Abstract
BACKGROUND
Single-institution data suggest that treatment with radiation and axillary lymph node dissection (ALND) may be an appropriate alternative to mastectomy for T0N+ breast cancer. Population-based multi-institutional data supporting this approach are lacking.
METHODS
The cause-specific survival (CSS) and overall survival (OS) of women with T0N+M0 ductal, lobular, or mixed breast cancer in the Surveillance, Epidemiology, and End Results database from 1983 to 2006 were analyzed. Groups were defined as: 1) no ALND, mastectomy, or RT (observation); 2) ALND only; 3) mastectomy plus ALND with or without postmastectomy radiation (Mast); and 4) breast-conserving therapy (BCT) with ALND and radiation (BCT).
RESULTS
In total, 750 of 770,030 patients with breast cancer had T0N+ M0 disease (incidence, 0.10%), and 596 of those patients underwent ALND (79.5%). Patients who underwent Mast or BCT (n = 470) had a 10-year OS rate of 64.9% compared with 58.5% for patients who underwent ALND only (n = 126; P = .02) and 47.5% for patients who underwent observation only (n = 94; P = .04). The 10-year CSS rate was 75.7% for patients who underwent BCT versus 73.9% for patients who underwent Mast (P = .55). In multivariate analysis of CSS for patients who underwent Mast or BCT, the following factors were correlated with an unfavorable outcome: positive estrogen receptor status (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.24–0.96; P = .04), ≥10 positive lymph nodes (HR, 5.7; 95%CI, 2.4–13.4; P ≤ .01), and <10 resected lymph nodes (HR, 42.9; 95%CI, 1.2–7.1; P = .02). Mast did not improve CSS compared with BCT (HR, 1.09; 95%CI, 0.57–2.1; P = .79).
CONCLUSIONS
Definitive locoregional treatment with either Mast or BCT improved the outcome of patients with T0N+breast cancer, and no difference in survival was observed between the treatments.
Keywords: occult breast cancer, axillary lymph node metastasis, breast-conserving therapy, mastectomy, Surveillance, Epidemiology, End Results
Patients with axillary lymph node metastasis from an occult primary breast cancer are a rare subset of patients. Prospective randomized trials have not been performed because of the scarcity of these patients; thus, locoregional treatment guidelines for this group are uncertain. Current National Comprehensive Cancer Network (NCCN) guidelines recommend magnetic resonance imaging (MRI) for these patients to identify neoplasms that are not identified on clinical examination or mammography. For patients who have normal MRI studies, the recommendation is to undergo either mastectomy with axillary lymph node dissection (ALND) with or without postmastectomy radiation or ALND with whole-breast irradiation with or without lymph node irradiation.1
The NCCN guidelines allowing for breast conservation were justified in part by small series from single institutions indicating that ALND and radiation therapy (RT) to the breast may be a viable alternative to mastectomy in this scenario. 2–4 Despite this evidence, large, population-based, multi-institutional analyses have not been conducted to validate earlier findings. The objective of the current study was to use a population-based database to determine the demographics and tumor characteristics, patterns of care, and treatment outcomes of patients with occult breast primary who had axillary metastasis.
MATERIALS AND METHODS
Data Source
The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute assembles information on cancer incidence and survival in the United States. The SEER Program registries routinely collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status. The registries that participate in the SEER Program capture approximately 97% of incident cases.5 The public-use data contain information on type of surgery performed and whether or not a patient received RT. However, the data do not contain information on comorbid conditions, imaging performed during staging evaluation, lymphovascular space invasion, surgical margins, RT details (such as dose and fields), systemic treatment (such as chemotherapy or hormone therapy), or locoregional control. The population residing within the areas served by the SEER cancer registries is comparable to the general US population with regard to measures of poverty and education but tends to be more urban and has a higher proportion of foreign-born individuals than the general US population.6 The catchments for the 17 SEER registries that were used in the current analysis comprise approximately 26% of the US population.7 Because this dataset is in the public domain, it was deemed exempt from institutional review board approval.
