Abstract
BACKGROUND
The purpose of the study was to describe the clinicopathologic characteristics and clinical outcomes of patients with primary breast angiosarcoma.
METHODS
The institutional database was searched to identify breast angiosarcoma patients seen between 1965 and 2002. Survival outcomes were estimated by the Kaplan-Meier method. The log-rank test was used to compare groups. Cox proportional hazards models were used for multivariate analysis.
RESULTS
In all, 69 patients were identified. Median follow-up was 40 months (range, 0–413 months). Median age was 46. Median tumor size at diagnosis was 5.5 cm. Thirteen (18.8%) patients received prior radiation for invasive breast carcinoma. Most patients underwent total mastectomy with (41%) or without (45%) axillary dissection. Regional metastasis to axillary lymph nodes was rare. There were 38 recurrences and 27 deaths. The 5-year overall (OS) and recurrence-free survival (RFS) rates were 61% (95% confidence interval [CI], 49%–76%) and 44% (95% CI, 33%–58%) with estimated medians of 100 and 37 months, respectively. In Cox proportional hazards models, OS and RFS were significantly associated only with T size and not with patient age, prior radiation, or chemotherapy administration. Of 29 patients treated with chemotherapy at recurrence, there were 4 complete and 10 partial responses (48%) with an anthracycline-ifosfamide or gemcitabine-taxane combination.
CONCLUSIONS
Breast angiosarcoma is frequently advanced at diagnosis and has a tendency for local-regional recurrence. A significant number of responses to chemotherapy was observed in the metastatic setting. These data suggest that a multidisciplinary therapeutic approach should be employed in high-risk patients with large primary tumors.
Keywords: breast cancer, angiosarcoma, therapy
Primary soft tissue breast sarcomas are malignant tumors arising from the connective tissue within the breast. They can arise de novo (primary) or as a consequence of treatment of an epithelial breast cancer (secondary).1–5 Breast sarcomas are rare tumors that account for less than 1% of all breast malignancies.6 In data compiled from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute, the annual incidence of breast sarcomas was 4.6 cases per million women.7 As with other nonbreast soft-tissue sarcomas, primary breast sarcomas are histologically heterogeneous. In general, fibrosarcomas, angiosarcomas, malignant fibrous histiocytomas (MFH), phyllodes tumor, and myxoid/round cell liposarcomas comprise the major histologic subtypes.8
Although the etiology of most soft-tissue sarcomas remains unknown, angiosarcoma of the breast has increasingly been associated with prior use of breast external beam radiation therapy and with lymphedema that occurs after surgery with or without radiation treatment for primary breast cancer.9–14 The SEER program data compiled by the National Cancer Institute included more than 194,000 women who were treated for breast carcinoma. Among patients in the radiotherapy cohort, the relative risk of developing angiosarcoma was 15.9.5
Because of the rarity of breast angiosarcoma, only small series of patients have been reported. To date the largest single-center series from a single institution described 55 patients of breast angiosarcoma.15 The aim of our retrospective review was to expand our series of breast angiosarcomas in order to study the behavior, clinical features, and outcome of this rare disease, as well as the impact of prior radiation therapy on the clinical features of angiosarcoma of the breast.
MATERIALS AND METHODS
This retrospective study included the 69 patients with primary angiosarcoma of the breast who were evaluated at M. D. Anderson Cancer Center between 1965 and 2002. Histological diagnosis was made by core-needle biopsy or excisional biopsy of the breast tumor. All pathologic specimens were reviewed by dedicated breast and sarcoma pathologists at M. D. Anderson. The histologic type of all tumors was defined according to the World Health Organization's classification system. The Institutional Review Board of M. D. Anderson approved the retrospective review of the medical records for the purposes of this report.
