic minor histocompatibility antigens are known to activate a much lower frequency of alloreactive effector T cells than intact allo-MHC molecules. Moreover, allo-MHC molecules, besides stimulating T cells by direct allorecognition, are also a major source of allogeneic peptides for indirect allopresentation. Accordingly, polyclonal effector T-cell populations should be less capable of rejecting minor histocompatibility-mismatched allografts, than fully MHC-mismatched skin allografts, as our results employing isolated CD4+CD25− T cells clearly confirm (Fig. 1b). Hence, our results showing a more potent effect of regulatory T cells in preventing skin allograft rejection when using B10.D2 grafts, when compared with fully MHC-mismatched C57BL/6 allografts, may be due, at least in part, to a smaller pool size of alloreactive CD4+CD25− T cells. To address this question, we employed an additional model using BM chimeric skin allograft donors. These BM chimeric donor mice, generated by reconstituting lethally irradiated DBA/2 (H-2d) mice with allogeneic recipient strain C57BL/6 (H-2b) BM, have an intact capacity to express H-2d class I and II molecules in their tissues, but lack donor-type allogeneic APCs, including Langerhans cells [33-35]. Thus, allografts harvested from chimeric donors should be capable of mediating powerful indirectly driven alloimmune responses, without activating alloreactive effector T cells via the direct pathway. In this system, indirectly driven effector immune responses had a similar capacity to induce graft rejection than directly plus indirectly triggered responses. In other words, the transfer of CD4+CD25− T cells alone into recipients bearing grafts harvested from unmanipulated MHC-mismatched donors or from BM chimeric donors resulted in a similar tempo of allograft rejection. Hence, in contrast to our first experimental setting, in this model using BM chimeric donors, the co-transfer of TRegs and CD4+CD25− T cells could provide more accurate information on the specific impact of direct and indirect allopresentation on TReg-suppressive function. Our results show that in the absence of donor-type APCs TReg function is maximized, suggesting that TRegs preferentially suppress indirectly driven responses. Taken together, our data indicate the absence of direct alloantigen presentation results in both: (i) a decreased alloreactive function of effector T cells (although rapid allograft rejection is still ensured if a potent indirectly driven alloimmune response is present); and (ii) more efficient TReg-mediated immunoregulatory effects. This conclusion is also supported by the experiments performed by utilizing Rag−/−/MHC-II−/− recipients, in which TRegs exerted weaker graft-protecting effects than when transferred into Rag−/−/MHC-II+/+ mice. However, the observation that in Rag−/−/MHC-II−/−) recipients TRegs could indeed inhibit skin allograft rejection if transferred at large numbers very likely indicates that a small fraction of TRegs has direct allospecificity.
The use of immunodeficient hosts lacking MHC class II raises the concern of whether the absence of MHC-II molecules might exert a different effect on the homeo-static proliferation of effector and regulatory T-cell sub-populations. Previously reported results indicate that CD4+CD25− and CD4+CD25+ T-cell homeostatic proliferations are equally susceptible to the absence of MHC-II molecules [45]. However, given the quantitative nature of our experimental system, it was imperative to directly assess this issue in an allograft model. Our data in skin allograft recipients indicate that lack of MHC-II molecules similarly impacts on the proliferation and survival of both effector and regulatory T-cell populations. This is the case not only in the spleen, but also in draining lymph nodes, which constitute the primary compartment in which allorecognition takes place. Taken together, our results suggest that differences in the immunoregulatory properties of TRegs observed after transfer into MHC-II+/+ and MHC-II−/− hosts are unlikely to be attributable to differences in cell expansion, survival or migratory patterns.
In short, while our experiments have been performed with a limited number of animals, our results clearly indicate that allorecognition pathways have a major impact on the balance between effector and regulatory T-cell populations. In the absence of direct pathway, the number of alloreactive effector T cells is reduced and TRegs exert optimal suppression. In contrast, pre-eminence of direct allopresentation leads to vigorous effector alloimmune responses in the face of suboptimal TReg function.