Description of Study Cohort and Treatment
In total, 1134 women with T0, axillary lymph node-positive, ductal, lobular, or mixed histology breast cancer among 770,030 patients who were diagnosed with breast cancer between January 1,1983 and December 31, 2006 were identified in the SEER database using SEER*Stat software (version 6.5.2; SEER Program, National Cancer Institute, Bethesda, Md).8 Patients who had distant metastatic disease (n = 364) or another primary tumor 6 months before or after diagnosis (n = 20) were excluded. Patient characteristics were identified, including age, race, year of diagnosis, tumor grade, number of lymph nodes resected and positive, and estrogen receptor (ER) and progesterone receptor (PR) status.
Treatment Course
Patients who underwent external-beam RT, mastectomy, and/or ALND were identified based on the SEER variables. Mastectomy was defined as modified radical, total, or extended radical mastectomy but not partial mastectomy. Patients who underwent modified radical mastectomy, extended mastectomy, partial mastectomy with ALND, regional lymph node surgery, or resection of ≥4 regional lymph nodes were categorized as having undergone ALND. For univariate and multivariate analyses, treatment groups were defined as 1) no ALND, RT, or mastectomy (the observation group); 2) ALND only; 3) mastectomy with ALND with or without postmastectomy RT (the mastectomy group); and 4) breast-conserving therapy with ALND and RT (the BCT group). Variables that indicated cancer stage were based on the American Joint Commission on Cancer (AJCC) Cancer Staging Manual, sixth edition.9
Outcomes
Survival was calculated from the date of diagnosis to the occurrence of the considered event through December 31, 2006. Cause-specific survival (CSS) was the primary endpoint and was defined as the time between diagnosis and death from breast cancer. Overall survival (OS) was defined as the time between diagnosis and death from any cause. Patients who died within 6 months of diagnosis were censored to reduce selection bias, because poor performance status and significant comorbidies may preclude these patients from receiving adequate therapy.10
Statistical Analysis
Data analysis was performed using Stata/SE 10.0 statistical software (StataCorp LP, College Station, Tex). The Pearson chi-square test was used to assess measures of univariate association in frequency tables. Unadjusted associations between treatment groups and outcomes were compared using survival analysis and the Kaplan-Meier log-rank test. A P value ≤.05 was considered statistically significant. Statistical tests were based on a 2-sided significance level.
A Cox proportional hazards model was used for both univariate and multivariate analyses to assess the effect of patient characteristics and other prognostic factors of significance on the endpoints. The endpoints for these analyses were OS and CSS. All variables were assessed on a univariate basis, and factors with a significance of ≤.25 were assessed for multivariate analysis using backward elimination. The Wald test and the likelihood-ratio test were used to assess the role of covariates in the model. The estimated hazard is reported.
Kaplan-Meier survival estimates were calculated for variables with missing values. These factors included ER status, PR status, tumor grade, the number of lymph nodes resected, and the number of positive lymph nodes resected. OS and breast CSS for patients who had missing values were similar to those for patients who had these values coded. Therefore, patients who had missing values were excluded from the Cox regression analysis. An additional check for missing values was carried out by comparing models and excluding patients who had missing values against models in which the missing values were included as an additional category. We observed that the 2 models were almost identical.
RESULTS
Patient Demographics and Treatments
In total, 750 of 770,030 patients with breast cancer (incidence, 0.10%) were included in the final analysis. Patient demographics and tumor characteristics are included in Table 1. The median age was 59 years. Of 750 patients, 276 patients underwent mastectomy (36.8%), 336 patients received RT (44.8%), and 220 patients received neither of these treatments (29.3%). In total, 596 patients underwent ALND (79.5%). In this group, 126 patients underwent ALND only (21.1%), 188 patients underwent mastectomy (31.5%), 202 patients received RT (33.9%), and 80 patients both underwent mastectomy and received RT (13.4%). Patient characteristics between the 4 treatment groups are presented in Table 2. Of 750 patients, 94 patients underwent observation, 6 patients underwent mastectomy only, 52 patients received RT only, 2 patients underwent mastectomy and received RT, 126 patients underwent ALND only, 268 patients underwent mastectomy, and 202 patients underwent BCT.
