Abstract
BACKGROUND
The objective of this study was to determine whether patients with breast cancer who received breast-conservation therapy after neoadjuvant chemotherapy had improved outcomes if radiopaque clips were placed to mark the primary tumor.
METHODS
The authors retrospectively reviewed the records of 410 patients with nonmetastatic breast cancer who received doxorubicin-based neoadjuvant chemotherapy and breast-conservation therapy from January 1990 to September 2005. Thirty-seven of those patients were omitted because of the inability to verify radiopaque clip placement in the primary tumor.
RESULTS
Of the 373 patients who were analyzed, 145 patients had radiopaque clips placed to mark the primary tumor before or during neoadjuvant chemotherapy, and 228 patients did not. The distribution of clinical T classification, nuclear grade, estrogen receptor status, final margin status, and extent of residual primary disease was similar between the 2 groups. After a median follow-up of 49 months (range, 20–177 months), 21 patients developed a local recurrence in the treated breast. The 5-year rate of local control was 98.6% in patients who had radiopaque clips placed versus 91.7% in patients who did not have tumor marker clips placed (P = .02; log-rank test). On multivariate analysis, the omission of tumor bed clips was associated with a hazard ratio of 3.69 for increased local recurrence compared with patients who did have radiopaque clip placement (P = .083; 95% confidence interval, 0.84–16.16).
CONCLUSIONS
The placement of radiopaque clips in patients who were receiving neoadjuvant chemotherapy and breast-conservation therapy was associated with better local control independent of stage and other clinicopathologic findings. The authors concluded that the placement of tumor-marker clips should be an integral part of the multidisciplinary approach in appropriate patients.
Keywords: breast cancer, neoadjuvant chemotherapy, breast-conservation therapy, locoregional recurrence, radiopaque clip
Neoadjuvant chemotherapy is the standard of care for patients with inoperable and locally advanced breast cancer, and its use is increasing in patients with earlier stage, operable breast cancer. From 80% to 90% of patients have a significant clinical response rate of the primary tumor to neoadjuvant chemotherapy. Although the dramatic response is desirable, and it has been demonstrated that a pathologic complete response (pCR) is prognostic, clinical and radiologic complete tumor response complicates the surgical excision because it is difficult to verify accurate localization of the site of the previous tumor.
The use of a radiopaque marker placed in the tumor bed has been reported as a safe and inexpensive technique that allows for subsequent localization of the tumor bed before surgical resection in patients who are receiving neoadjuvant chemotherapy.1–6 A report on 28 patients who underwent clip placement and preoperative chemotherapy indicated that preoperative wire localization of the tumor bed would have been impossible in 35.7% of patients and difficult in 21.4% of patients without the aid of the clip. Dash et al. concluded that the clip placement was valuable in 57% of patients at the time of preoperative needle localization.4 Edeiken and colleagues reported a similar experience with ultrasound-guided implantation of metallic markers in 49 patients to mark the tumor bed in anticipation of complete or near complete response to neoadjuvant chemotherapy. The markers reportedly were the only remaining evidence of the original tumor site in 23 of 49 patients (47%), and the authors concluded that this technique effectively addresses the problem of preoperative localization of the tumor bed in patients who are expected to achieve a complete or near complete response to neoadjuvant chemotherapy.5
Although the studies described above support the utility of clip placement in approximately 50% of individuals for preoperative tumor bed localization in patients who are receiving neoadjuvant chemotherapy, the potential impact on locoregional control currently is unknown. The objective of this study was to determine whether patients who were treated with breast conservation after neoadjuvant chemotherapy had improved local control if radiopaque coils/clips were placed to mark the primary tumor.
MATERIALS AND METHODS
The medical records of 410 patients with nonmetastatic breast cancer who were treated from January 1990 to September 2005 with doxorubicin-based neoadjuvant chemotherapy followed by breast-conservation surgery and whole-breast radiation were reviewed retrospectively. The placement of a tumor bed coil/clip was determined through the review of radiology reports. Thirty-seven patients were omitted because of the inability to verify the presence or absence of radiopaque clips, and 373 analyzable patients were left. Data from 248 patients who were included in this analysis were analyzed in a previous publication from our group.7 For the purpose of this study, we updated the database of these patients to include the presence or absence of radiopaque clips and the long-term clinical outcome.