The observation that most TRegs lack direct allospecificity could explain why, in unmanipulated recipients, TRegs are unable to prevent acute allograft rejection, a response dominated by directly activated effector T cells, unless a provision is made to inactivate a substantial proportion of the allo-aggressive T cells [44,46]. Similarly, the requirement for high numbers of infused TRegs to prevent graft-versus-host disease in MHC-mismatched situations is consistent with a low frequency of CD4+CD25+ T cells exhibiting direct allospecificity [22,47,48]. Our data also provide for a reinterpretation of several experiments reported decades ago. For example, the model herein proposed can explain why, under certain circumstances, minor histocompatibility mismatched grafts, in which only indirect allopresentation is available, are spontaneously accepted [49]. In addition, the reason why F1 kidney allografts ‘parked’ into immunodeficient hosts can be subsequently engrafted into immunocompetent recipients in the absence of immunosuppression, may be accounted for by the preeminence of the indirect allorecognition pathway in a situation in which donor strain dendritic cells have been depleted [50]. More recently, the observation that tolerance to MHC-mismatched allografts cannot be achieved in MHC-II-deficient recipients via costimulation blockade can also be explained at least in part by impaired TReg function in the absence of indirect presentation [6]. The failure of directly presented alloantigens to elicit a substantial TReg-meditated immunoregulatory response has also important clinical implications. For instance, attempts at inducing transplantation tolerance via administration of donor-type tolerogenic dendritic cells may not be entirely successful, unless some kind of MHC matching has been ensured. The same concerns are valid for strategies aiming at the ex vivo expansion of recipient donor-specific TRegs for immunotherapeutical purposes in transplantation. Thus, based on our results, we predict that large numbers of polyclonal TRegs would have to be transferred to provide adequate immunoregulation, unless an effective way to generate MHC-reactive TRegs is found. Furthermore, our data may warrant the use of effective immunosuppression post-transplantation to delay the administration of tolerogenic treatments until replacement of donor APCs with recipient-type APCs has taken place. Finally, our data emphasize the need to take into account indirect allopresentation for the design of CD4+CD25+ TReg-based tolerance-monitoring assays in clinical transplantation.
Acknowledgments
We thank Yan Tian for excellent technical assistance and Bill Nostrom and John Tigges for their help in cell-sorting experiments. Support for this work was provided by grants from the Juvenile Diabetes Research Foundation (to X.X.Z), the National Institute of Allergy and Infectious Diseases (to T.B.S), and the Juvenile Diabetes Research Foundation Center for Islet Transplantation at Harvard Medical School (to T.B.S). A.S-F was recipient of a postdoctoral research fellowship from the Juvenile Diabetes Research Foundation.
Footnotes
Authorship
ASF and CMD designed and performed research; analyzed and wrote the paper. CM and SA helped perform research. XXZ and TBS provided overall research advice and guidance.
References
- 1.Auchincloss H, Jr, Sultan H. Antigen processing and presentation in transplantation. Curr Opin Immunol. 1996;8:681. doi: 10.1016/s0952-7915(96)80086-0. [DOI] [PubMed] [Google Scholar]
- 2.Lombardi G, Sidhu S, Daly M, Batchelor JR, Makgoba W, Lechler RI. Are primary alloresponses truly primary? Int Immunol. 1990;2:9. doi: 10.1093/intimm/2.1.9. [DOI] [PubMed] [Google Scholar]
- 3.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352. [PubMed] [Google Scholar]
- 4.Braun MY, Grandjean I, Feunou P, et al. Acute rejection in the absence of cognate recognition of allograft by T cells. J Immunol. 2001;166:4879. doi: 10.4049/jimmunol.166.8.4879. [DOI] [PubMed] [Google Scholar]
- 5.Wise MP, Bemelman F, Cobbold SP, Waldmann H. Linked suppression of skin graft rejection can operate through indirect recognition. J Immunol. 1998;161:5813. [PubMed] [Google Scholar]
- 6.Yamada A, Chandraker A, Laufer TM, Gerth AJ, Sayegh MH, Auchincloss H., Jr Recipient MHC class II expression is required to achieve long-term survival of murine cardiac allografts after costimulatory blockade. J Immunol. 2001;167:5522. doi: 10.4049/jimmunol.167.10.5522. [DOI] [PubMed] [Google Scholar]
- 7.Sayegh MH, Khoury SJ, Hancock WW, Weiner HL, Carpenter CB. Induction of immunity and oral tolerance with polymorphic class II major histocompatibility complex allopeptides in the rat. Proc Natl Acad Sci USA. 1992;89:7762. doi: 10.1073/pnas.89.16.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederkorn JY, Mayhew E. Phenotypic analysis of oral tolerance to alloantigens: evidence that the indirect pathway of antigen presentation is involved. Transplantation. 2002;73:1493. doi: 10.1097/00007890-200205150-00021. [DOI] [PubMed] [Google Scholar]
- 9.Hara M, Kingsley CI, Niimi M, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Sakaguchi N. Thymus and autoimmunity: capacity of the normal thymus to produce pathogenic self-reactive T cells and conditions required for their induction of autoimmune disease. J Exp Med. 1990;172:537. doi: 10.1084/jem.172.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151. [PubMed] [Google Scholar]