Table 1.
Patient Demographics and Tumor Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Age, y | ||
| <40 | 42 | 5.6 |
| 40–49 | 139 | 18.5 |
| 50–59 | 200 | 26.7 |
| 60–69 | 194 | 25.9 |
| ≥70 | 175 | 23.3 |
| Mean | 59 | |
| Median | 59 | |
| Race | ||
| White | 616 | 82.1 |
| Black | 72 | 9.6 |
| Asian or Pacific Islander | 47 | 6.3 |
| American Indian/Alaska Native | 11 | 1.5 |
| Unknown/other | 4 | 0.5 |
| Grade | ||
| 1 | 6 | 0.8 |
| 2 | 46 | 6.1 |
| 3 | 246 | 32.8 |
| Unknown | 452 | 60.3 |
| Hormone status | ||
| ER-positive | 286 | 38.1 |
| ER-negative | 242 | 32.3 |
| ER unknown | 222 | 29.6 |
| PR-positive | 211 | 28.1 |
| PR-negative | 296 | 39.5 |
| PR unknown | 243 | 32.4 |
| No. of resected lymph nodes | ||
| 1–3 | 140 | 18.7 |
| 4–9 | 88 | 11.7 |
| ≥10 | 338 | 45.1 |
| Unknown | 184 | 24.5 |
| No. of positive lymph nodes | ||
| 1–3 | 367 | 48.9 |
| 4–9 | 112 | 14.9 |
| ≥10 | 91 | 12.1 |
| Unknown | 180 | 24 |
| Year of diagnosis | ||
| 1983–1997 | 266 | 35.5 |
| 1998–2006 | 484 | 64.5 |
| Laterality | ||
| Left | 387 | 51.6 |
| Right | 327 | 43.6 |
| Unknown | 36 | 4.8 |
ER indicates estrogen receptor; PR, progesterone receptor
Table 2.
Covariate Incidence in the No Axillary Lymph Node Dissection (ALND), No Mastectomy, No Radiation (Observation) Group; the ALND Only Group; the ALND and Mastectomy With or Without Radiation (Mastectomy) Group, and the ALND and Radiation Only (Breast-Conserving Therapy) Group
| Observation (n = 94) |
ALND Only (n = 126) |
Mastectomy Group (n = 268) |
BCT Group (n = 202) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | No. | % | No. | % | No. | % | No. | % | P | ||||
| Age, y | .11 | ||||||||||||
| <50 | 17 | 18.1 | 22 | 17.5 | 73 | 27.2 | 52 | 25.7 | |||||
| ≥50 | 77 | 81.9 | 103 | 81.7 | 195 | 72.8 | 150 | 74.3 | |||||
| Median | 62 | 62 | 59 | 57 | |||||||||
| Grade | .39 | ||||||||||||
| 1 | 0 | 0 | 0 | 0 | 5 | 1.9 | 1 | 0.5 | |||||
| 2 | 5 | 5.3 | 8 | 6.3 | 19 | 7.1 | 10 | 5 | |||||
| 3 | 26 | 27.7 | 45 | 35.7 | 86 | 32.1 | 71 | 35.1 | |||||
| Unknown | 63 | 67 | 73 | 57.9 | 158 | 59 | 120 | 59.4 | |||||
| ER status | .64 | ||||||||||||
| Positive | 37 | 39.4 | 33 | 26.2 | 96 | 81 | 40.1 | ||||||
| Negative | 30 | 31.9 | 47 | 37.3 | 79 | 80 | 39.6 | ||||||
| Unknown | 27 | 28.7 | 46 | 36.5 | 93 | 41 | 20.3 | ||||||
| No. of lymph nodes resected | .04 | ||||||||||||
| 1−9 | NA | 39 | 31 | 55 | 20.5 | 25.7 | |||||||
| ≥10 | NA | 56 | 44.4 | 153 | 57.1 | 56.9 | |||||||
| No. of lymph nodes positive | .04 | ||||||||||||
| 1−9 | NA | 88 | 69.8 | 167 | 62.3 | 133 | 66.8 | ||||||
| ≥10 | NA | 9 | 7.1 | 43 | 16 | 34 | 16.8 | ||||||
| Year diagnosed | .17 | ||||||||||||
| 1983–1997 | 29 | 30.9 | 43 | 34.1 | 108 | 40.3 | 64 | 31.7 | |||||
| 1998–2006 | 65 | 69.1 | 83 | 65.9 | 160 | 59.7 | 138 | 68.3 | |||||
BCT indicates breast-conserving therapy; ER, estrogen receptor.