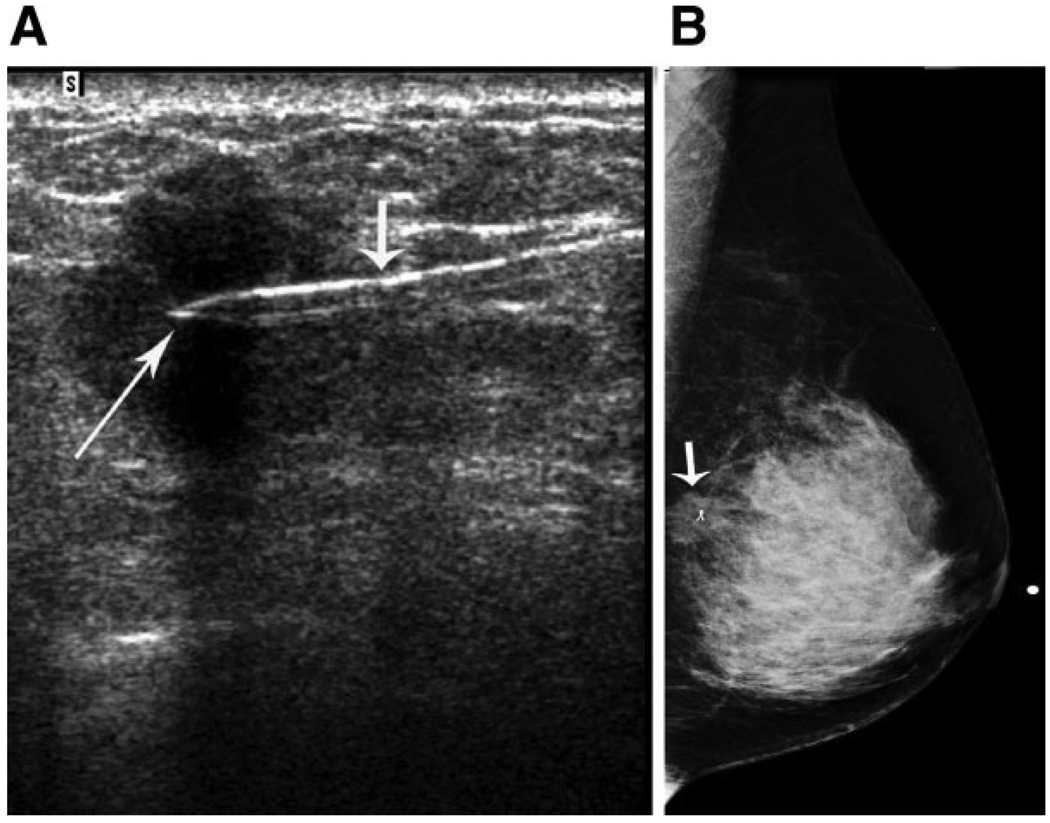
The neoadjuvant chemotherapy regimen consisted of 4 to 6 cycles of combined 5-fluorouracil, doxorubicin, and cyclophosphamide, and 53% of patients received additional taxane therapy. During the study period, general guidelines within the institution for placement of radiopaque clips in the tumor bed included the following (tumor measurements were based on physical examination and imaging studies): 1) for patients with advanced lymph node disease, clip placement was recommended when the tumor measured ≤2 cm at the initiation of chemotherapy; 2) for patients with tumors that measured >2 cm, clip placement was recommended when a 50% reduction in tumor size occurred; and 3) for patients with multifocal breast cancer, clip placement was recommended in the primary tumor and in any satellite lesions at the initiation of chemotherapy. In general, patients who had microcalcifications that fully delineated the area of the primary tumor or who had a clip placed at the time of the initial diagnostic biopsy did not have clips placed. Although these were the general guidelines to select appropriate patients for radiopaque clip placement, there were patients who met the criteria but did not have clip placement for unknown reasons. The technique of marker implantation under ultrasound guidance has been reported previously and is summarized for this report.5 The marker clips typically were placed in the center of the tumor mass but, at the discretion of the surgeon and/or radiologist, could be placed alternatively at the periphery of the tumor mass. The marker clips were placed under sonographic guidance, and postprocedural mammograms were obtained to confirm that the clips were placed in the appropriate location (Fig. 1). The records verifying placement of radiopaque clips to mark the tumor bed were identified by detailed radiology reports that described the clip placement and mammographic confirmation of the markers in the breast.
FIGURE 1.