Patient characteristics and treatments received were tabulated or described by their median and range, as appropriate. Characteristics were compared between groups with and without prior radiation therapy with the chi-squared test, Fisher exact test, or the Wilcoxon rank sum test, as appropriate. Overall survival (OS) was measured from the date of surgery to the date of last follow-up. Recurrence-free survival (RFS) was measured from the date of surgery to the date of disease recurrence or last follow-up. Patients who died before experiencing a disease recurrence were considered censored at their date of death. Locoregional recurrence was measured from the date of surgery to the date of locoregional recurrence. Patients who experienced distant disease recurrence were considered censored at their recurrence date and patients who died before experiencing a disease recurrence were considered censored at their date of death. Median follow-up was calculated as the median observation time among all patients. Survival outcomes were estimated by the Kaplan-Meier method and the log-rank statistic was used to compare groups. Cox proportional hazards models were fit to determine the association between continuous characteristics and survival outcomes and also to explore the association between prior radiation therapy and survival outcomes after adjustment for other characteristics. P-values less than 0.05 were considered statistically significant. Analyses were performed with S-plus 7.0 (Insightful, Seattle Wash) software.
RESULTS
We identified 69 patients with nonmetastatic (M0) angiosarcoma of the breast at diagnosis. Table 1 shows the tabulation of patient characteristics and treatments received. All patients were female, most were white (88%), and the median age at diagnosis was 46. The median tumor size at diagnosis (pathology specimen) was 5.5 cm. Because of the histologic diagnosis of angiosarcoma, most patients did not have estrogen (ER) or progesterone receptor (PR) assayed. However, among 13 patients that did have ER or PR information, most were negative (91.7%). Most patients (86%) received mastectomy with (n = 28) or without (n = 31) axillary node dissection. Among patients that received axillary dissection, the median number of nodes removed was 10 and only 2 patients had lymph nodes that were pathologically positive for metastatic angiosarcoma. Fifteen (22%) patients received neoadjuvant chemotherapy, 30 (44%) received adjuvant chemotherapy, 1 patient received neoadjuvant radiotherapy, and 21 (31%) patients received adjuvant radiotherapy.
TABLE 1.
Patient and Tumor Characteristics
| Characteristic | N = 69* no. | % | |
|---|---|---|---|
| Age | Minimum | 15 | |
| Median | 46 | ||
| Maximum | 82 | ||
| Race | White | 61 | 88.40 |
| Black | 3 | 4.30 | |
| Hispanic | 4 | 5.80 | |
| Other | 1 | 1.40 | |
| Site | Right | 36 | 52.20 |
| Left | 32 | 46.30 | |
| Bilateral | 1 | 1.40 | |
| Tumor size (cm) | Minimum | 1 | |
| Median | 5.5 | ||
| Maximum | 14 | ||
| Tumor grade | High | 52 | 74.40 |
| Low | 17 | 25.60 | |
| T stage | T1 | 24 | 49.20 |
| T2 | 25 | 50.70 | |
| Lymph nodes dissection | Yes | 31 | 44.90 |
| No | 38 | 55.10 | |
| Lymph nodes | Positive | 2 | 6.5 |
| Negative | 29 | 93.5 | |
| Neoadjuvant chemotherapy | Yes | 15 | 21.70 |
| No | 54 | 78.30 | |
| Adjuvant chemotherapy | Yes | 30 | 44.10 |
| No | 38 | 55.90 | |
| Adjuvant radiotherapy | Yes | 46 | 67.70 |
| No | 21 | 39.90 | |
| Previous radiotherapy | Yes | 13 | 18.80 |
| No | 56 | 81.20 |
Due to missing data for some patients, the total numbers do not always add up to 69.
Thirteen (19%) patients had received prior breast radiation for carcinoma. The median interval between initiation of radiotherapy and development of breast angiosarcoma was 7 years. Among patients with no prior radiotherapy the median age was 42 (range, 15–70), and among those with prior radio-therapy the median age was 72 (range, 48–82, P < .0001). The angiosarcoma tumor size at diagnosis was not significantly different between the 2 groups defined by prior radiation therapy administration for invasive breast cancer. Those having had prior breast irradiation for breast carcinoma did not receive adjuvant radiotherapy after primary surgery for breast angiosarcoma, in contrast to those patients who had not received prior breast radiation (0/13 vs 21/54, P = .01). Patients who had received prior radiation also tended to undergo mastectomy for angiosarcoma more frequently than other patients (12/13 vs 47/56, P = .67) and were more likely to receive a taxane in the (neo)adjuvant setting, although these associations did not achieve statistical significance.