Patterns of Care
Treatment patterns changed significantly over the years of this study. Among the patients who underwent ALND, the use of BCT increased over time. Only 29.8% received BCT before 1998 compared with 36.2% during or after 1998 (P = .11). Among the 750 patients, 50.2% underwent mastectomy before 1998 compared with 42% during and after 1998 (P = .05).
Predictors of Failure
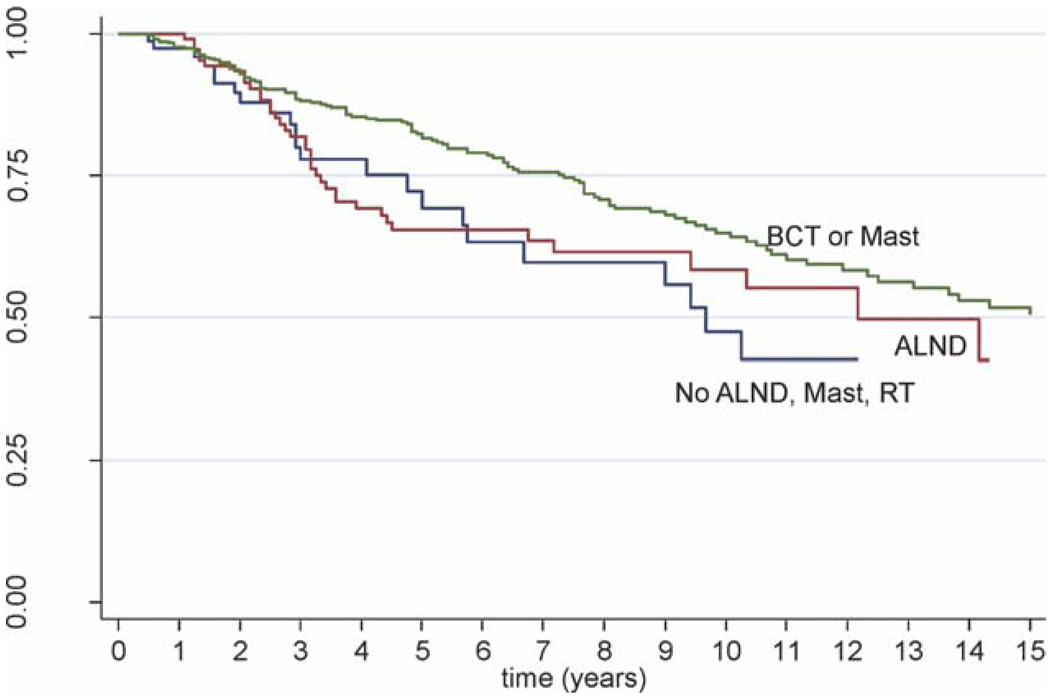
In total, 201 of 750 patients (27%) died from all causes, and 128 patients (17%) died of breast cancer during a median follow-up of 4 years (range, 0.08–21.8 years). Patients who received less that optimal locoregional therapy according to NCCN guidelines had worse outcomes. Specifically, the patients who underwent BCT or mastectomy (n = 470) had a 10-year OS rate of 64.9% compared with 58.5% for patients who underwent ALND only (n = 126; log-rank P = .02) and 47.5% for patients who underwent observation (n = 94; P = .04) (Fig. 1). The 10-year CSS rate for patients who underwent BCT or mastectomy was 74.6% compared with 71.2% for patients who underwent ALND only (P = .09) and 71.9% for patients who underwent observation (P = .69).