Placement and postprocedure mammogram of radiopaque clip in a known invasive ductal carcinoma in a woman aged 48 years. (A) Transverse left breast sonography shows the introducer (short arrow) and the clip marker (long arrow; UltraCLIP II Tissue Marker; INRAD, Inc., Grand Rapids, Mich) in the hypoechoic mass representing the known invasive ductal carcinoma. (B) Left lateromedial mammogram shows the clip marker within the known invasive ductal carcinoma (arrow). The patient was treated with preoperative paclitaxel and combined 5-fluorouracil, doxorubicin and cyclophosphamide chemotherapy. Left breast segmental mastectomy demonstrated residual invasive ductal carcinoma, and left axillary dissection revealed 1 metastatic lymph node of 18 lymph nodes.
Locoregional therapy consisted of a segmental mastectomy and a Level I and II axillary lymph node dissection or sentinel lymph node dissection followed by adjuvant irradiation of the intact breast with or without the regional lymphatics, depending on an individual patient’s risk. At the time of surgery, the markers were identified with ultrasound; however, in select patients in whom ultrasound could not depict the markers or residual tumor adequately, preoperative, mammographically guided needle localization of the markers was performed. Before pathologic evaluation for residual carcinoma and surgical margins, a specimen radiograph was obtained to confirm successful excision of the tumor bed and all implanted markers.5 An important surgical consideration to note is that no attempt is made to remove the prechemotherapy volume of disease. Instead, the aim of a segmental mastectomy after neoadjuvant chemotherapy is to remove all residual foci of clinically evident or radiographically visible disease with negative surgical margins. The target volume of resection is the postchemotherapy abnormality with all tumor bed clips.8 The median dose to the breast was 50 Gray (Gy): A 10-Gy electron boost was delivered to the lumpectomy bed in patients who had negative margins, or a 14-Gy lumpectomy boost was delivered to patients who had margins that measured <2 mm. Positive margins received a 16-Gy lumpectomy boost. Re-excision was undertaken for patients with initial close/positive margins unless the patient refused or the surgeon believed that additional surgery would lead to an unacceptable cosmetic outcome. Mammogram and ultrasound studies were obtained at the time of initial presentation, at the time of radiopaque clip implantation (when performed), midway during the planned chemotherapy regimen, and again before surgical intervention. Clinical examination, mammography, and ultrasound reports were used to measure tumor size, assess response to chemotherapy, and verify the presence or the absence of radiopaque clips.
Data analysis was performed using Stata 9.2 statistical software. Tumor characteristics were compared between groups using the chi-square statistic, and univariate analysis was performed using the Kaplan-Meier method. The Cox proportional hazards model was used for multivariate analysis to assess the effect of patient characteristics and other prognostic factors on local tumor control. All variables with a significance level ≥.25 on univariate analysis were entered into the model, and a backward elimination was carried out. The final model consisted of variables with a significance value ≤.05 or that had biologic significance to the model. The estimated hazard is reported. The Wald test was used to assess the role of covariates in the model.9
RESULTS
Clinical Features
The median follow-up was 49 months (range, 20–177 months). The median patient age was 47 years (range, 25–84 years). The majority of patients (62.7%) presented with T2 tumors. Clinical T3 tumors were present in 19.8% of patients, and 13.7% had T1 tumors. Nearly 4% of patients presented with clinical T4b tumors with various degrees of skin edema; and, in 1 patient, a focus of skin ulceration was present. Although patients with T4 tumors typically undergo mastectomy, these 14 patients were seen in the multidisciplinary clinic and, because of limited skin involvement or a dramatic response to neoadjuvant chemotherapy, were selected carefully for breast-conservation therapy. The extent of regional lymph node disease at presentation was N0 or N1 disease in 80% of patients, N2 disease in 12% of patients, and N3 disease in 8% of patients. Overall clinical stage at presentation was 63.5% stage II, 31% stage III, and 5.5% stage I.
Clinical tumor size, lymph node status, and overall stage at presentation were compared between the patients who did or did not have placement of radiopaque clips (Table 1). Although the T classification was balanced between the patients with or without clips placed, the patients without clip placement had more advanced lymph node disease. Among the patients who had clips placed, 87% had N0 or N1 lymph node disease, and 13% had N2 or N3 disease. Among patients without clip placement, 75.5% presented with N0 or N1 lymph node disease, and 24.5% presented with N2 or N3 disease (P < .001). When comparing overall stage, stage I, II, and III represented 7%, 73%, and 20% of patients with radiopaque clip placement, respectively, compared with 4.8%, 57.5%, and 37.7%, respectively, of patients without clip placement (P = .001).
TABLE 1.