The median follow-up of all patients was 40 months (range, 0–413 months). Table 2 shows the Kaplan-Meier estimates of OS and RFS as well as the univariable association of administered chemotherapy and radiotherapy on survival rates (Figs. 1–3). Adjuvant chemotherapy and adjuvant radiotherapy were included in this analysis, although these treatment decisions have the potential to be biased by multiple factors (age and comorbid conditions). The OS of all patients is shown in Table 2. Twenty-seven patients have died and the median OS was 100 months. OS was not significantly associated with prior radiation in this univariable analysis. Table 3 shows the results of the multivariate analysis for OS. The axillary lymph node status was not included in this analysis because only 2 patients had positive axillary lymph nodes. Adjuvant radiotherapy was not included in the model because it was collinear with prior radiation. After adjustment for prior radiation, age at diagnosis, and use of chemotherapy, tumor size remained significantly associated with OS (hazard ratio [HR], 1.34, 95% CI (1.09, 1.65), P = .006). Age at diagnosis was not significantly associated with OS (HR, 0.98, 95% CI (0.95, 1.02), P = .39).
TABLE 2.
Survival Estimates
| Survival estimates | No. | No. events | Median, mo | 5-y estimate | 95% CI | 10-y estimate | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| Overall survival | ||||||||
| All patients | 69 | 27 | 100 | 61% | 48.5%, 75.9% | 41% | 27.8%, 60.9% | |
| Adjuvant chemotherapy | ||||||||
| No | 30 | 13 | 100 | 60% | 42.6%, 83% | 39% | 21.1%, 70.7% | |
| Yes | 38 | 14 | 79 | 61% | 44.6%, 83.2% | 42% | 24%, 72% | .59 |
| Any chemotherapy | ||||||||
| No | 31 | 11 | 119 | 65% | 47.4%, 88.5% | 44% | 25.5%, 76.6% | |
| Yes | 38 | 16 | 100 | 58% | 42.7%, 78.8% | 38% | 20.9%, 68% | .43 |
| Adjuvant Radiotherapy | ||||||||
| No | 21 | 11 | 57 | 50% | 30.9%, 79.4% | 33% | 15.8%, 69% | |
| Yes | 46 | 16 | 100 | 65% | 50.3%, 84.3% | 44% | 27.3%, 70.4% | .51 |
| Recurrence-free survival | ||||||||
| All patients | 69 | 38 | 37 | 44% | 32.8%, 58.4% | 34% | 22.3%, 51.5% | |
| Adjuvant chemotherapy | ||||||||
| No | 30 | 15 | 42 | 42% | 25.9%, 66.9% | 42% | 25.9%, 66.9% | |
| Yes | 38 | 23 | 37 | 45% | 31%, 64.5% | 28% | 14%, 54.4% | .46 |
| Any chemotherapy | ||||||||
| No | 31 | 19 | 24 | 46% | 31%, 68.1% | 28% | 14.2%, 56.7% | |
| Yes | 38 | 19 | 37 | 41% | 26.9%, 63.2% | 41% | 26.9%, 63.2% | .51 |
| Adjuvant radiotherapy | ||||||||
| No | 21 | 15 | 24 | 33% | 18.2%, 61% | 25% | 10.9%, 57.2% | |
| Yes | 46 | 23 | 45 | 47% | 33.6%, 66.4% | 37% | 22.5%, 60.3% | .28 |
| Local-regional recurrence-free survival | ||||||||
| Adjuvant radiotherapy | ||||||||
| No | 15 | 8 | 24 | 20% | 3.92%, 99% | – | – | |
| Yes | 23 | 14 | 22 | 24% | 8.43%, 66.3% | – | – | .64 |
CI indicates confidence interval.
FIGURE 1.
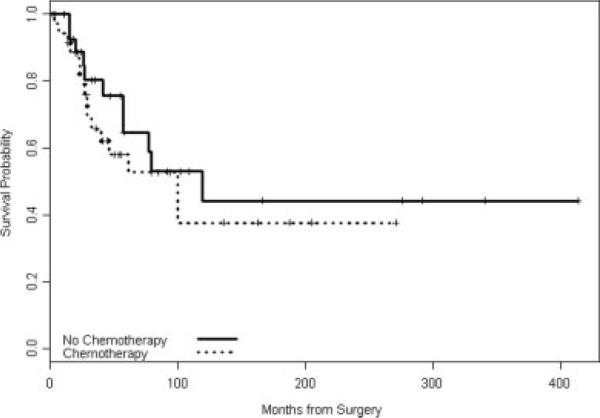
Overall survival by any chemotherapy.