Figure 1.
This Kaplan-Meier curve illustrates overall survival for patients who underwent breast-conserving therapy (BCT) or mastectomy (Mast) (green line); axillary lymph node dissection (ALND) (red line); and no ALND, Mast, or radiation therapy (RT) (blue line).
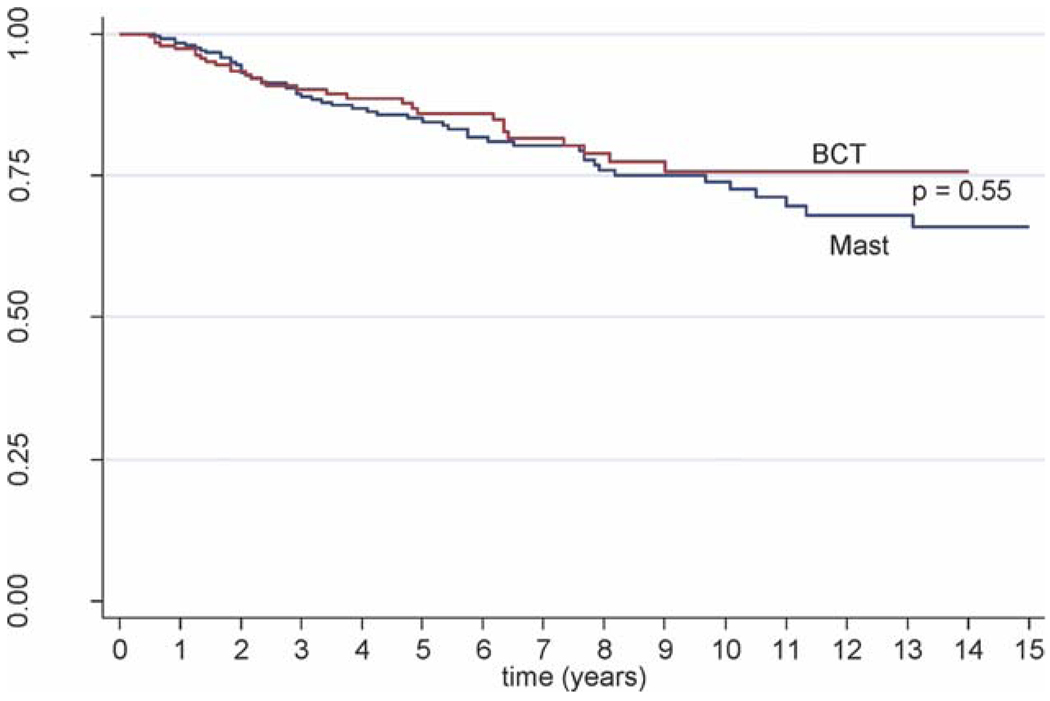
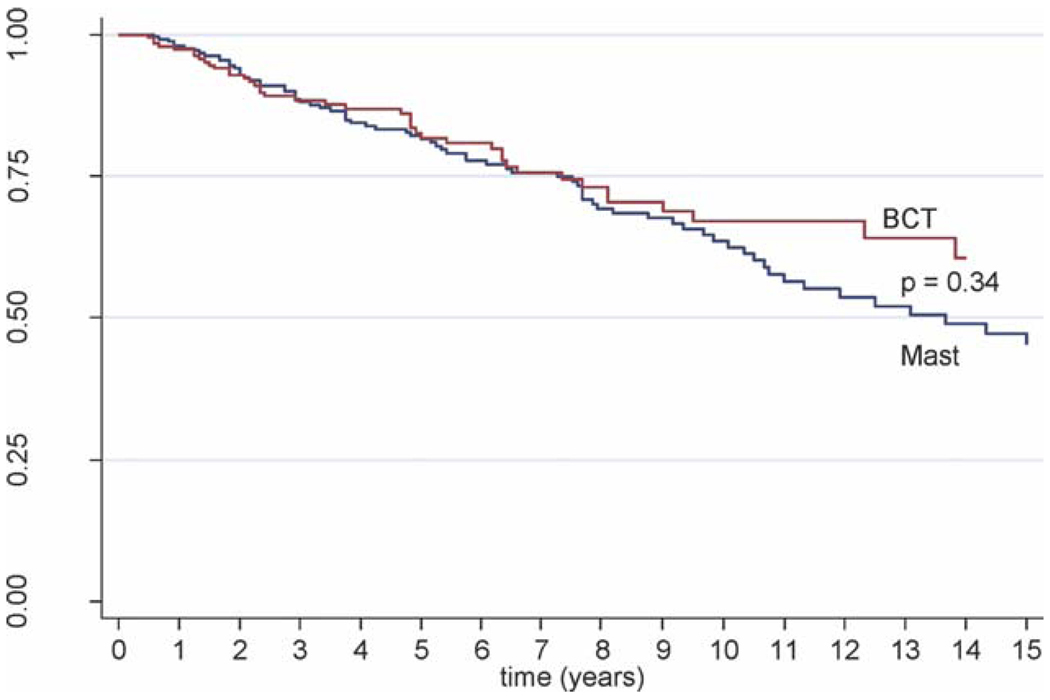
For patients who underwent ALND and received additional local treatments, no differences in CSS or OS rates were observed between patients who underwent mastectomy and patients who underwent BCT. The 10-year CSS rate was 73.9% for patients who underwent mastectomy versus 75.7% for patients who underwent BCT (P = .55) (Fig. 2). The 10-year OS rate was 63.5% for patients who underwent mastectomy and 67.1% for patients who underwent BCT (P = .34) (Fig. 3).
Figure 2.
This Kaplan-Meier curve illustrates cause-specific survival for patients who underwent breast-conserving therapy (BCT) (red line) and patients who underwent mastectomy (Mast) (blue line).
Figure 3.
This Kaplan-Meier curve illustrates overall survival for patients who underwent breast-conserving therapy (BCT) (red line) and patients who underwent mastectomy (Mast) (blue line).
Lymph Node Involvement and Hormone Status
Information on patients’ lymph node status coded from 1983 to 2003 grouped axillary, infraclavicular, and/or internal mammary lymph nodes into the same category, thus making it impossible to extrapolate their lymph node status into the current TNM staging system. Patients who were treated between 2004 and 2006 had their lymph node status coded properly based on the current AJCC staging system.9 By using the 185 patients in the latter group, there was no difference in CSS or OS between patients with N2–N3 disease who underwent mastectomy and patients who underwent BCT, although the dataset had follow-up only through the end of 2006.