Patient and Tumor Characteristics
| No. of patients (%) | |||
|---|---|---|---|
| Characteristic | With clips | Without clips | P |
| Median age, y | 47 | 45 | — |
| Tumor classification | .079 | ||
| T1 | 22 (15.2) | 29 (12.7) | |
| T2 | 99 (68.3) | 135 (59.2) | |
| T3 | 21 (14.5) | 53 (23.2) | |
| T4 | 3 (2.1) | 11 (4.8) | |
| Lymph node classification | <.001 | ||
| N0 | 75 (51.7) | 70 (30.7) | |
| N1 | 51 (35.2) | 102 (44.7) | |
| N2 | 9 (6.2) | 37 (16.2) | |
| N3 | 10 (6.9) | 19 (8.3) | |
| Stage | .001 | ||
| I | 10 (6.9) | 11 (4.8) | |
| II | 106 (73.1) | 131 (57.5) | |
| III | 29 (20) | 86 (37.7) | |
| Margins | .368 | ||
| Negative | 129 (89) | 191 (83.8) | |
| Close | 12 (8.3) | 23 (10.1) | |
| Positive | 4 (2.8) | 12 (5.3) | |
| Estrogen receptor status | .250 | ||
| Positive | 65 (44.8) | 96 (42.1) | |
| Negative | 76 (52.4) | 117 (51.3) | |
| Unknown | 4 (2.8) | 15 (6.6) | |
| Nuclear grade | .697 | ||
| 1 | 8 (5.5) | 16 (7) | |
| 2 | 51 (35.2) | 72 (31.6) | |
| 3 | 86 (59.3) | 140 (61.4) | |
| LVSI | .005 | ||
| Positive | 17 (11.7) | 48 (21.1) | |
| Negative | 101 (69.7) | 159 (69.7) | |
| Unknown | 27 (18.6) | 21 (9.2) | |
| Total | 145 | 228 | |
LVSI indicates lymphovascular space invasion.
We also examined estrogen receptor status, tumor nuclear grade, and the presence or absence of lymphovascular space invasion. The estrogen receptor status was known for 354 of 373 patients. The rate of estrogen receptor-positive tumors among the patients who had radiopaque clip placement was 45% compared with 42% in patients without clips; estrogen receptor-negative tumors comprised 52.4% and 51.3% of patients with and without clips, respectively (P = .258). The tumor nuclear grade also was balanced well between the 2 groups. In patients with clip placement, the percentages with nuclear grade 1, 2, and 3 tumors were 5.5%, 35.2%, and 59.3%, respectively. In patients without a tumor marker placed, the tumor grade distribution was very similar (7%, 31.6%, and 61.4%, respectively; P = .697).
The status of lymphovascular invasion required specific documentation in pathology reports as either positive or negative. If there was no mention of lymphovascular invasion, then these patients were coded as unknown and made up 13% of the study population. Among the patients who had radiopaque clip placement, 70% were negative, 12% were positive, and 18% were unknown for lymphovascular invasion compared with patients without clip placement (70% negative, 21% positive, and 9% unknown for lymphovascular invasion; P = .005). The overall number of patients with lymphovascular invasion was modest (65 of 373 patients; 17%). The 2 groups were balanced with regard to the number of patients negative for lymphovascular space invasion (69.7% in both groups) (Table 1).
Imaging Findings
The incidence of radiopaque clip placement among all 373 patients was 39%. The reason for omission of clips was not clear in the majority of these patients. Only 6 of 21 patients (28.6%) who did not have clips and who had an ipsilateral breast tumor recurrence had microcalcifications associated with the index breast lesion on initial mammograms.
Response to Chemotherapy
Pathologic response of the breast tumor after neoadjuvant chemotherapy was reported in all but 3.8% of patients. Pathologic responses were grouped into 3 categories: pCR when no residual invasive tumor was identified in the primary tumor, near pCR when the residual tumor measured ≤1 cm in greatest dimension, and residual disease for any specimen with >1 cm of residual invasive carcinoma. The pathologic response to chemotherapy was balanced between the patients with and without clip placement (P = .257) (Table 2). We observed an overall pCR rate of 23.3% (25.5% in patients with clips, 21.9% in patients without clips; P = .257), a near pCR rate of 27.3% (30.3% with clips, 25.4% without clips), and a residual disease rate of 45.6% (39.3% with clips, 49.6% without clips). Surgical margin status was not statistically different between the groups with and without radiopaque clip placement (P = .368). The rates of negative surgical margins after segmental mastectomy were 89% and 83.8% in patients with and without clips, respectively. Close margins, which we defined as <2 mm, were present in 8.3% and 10.1% of patients with and without clips, respectively. Positive margins were present in 2.8% of patients with clips and in 5.3% of patients without clips (Table 1).