FIGURE 3.

Local recurrence-free survival by radiotherapy.
TABLE 3.
Multivariable Model Results for Overall and Recurrence-Free Survival
| Hazard ratio | 95% CI | P | |
|---|---|---|---|
| Overall survival | |||
| Prior radiation (yes vs no) | 2.71 | 0.39, 18.86 | .31 |
| Age at diagnosis (continuous) | 0.98 | 0.95, 1.02 | .39 |
| Tumor size (continuous) | 1.34 | 1.09, 1.65 | .006 |
| Any chemotherapy (yes vs no) | 0.67 | 0.22, 2.05 | .48 |
| Recurrence-free survival | |||
| Prior radiation (yes vs no) | 1.56 | 0.26, 9.22 | .63 |
| Age at diagnosis (continuous) | 0.97 | 0.94, 1 | .09 |
| Tumor size (continuous) | 1.18 | 1, 1.39 | .05 |
| Any chemotherapy (yes vs no) | 0.47 | 0.19, 1.18 | .11 |
CI indicates confidence interval.
RFS among all patients is shown in Table 2. Thirty-eight patients experienced a disease recurrence and the median RFS was 37 months. The most common site of recurrence was local-regional (52.6%) followed by liver (13.2%), bone (10.5%), and lung (10.5%). RFS was not significantly associated with chemotherapy or radiotherapy administration (Table 2) or with history of prior radiation therapy in the univariable analysis. In the multivariate analysis (Table 3) prior radiation, use of chemotherapy, and age at diagnosis (HR, 0.97, 95% CI (0.94, 1.0), P = .09) were not significantly associated with RFS; tumor size remained statistically significant (HR, 1.18, 95% CI (1.0, 1.39), P = .05). Of 5 patients who had received prior radiation and who experienced a disease recurrence, all had a local-regional recurrence. Among 33 patients who had not received prior radiation and who experienced a disease recurrence 45.5% had a local-regional recurrence, 15.2% recurred in the liver, 12.1% recurred in the bone, and 12.1% recurred in the lung. In a uni-variable analysis it was determined that adjuvant radiation therapy administration was not significantly associated with local-regional RFS (Table 2).
Finally, the responsiveness of metastatic angiosarcoma of the breast to first-line cytotoxic chemo-therapy was examined retrospectively. Of 29 patients treated with a first-line anthracycline-ifosfamide or gemcitabine-taxane chemotherapy combination at disease recurrence, there were 4 complete and 10 partial responses (48% overall response rate) determined from radiographic and/or clinical examinations that were documented in the clinical records.
DISCUSSION
In our analysis of 69 patients with nonmetastatic angiosarcoma of the breast, the estimated median overall and recurrence-free survival times were 100 and 37 months, respectively, suggesting that the disease is aggressive despite the rarity of angiosarcoma metastasis to the regional lymph nodes at diagnosis. We noted recurrent disease in 55% of patients after a median follow-up of only 40 months. OS and RFS were significantly associated only with primary tumor size on multivariate analysis, a finding consistent with other studies.6,16 Survival was not favorably associated with (neo)adjuvant chemotherapy or radiotherapy administration in our study. However, this is not conclusive evidence that breast angiosarcoma is not a chemosensitive disease. The lack of a survival association with (neo)adjuvant chemotherapy administration may be due to the retrospective nature of the study, the relatively small number of patients, and to selection bias favoring chemotherapy in those patients with greater burden of disease and/or poorly differentiated tumors. Indeed, the responsiveness of metastatic breast angiosarcoma to combination cytotoxic chemotherapy (48% overall response rate) suggests that it is likely to be a chemosensitive disease, specifically in terms of anthracycline-ifosfamide and gemcitabine-taxane combinations.