In the cohort of 470 patients who underwent ALND and received additional local therapy, lymph node status and hormone receptor status were associated with outcomes. Patients who had <10 positive lymph nodes had a 10-year CSS rate of 82.4% compared with 63.3% for patients who had ≥10 positive lymph nodes (P = .0008). Patients who had <10 positive lymph nodes had a 10-year OS rate of 72.2% compared with 52.1% for patients who had >10 positive lymph nodes (P = .003). Patients who had ≥10 lymph nodes resected had a 10-year CSS rate of 81.1% compared with 73.7% for patients who had <10 lymph nodes resected (P = .02). Patients who had ≥10 lymph nodes resected had a 10-year OS rate of 70.9% compared with 61.9% for patients who had <10 lymph nodes resected (P = .02) There was no statistically significant difference in CSS or OS between patients who had ER-positive disease and patients who had ER-negative disease.
Multivariate Analysis
A multivariate Cox regression analysis was performed for patients who underwent mastectomy or BCT. The following factors were correlated with an unfavorable CSS: ER-negative disease (hazard ratio [HR], 2.08; 95% confidence interval [CI], 1.04–4.2; P = .04), ≥10 positive lymph nodes (HR, 5.7; 95%CI, 2.4–13.4; P ≤ .01), and <10 lymph nodes resected (HR, 2.9; 95%CI, 1.2–7.1; P = .02). Undergoing mastectomy did not improve CSS in this group (HR, 1.09; 95%CI, 0.57–2.1; P = .79) (Table 3). Factors that were correlated with an unfavorable OS were age at diagnosis (continuous HR, 1.06; 95%CI, 1.04–1.08; P ≤ .001) and ≥10 positive lymph nodes (HR, 2.04; 95%CI, 1.2–3.4; P = .005). Undergoing mastectomy did not improve OS in this group (HR, 0.92; 95%CI, 0.57–1.5; P = .72).