TABLE 2.
Pathologic Response to Neoadjuvant Chemotherapy
| No. of patients (%) | ||
|---|---|---|
| Response after neoadjuvant chemotherapy | With clips | Without clips |
| pCR, no residual | 37 (25.5) | 50 (21.9) |
| Near pCR, ≤1 cm residual | 44 (30.3) | 58 (25.4) |
| Residual disease, >1 cm residual | 57 (39.3) | 113 (49.6) |
| Unknown | 7 (4.8) | 7 (3.1) |
pCR indicates pathologic complete response.
Local, Regional, and Systemic Failures
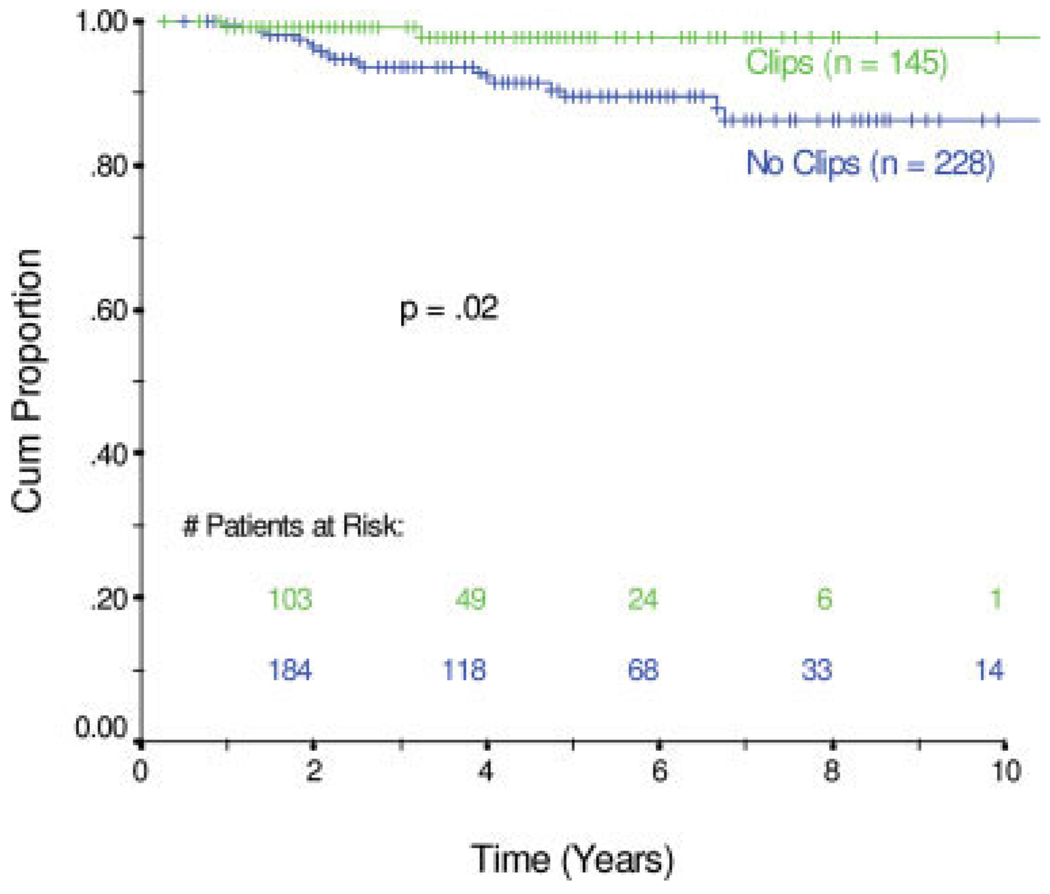
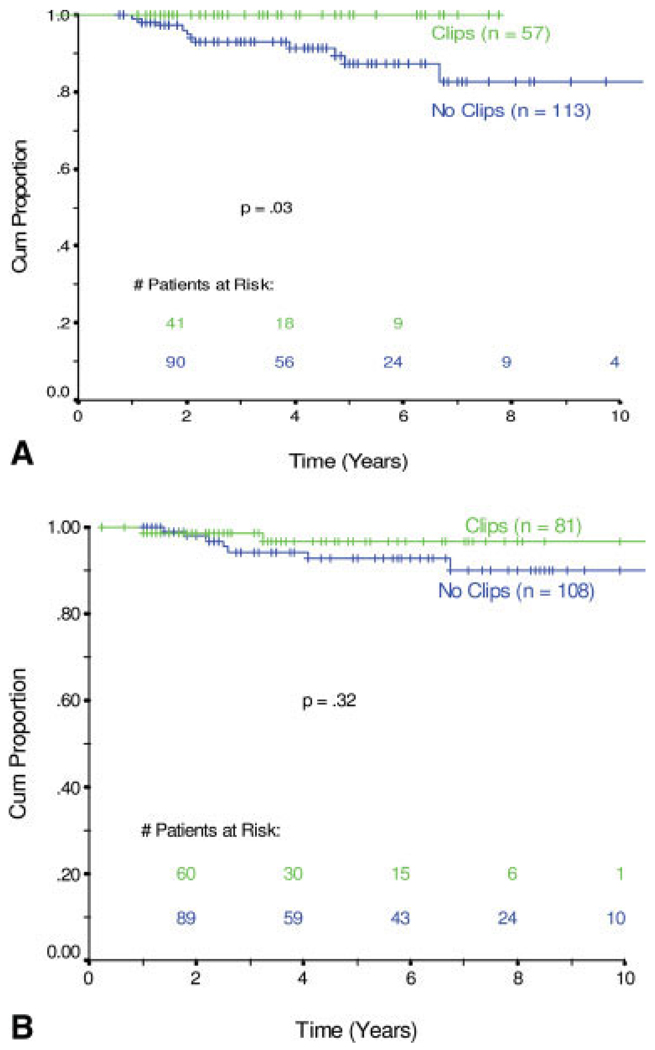
In total, 21 patients developed a local recurrence in the treated breast; 3 patients also had a simultaneous regional recurrence (2 supraclavicular lymph node and 1 internal mammary lymph node) (Table 3). Among the 145 patients who had placement of radiopaque clips, 2 patients had recurrences in the treated breast compared with 19 breast recurrences among the 228 patients without clip placement. The actuarial rate of ipsilateral breast tumor control at 5 years was 98.6% compared with 91.7%, respectively (P = .0200) (Fig. 2). When patients were grouped according to their pathologic response to neoadjuvant chemotherapy, the 170 patients who had pathologic residual disease that measured >1 cm had no local recurrences if they had radiopaque tumor clips placed (0 of 54 patients), whereas 11 of 113 patients without clip placement developed local recurrence (P = .0334) (Fig. 3). In the 189 patients who achieved a pCR or a near pCR to neoadjuvant chemotherapy, 2 of 81 patients with clip placement had a breast recurrence, and 7 of 108 patients without clip placement had a breast recurrence. Their rates of local control did not differ statistically (P = .3208) (Fig. 3A).