Angiosarcoma of the breast is a rare disease. The true incidence of angiosarcoma after previous treatment for breast cancer is unknown but is thought to be exceedingly low. In a case-control study Cozen et al.13 reviewed the incidence of angiosarcoma in women in Los Angeles County with a history of invasive breast carcinoma. The odds ratio was 59 for developing an upper extremity angiosarcoma and 11.6 for developing either a chest wall or breast angiosarcoma. Marchal et al.14 surveyed the French Comprehensive Cancer Center experience with angiosarcoma after breast-conserving therapy and reported an approximate prevalence of 5 per 10,000. Another series reported that angiosarcoma constitutes more than 50% of all the sarcomas identified in patients previously treated with radiation therapy, but only 20% of sarcomas identified in patients who have not been previously treated with radiation therapy.9
In our study population, patients who had prior radiation-associated (secondary) breast angiosarcoma were significantly older than those patients who had primary or de novo tumors. The median interval between initiation of prior breast radiotherapy and the development of breast angiosarcoma was 7 years in our study; a similar interval has been reported in other series of breast sarcoma.17–19 However, the median duration of development of radiation-associated sarcomas in other soft-tissue sites has been reported to be 10.3 to 14 years.20,21 The multivariate model in our study suggested, after adjustment for other characteristics, that patients who received prior radiotherapy had 2.71 times the risk of death and 1.56 times the risk of disease recurrence compared with patients who had not received prior radiotherapy, although this effect did not attain statistical significance, (Table 3).
Given the rarity of breast angiosarcoma and the retrospective nature of the analysis, this study primarily is a descriptive one. However, a number of observations can be made. Breast angiosarcoma is an aggressive disease with a tendency to local-regional recurrence. The most significant feature at diagnosis associated with prognosis appears to be tumor size. The apparent lack of a survival benefit from (neo)adjuvant chemotherapy or adjuvant radiation therapy is likely to be the result of small sample size and patient selection bias. Given the activity of cytotoxic chemotherapy in metastatic angiosarcoma of the breast, this therapeutic modality should certainly be considered for patients with high-risk localized breast angiosarcoma, and anthracycline-ifosfamide and gemcitabine-taxane chemotherapy regimens appear to be highly active. Due to the propensity for local-regional failure in this disease, local therapies should be a fully optimized component of the multi-disciplinary care of this disease. There are certain features of radiation-related breast angiosarcoma that suggest it may have a different natural history to primary or de novo breast angiosarcoma including older age at presentation and higher risk of systemic disease at presentation.15
FIGURE 2.
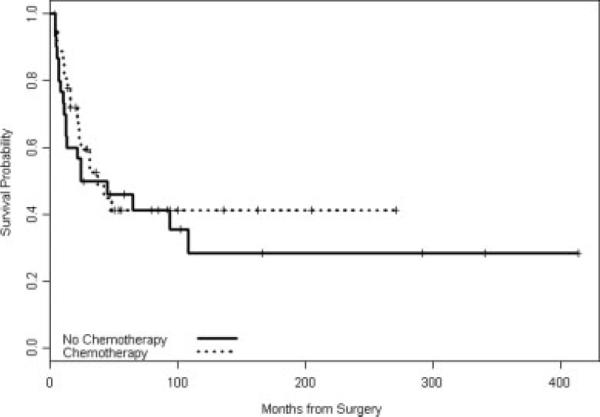
Recurrence-free survival by any chemotherapy.
Acknowledgments
Supported by the Nellie B. Connally Breast Cancer Research Fund. Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, Atlanta, Georgia, June, 2006.