Table 3.
Results From Univariate/Multivariate Analyses of Patients Who Underwent Breast-Conserving Therapy or Mastectomy With Cause-Specific Survival as the Endpoint
| Multivariate | ||||
|---|---|---|---|---|
| Variable | Univariate P |
P | RR | 95%CI |
| Full model | ||||
| Final model | ||||
| ER positive (yes vs. no) | .01 | .04 | 0.48 | 0.24–0.96 |
| <LN resected (yes vs no) | .09 | .02 | 2.93 | 1.2–7.1 |
| ≥LN positive (yes vs no) | .003 | <.01 | 5.72 | 2.4–13-3 |
| Mastectomy (yes vs no) | .55 | .79 | 1.09 | 0.57–2.1 |
| Age (continuous) | .02 | .057 | 1.03 | 1.0–1.06 |
| PR positive (yes vs no) | .64 | |||
| Tumor grade (3 vs 1–2) | .97 | |||
| Diagnosis y (continuous) | .76 | |||
| Diagnosis y (≥1998 vs <1998) | .78 | |||
| Age (≥50 y vs <50 y) | .12 | |||
RR indicates relative risk; CI, confidence interval; ER, estrogen receptor; LN, lymph node; PR, progesterone receptor.
DISCUSSION
To our knowledge, this report represents the largest published series to date reporting the outcome of patients with breast cancer who had occult primary tumors and presented with axillary lymph node metastasis. The data from this study further support the NCCN treatment guideline recommendations for locoregional treatment. Specifically, these data indicate that definitive locoregional treatment with either mastectomy or RT improves OS in patients with occult breast cancer and axillary metastasis who undergo ALND. In addition, our analysis indicates that BCT approaches with RT are safe and justifiable. In this study, the OS and CSS of patients who underwent BCT were comparable to the OS and CSS of patients who underwent mastectomy in both univariate and multivariate analyses. Because of the rarity of this clinical presentation, it is unlikely that prospective comparisons of BCT and mastectomy will ever be performed.
Few published data have been available to indicate practice patterns for patients with occult breast primaries. We observed that, in recent years, more patients received RT without mastectomy. This result most likely represents the extrapolation of prospective, randomized controlled trials indicating that BCT produces outcomes comparable to those produced by mastectomy in patients who have stage I and II breast cancer and the single-institution reports indicating success with this approach.11–17
Although the current results are compelling, there are significant obstacles to answering these questions using the SEER dataset alone. One bias that exists between the patients who undergo mastectomy compared with those who receive RT is that, after mastectomy, approximately 33% of patients with occult malignancies will have primary tumors discovered pathologically.12 Earlier series reported higher statistics, but it is unclear how accurate those numbers were given the poorer quality imaging at the time. These patients no longer would be staged as T0. In contrast, these malignancies would remain in the T0 category among patients who receive RT. With this knowledge, it seems reasonable to conclude that a higher percentage of patients in the RT group have microscopic disease in the breast. Although microscopic tumor may exist, it is unclear how outcomes in this group may differ from the outcomes of patients without microscopic tumor or how these small tumors behave compared with their larger counterparts. The second bias is the use of OS as an outcome. Patients with poorer performance status or significant comorbidities may be less likely to undergo aggressive therapy like surgery or RT, thus biasing any interpretation of treatment outcomes using this endpoint. In addition, patient and provider biases are present in choosing who should receive locoregional treatment. Finally, as indicated previously above, the endpoint of locoregional recurrence is not captured in SEER, and, as such, we are limited to the evaluation of CSS and OS. In our analysis, patients in the mastectomy group and the BCT group had more lymph nodes resected than patients in the ALND group, which may explain their improved CSS and OS, although patients in this latter group had fewer positive lymph nodes.