TABLE 3.
Pattern of Failure by Site
| Site of locoregional recurrence | With clips | Without clips |
|---|---|---|
| Breast | 2 | 16 |
| Axillary lymph nodes | 0 | 4 |
| IMC | 0 | 2 |
| SCV | 2 | 5 |
| Breast and IMC | 0 | 1 |
| Breast and SCV | 0 | 2 |
IMC indicates internal mammary lymph nodes; SCV, supraclavicular lymph nodes.
FIGURE 2.
Local control over the 10-year study period. Cum Proportion indicates cumulative proportion.
FIGURE 3.
(A) Local control in patients with residual disease >1 cm after neoadjuvant chemotherapy. (B) Local control in patients with pathologically complete or nearly complete response (median size = 1 mm) after neoadjuvant chemotherapy. Cum Proportion indicates cumulative proportion.
Regarding recurrences in the ipsilateral regional lymphatics, 14 occurred in patients without radiopaque clips placed, and 2 occurred in patients with clips placed. The regional recurrences were distributed as follows: Two patients with clip placement had an isolated supraclavicular recurrence, and the remaining regional recurrences were in patients without clip placement in the supraclavicular lymph nodes (8 patients), the axillary lymph nodes (4 patients) and the internal mammary lymph nodes (3 patients) (Table 3). In total, 50 patients (13.4%) developed distant metastases to lung, liver, brain, or bone. Overall survival calculated by Kaplan-Meier analysis was 96.6% and 84.7% in patients with and without placement of radiopaque clips, respectively (P = .0105).