REFERENCES
- 1.Karlsson P, Holmberg E, Johansson KA, Kindblom LG, Carstensen J, Wallgren A. Soft tissue sarcoma after treatment for breast cancer. Radiother Oncol. 1996;38:25–31. doi: 10.1016/0167-8140(95)01663-5. [DOI] [PubMed] [Google Scholar]
- 2.Brady MS, Garfein CF, Petrek JA, Brennan MF. Post-treatment sarcoma in breast cancer patients. Ann Surg Oncol. 1994;1:66–72. doi: 10.1007/BF02303543. [DOI] [PubMed] [Google Scholar]
- 3.Lagrange JL, Ramaioli A, Chateau MC, et al. Sarcoma after radiation therapy. Radiology. 2000;216:197–205. doi: 10.1148/radiology.216.1.r00jl02197. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard DK, Reynolds C, Grant CS, Farley DR, Donohue JH. Radiation-induced breast sarcoma. Am J Surg. 2002;184:356–358. doi: 10.1016/s0002-9610(02)00943-1. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer. 2001;92:172–180. doi: 10.1002/1097-0142(20010701)92:1<172::aid-cncr1306>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.McGowan TS, Cummings BJ, O'Sullivan B, Catton CN, Miller N, Panzarella T. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys. 2000;46:383–390. doi: 10.1016/s0360-3016(99)00444-7. [DOI] [PubMed] [Google Scholar]
- 7.Zelek L, Llombart-Cussac A, Terrier P, et al. Prognostic factors in primary breast sarcomas. J Clin Oncol. 2003;21:2583–2588. doi: 10.1200/JCO.2003.06.080. [DOI] [PubMed] [Google Scholar]
- 8.Adem C, Reynolds C, Ingle JN, Nascimento AG. Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer. 2004;91:237–241. doi: 10.1038/sj.bjc.6601920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap J, Chuba PJ, Thomas R, et al. Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiation Oncol Biol Phys. 2002;52:1231–1237. doi: 10.1016/s0360-3016(01)02799-7. [DOI] [PubMed] [Google Scholar]
- 10.Pendlebury SC, Bilous M, Langlands AO. Sarcoma following radiation therapy for breast cancer: a report of three cases and review of the literature. Int J Radiat Oncol Biol Phys. 1995;31:405–410. doi: 10.1016/0360-3016(95)93157-3. [DOI] [PubMed] [Google Scholar]
- 11.Hartfield PM, Schultz MD. Post-irradiation sarcoma. Including 5 cases after X-ray therapy of breast carcinoma. Radiology. 1970;96:593–602. doi: 10.1148/96.3.593. [DOI] [PubMed] [Google Scholar]
- 12.Taghian A, de Vathaire, Terrier P, et al. Long term risk of sarcoma following radiation treatment for breast cancer. Int J Radiat Oncol Biol Phys. 1991;21:361–367. doi: 10.1016/0360-3016(91)90783-z. [DOI] [PubMed] [Google Scholar]
- 13.Cozen W, Bernstein L, Wang F, Press MF, Mack TM. The risk of angiosarcoma following primary breast cancer. Br J Cancer. 1999;81:532–536. doi: 10.1038/sj.bjc.6690726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchal C, Weber B, De Lafontan B, et al. Nine breast angiosarcomas after conservative treatment for breast carcinoma: a survey from French Comprehensive Cancer Center. Int J Radiat Oncol Biol Phys. 1999;44:113–119. doi: 10.1016/s0360-3016(98)00537-9. [DOI] [PubMed] [Google Scholar]
- 15.Vorburger SA, Xing Y, Hunt KK, et al. Angiosarcoma of the breast. Cancer. 2005;104:2682–2688. doi: 10.1002/cncr.21531. [DOI] [PubMed] [Google Scholar]
- 16.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 17.Stokkel MP, Peterse HL. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69:2965–2968. doi: 10.1002/1097-0142(19920615)69:12<2965::aid-cncr2820691216>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Otis CN, Peschel R, McKhann C, et al. The rapid onset cutaneous angiosarcoma after radiotherapy for breast carcinoma. Cancer. 1986;57:2130–2134. doi: 10.1002/1097-0142(19860601)57:11<2130::aid-cncr2820571108>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Cafiero F, Gipponi M, Peressini A, et al. Radiation-associated angiosarcoma: diagnostic and therapeutic implications—two case reports and a review of the literature. Cancer. 1996;77:2496–2502. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2496::AID-CNCR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Brady MS, Gaynor JJ, Bernnan MF. Radiation-associated sarcoma of bone and soft tissue. Arch Surg. 1992;127:1379–1385. doi: 10.1001/archsurg.1992.01420120013002. [DOI] [PubMed] [Google Scholar]
- 21.Mark RJ, Poen J, Tran LM, et al. Postirradiation sarcomas. A single-institution study and review of the literature. Cancer. 1994;73:2653–2662. doi: 10.1002/1097-0142(19940515)73:10<2653::aid-cncr2820731030>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]