- 12.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 13.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 14.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Rasmussen J, Rudensky A. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 16.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10- dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Fueyo A, Weber M, Domenig C, Strom TB, Zheng XX. Tracking the immunoregulatory mechanisms active during allograft tolerance. J Immunol. 2002;168:2274. doi: 10.4049/jimmunol.168.5.2274. [DOI] [PubMed] [Google Scholar]
- 18.Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H. Both CD4(+)CD25(+) and CD4(+)CD25(−) regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002;168:5558. doi: 10.4049/jimmunol.168.11.5558. [DOI] [PubMed] [Google Scholar]
- 19.Lechler RI, Garden OA, Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nat Rev Immunol. 2003;3:147. doi: 10.1038/nri1002. [DOI] [PubMed] [Google Scholar]
- 20.Jiang S, Herrera O, Lechler RI. New spectrum of allorecognition pathways: implications for graft rejection and transplantation tolerance. Curr Opin Immunol. 2004;16:550. doi: 10.1016/j.coi.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunological self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 22.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 23.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 24.Apostolou I, Sarukhan A, Klein L, Von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 25.von Boehmer H. Dynamics of suppressor T cells: in vivo veritas. J Exp Med. 2003;198:845. doi: 10.1084/jem.20031358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romagnoli P, Hudrisier D, van Meerwijk JP. Preferential recognition of self antigens despite normal thymic deletion of CD4(+)CD25(+) regulatory T cells. J Immunol. 2002;168:1644. doi: 10.4049/jimmunol.168.4.1644. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Karim M, Kingsley CI, Bushell AR, Sawitzki BS, Wood KJ. Alloantigen-induced CD4+CD25+ regulatory T cells can develop in vivo from CD25-CD4+ precursors in a thymus-independent process. J Immunol. 2004;172:923. doi: 10.4049/jimmunol.172.2.923. [DOI] [PubMed] [Google Scholar]
- 29.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 31.Auchincloss H, Jr, Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of ‘indirect’ recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc Natl Acad Sci USA. 1993;90:3373. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada A, Laufer TM, Gerth AJ, et al. Further analysis of the T-cell subsets and pathways of murine cardiac allograft rejection. Am J Transplant. 2003;3:23. doi: 10.1034/j.1600-6143.2003.30105.x. [DOI] [PubMed] [Google Scholar]
- 33.Katz SI, Tamaki K, Sachs DH. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979;282:324. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- 34.Stingl G, Tamaki K, Katz SI. Origin and function of epidermal Langerhans cells. Immunol Rev. 1980;53:149. doi: 10.1111/j.1600-065x.1980.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 35.Merad M, Manz MG, Karsunky H, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 37.Lagaaij EL, Hennemann IP, Ruigrok M, et al. Effect of one-HLA-DR-antigen-matched and completely HLA-DR-mismatched blood transfusions on survival of heart and kidney allografts. N Engl J Med. 1989;321:701. doi: 10.1056/NEJM198909143211101. [DOI] [PubMed] [Google Scholar]
- 38.Game DS, Hernandez-Fuentes MP, Chaudhry AN. Lechler RI. CD4+CD25+ regulatory T cells do not significantly contribute to direct pathway hyporesponsiveness in stable renal transplant patients. J Am Soc Nephrol. 2003;14:1652. doi: 10.1097/01.asn.0000067411.03024.a9. [DOI] [PubMed] [Google Scholar]
- 39.Salama AD, Najafian N, Clarkson MR, Harmon WE, Sayegh MH. Regulatory CD25+ T cells in human kidney transplant recipients. J Am Soc Nephrol. 2003;14:1643. doi: 10.1097/01.asn.0000057540.98231.c1. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 41.Teh HS, Harley E, Phillips RA, Miller RG. Quantitative studies on the precursors of cytotoxic lymphocytes. I. Characterization of a clonal assay and determination of the size of clones derived from single precursors. J Immunol. 1977;118:1049. [PubMed] [Google Scholar]
- 42.Teh HS, Phillips RA, Miller RG. Quantitative studies on the precursors of cytotoxic lymphocytes. II. Specificity of precursors responsive to alloantigens and to concanavalin A. J Immunol. 1977;118:1057. [PubMed] [Google Scholar]
- 43.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 44.Li XC, Strom TB, Turka LA, Wells AD. T cell death and transplantation tolerance. Immunity. 2001;14:407. doi: 10.1016/s1074-7613(01)00121-2. [DOI] [PubMed] [Google Scholar]
- 45.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 46.Zheng XX, Sanchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19:503. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 49.Peugh WN, Superina RA, Wood KJ, Morris PJ. The role of H-2 and non-H-2 antigens and genes in the rejection of murine cardiac allografts. Immunogenetics. 1986;23:30. doi: 10.1007/BF00376519. [DOI] [PubMed] [Google Scholar]
- 50.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]