Univariate and Multivariate Analyses
Results of the Cox proportional hazards model are shown in Table 4. The absence of radiopaque clips was highly statistically significant on univariate analysis with a relative risk (RR) of 4.8 for increased local recurrence (P = .04; 95% confidence interval [95% CI], 1.11–20.65). On multivariate analysis, the absence of radiopaque clips continued to have a strong trend toward significance with an RR of 3.69 (P = .083; 95% CI, 0.84–16.16) (Table 4). Three additional factors that were associated with an increased hazard for local recurrence reached statistical significance. Patients who had close or positive surgical margins had an RR of 3.37 (P = .03; 95% CI, 1.13–10.07) compared with patients who had negative margins. Patients who had an advanced primary clinical tumor stage (designated as T3 or T4) had an RR of 2.66 (P = .04; 95% CI, 1.03–6.86) compared with patients who had clinical stage T1 and T2 tumors. Modified Black nuclear grade 3 (MBNG 3), compared with MBNG 1 or 2, also was associated with a statistically significant increased risk of local recurrence with an RR of 3.86 (P = .03; 95% CI, 1.13–13.22).
TABLE 4.
Cox Regression Analysis With Local Control as the Endpoint
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Variable | RR | P | 95% CI | RR | P | 95% CI | Comparison variable |
| Positive/close margin | 2.57 | .07 | 0.93–7.08 | 3.37 | .03* | 1.13–10.07 | Negative margin |
| T3–T4 | 2.93 | .01 | 1.24–6.91 | 2.66 | .04* | 1.03–6.86 | T1–T2 |
| No clips | 4.80 | .04 | 1.11–20.65 | 3.69 | .083 | 0.84–16.16 | Clips |
| MBNG 3 | 4.15 | .02 | 1.22–14.09 | 3.86 | .03* | 1.13–13.22 | MBNG 1–2 |
| Right side | 0.61 | .27 | 0.25–1.47 | — | — | — | Center side |
| Caucasian | 0.60 | .39 | 0.19–1.91 | — | — | — | African American |
| ER negative | 2.70 | .08 | 0.88–8.27 | — | — | — | ER positive |
| LVSI positive | 3.28 | .01 | 1.32–8.16 | — | — | — | LVSI negative |
| Lymph node negative | 1.89 | .22 | 0.69–5.16 | — | — | — | Lymph node positive |
| N2–N3 | 3.05 | .01 | 1.28–7.25 | — | — | — | N0–N1 |
| Stage III | 3.73 | .004 | 1.54–9.02 | — | — | — | Stage I–II |
| Path residual Ca | 1.50 | .37 | 0.62–3.64 | — | — | — | Near CR or Pcr |
| Age | 0.96 | .05 | 0.91–1.00 | — | — | — | Continuous variable |
RR indicates relative risk; 95% CI, 95% confidence interval; T, tumor classification; MBNG, modified Black nuclear grade; ER estrogen receptor; LVSI, lymphovascular space invasion; N, lymph node status; Path, pathologic; CA, cancer; CR complete response; pCR, pathologic complete response.
Significant difference.
DISCUSSION
To our knowledge, this is the first report describing an increased incidence of local failure associated with the omission of radiopaque clip placement in the tumor bed before or during neoadjuvant chemotherapy and breast-conservation therapy. This association was highly significant on univariate analysis and trended toward improved local control in multivariate analysis. Our findings fill the knowledge gap that existed regarding the impact of radiopaque clips on local control in patients who receive neoadjuvant chemotherapy and undergo breast-conservative surgery. On the basis of our findings, we believe that placement of radiopaque clips should be an integral part of the multidisciplinary approach in these patients.
Since preliminary results from our study became available, the incidence of tumor clip placement in patients undergoing neoadjuvant chemotherapy and breast-conservation therapy at our institution has increased by approximately 20%. At the M. D. Anderson Cancer Center, the request for clip placement in the primary tumor bed typically is coordinated by the treating medical oncologist and the radiologist. When the patient is seen for surgical consultation, the surgeon also may request clip placement as part of his/her surgical planning. It has been our practice to recommend ultrasound-guided clip placement when the tumor becomes difficult to palpate or if the mass has regressed in size to approximately 1 or 2 cm on serial imaging studies. A possible explanation for some patients in whom no radiopaque marker was placed is that the tumor was associated with diffuse calcifications; however, calcifications associated with the tumor mass were observed in only a minority of patients. Furthermore, the reliability on calcifications depends on whether or not the calcifications accurately outlined the tumor bed.
Our guidelines support the practice of clip placement in small or rapidly regressing tumors and omit clip placement in patients with large and nonresponsive tumors based on the assumption that patients with larger tumors will likely have visible residual tumor at the time of surgery and that small or rapidly regressing tumors are at the greatest risk of disappearing before surgery. We expected that the data would demonstrate the highest local recurrence rate among those patients who had an excellent response to neoadjuvant chemotherapy without radiopaque clip placement. However, the patients who attained a pCR or those who had minimal residual disease demonstrated excellent local control whether or not they had radiopaque clips placed. We observed a more robust improvement in local control among the patients who had pathologic residual disease. With the same median size of pathologic residual disease (2 cm), these patients had a statistically significant improvement in local control if they had clips placed. There were no breast recurrences in patients with clips versus a 10% local failure rate in those without clips. In this cohort of patients with residual disease, the local control benefit of clip placement was very evident.
Because our cohort was heterogeneous, we did not have complete information on every patient, such as lymphovascular space invasion, and only 50% of the patients received additional taxane chemotherapy as a component of their neoadjuvant chemotherapy. Nonetheless, on multivariate analysis with the given data, the absence of radiopaque tumor clips remained associated with an increased local recurrence rate. Other limitations of this study are its retrospective design and the unforeseen imbalances that are inherent to such a study. For example, the patients in whom radiopaque clip placement was omitted had a higher percentage of advanced lymph node disease at presentation. This most likely explains the inferior overall survival observed in patients without clips placed versus patients with clip placement.
How should the finding of an association between the omission of clips and an increased rate of local recurrence be incorporated into clinical practice? Although it is unlikely that all patients planned for neoadjuvant chemotherapy need to be referred for placement of radiopaque markers, the anticipation of response to chemotherapy and the possible disappearance of small tumors may be insufficient for selecting all appropriate patients. In most patients, the benefits of clip placement outweigh the omission of clip placement. Any concerns regarding additional resources required for clip placement have not been observed in our experience. The ultrasound-guided clip placement takes only a few minutes in an ultrasound procedure room; and, currently, the growing trend is to place the clip marker immediately after the biopsy. This cuts down on the costs involved in having the patient return for a separate study to place the clip.
In conclusion, the current study underscores the importance of marking the tumor bed before or during neoadjuvant chemotherapy for optimal local control. Early placement of radiopaque clips in the tumor bed is advised to facilitate accurate tumor bed localization and to reduce the risk of breast tumor recurrence. Proper selection of appropriate patients and the timing of radiopaque clip placement are optimized by using a multidisciplinary approach to patient care.
REFERENCES
- 1.Alonso-Bartolome P, Garcia EO, Ayensa FG, et al. Utility of the tumor bed marker in patients with breast cancer receiving induction chemotherapy. Acta Radiol. 2002;43:29–33. doi: 10.1080/028418502127347600. [DOI] [PubMed] [Google Scholar]
- 2.Baron L, Baron P, Ackerman S, et al. Sonographically guided clip placement facilitates localization of breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2000;174:539–540. doi: 10.2214/ajr.174.2.1740539. [DOI] [PubMed] [Google Scholar]
- 3.Braeuning MP, Burke E, Pisano E. Embolization coils as tumor markers for mammography in patients undergoing neoadjuvant chemotherapy for carcinoma of the breast. AJR Am Roentgenol. 2000;174:251–252. doi: 10.2214/ajr.174.1.1740251. [DOI] [PubMed] [Google Scholar]
- 4.Dash N, Chafin S, Johnson R, et al. Usefulness of tissue marker clips in patients undergoing neoadjuvant chemotherapy for breast cancer. AJR Am J Roentgenol. 1999;173:911–917. doi: 10.2214/ajr.173.4.10511147. [DOI] [PubMed] [Google Scholar]
- 5.Edeiken BS, Fornage BD, Bedi DG, et al. US-guided implantation of metallic markers for permanent localization of the tumor bed in patients with breast cancer who undergo preoperative chemotherapy. Radiology. 1999;213:895–900. doi: 10.1148/radiology.213.3.r99dc34895. [DOI] [PubMed] [Google Scholar]
- 6.Sever AR, O’Brien ME, Humphreys S, et al. Radiopaque coil insertion into breast cancers prior to neoadjuvant chemotherapy. Breast. 2005;14:108–117. doi: 10.1016/j.breast.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Chen AM, Meric-Bernstein F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the M.D. Anderson Cancer Center experiencem. J Clin Oncol. 2004;22:2303–2312. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 8.Whitman GJ, Iyer RB, Reeve CJ, et al. Assessment of response to neoadjuvant chemotherapy in breast cancer: imaging considerations. Semin Breast Dis. 2004;7:61–74. [Google Scholar]
- 9.Stata Corporation. Stata Statistical Software: Release 9. College Station, Tex: Stata Corporation LP; 2005. [Google Scholar]