Various authors have reported single-institution results in this clinical subset with 5-year OS rates that range from 76% to 100% (Table 4).2–4,12,18–22 Two of those studies produced an improved recurrence-free survival among patients who received RT to the breast compared with patients who underwent observation only.2–4 Although the number of patients in these studies was very small, none demonstrated a benefit from mastectomy compared with BCT plus RT. The proportion of patients who underwent observation only in those studies ranged from 0% to 38%. In our current SEER analysis, 12.5% of patients underwent observation only, which is a reasonable proportion in light of the earlier series.
Table 4.
Previous Retrospective Series of Patients With Occult Breast Cancer and Axillary Metastasis
| Mast | BCT With RT |
No Mast or BCT With RT |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Median Follow-Up, y |
No. | % | No. | % | No. | % | No. | 5-Year LRR, % |
5-Year OS, % |
| Vilcoq 198220 | >5 | 11 | 0 | 0 | 100 | 11 | 0 | 0 | 9.1 | 90.9 |
| Campana 198919 | 9 | 31 | 3 | 1 | 97 | 30 | 0 | 0 | 12.9 | 76 |
| Merson 199118 | 6 | 60 | 48 | 29 | 10 | 6 | 28 | 17 | NA | 76.6 |
| Foroudi & Tiver 20003 | Mean, 6.1 | 20 | 10 | 2 | 60 | 12 | 30 | 6 | 45 | NA |
| Vlastos 20012 | 7 | 45 | 29 | 13 | 56 | 25 | 22 | 10 | 13–15 | NA |
| Shannon 20024 | 3.7 | 29 | 0 | 0 | 55 | 16 | 38 | 11 | NA | 88 |
| Blanchard & Farley 200412 | 3.4 | 35 | 51 | 18 | NA | 27 | 23 | 8 | 58 | NA |
| Galimberti 200422 | Mean, 3.4 | 27 | 0 | 0 | 100 | 0 | 0 | 16 | NA | |
| Varadarajan 200621 | 4.8 | 10 | 10 | 1 | 80 | 8 | 0 | 0 | 0 | 100 |
Mast indicates mastectomy; BCT, breast-conserving therapy; RT, radiation therapy; LRR, locoregional recurrence; OS, overall survival; NA, not available.
The vast majority of patients in this series were treated during an era that predated the routine use of breast MRI. MRI reportedly can detect tumor within the breast in 62% to 70% of patients with axillary metastasis and normal mammograms.23,24 The percentage of patients without malignancy identified on MRI but with microscopic disease in a surgical specimen also is unclear. One study demonstrated that, among those with a negative MRI, 2 of 8 patients (25%) had tumor identified in a pathology specimen from mastectomy.24
In conclusion, these results from the SEER dataset provide evidence that occult breast cancer with axillary lymph node metastasis is a rare clinical presentation that warrants definitive locoregional therapy. BCT with RT and ALND appears to provide equal OS and CSS compared with mastectomy.
Acknowledgments
We acknowledge Pamela Allen, PhD, for her contribution to the multivariate statistical analysis.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology, Breast Cancer v1.2009. [Accessed August 28, 2009]; Available at: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. [Google Scholar]
- 2.Vlastos G, Jean ME, Mirza AN, et al. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol. 2001;8:425–431. doi: 10.1007/s10434-001-0425-6. [DOI] [PubMed] [Google Scholar]
- 3.Foroudi F, Tiver KW. Occult breast carcinoma presenting as axillary metastases. Int J Radiat Oncol Biol Phys. 2000;47:143–147. doi: 10.1016/s0360-3016(99)00542-8. [DOI] [PubMed] [Google Scholar]
- 4.Shannon C, Walsh G, Sapunar F, A’Hern R, Smith I. Occult primary breast carcinoma presenting as axillary lymphadenopathy. Breast. 2002;11:414–418. doi: 10.1054/brst.2002.0455. [DOI] [PubMed] [Google Scholar]
- 5.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute. SEER Registries—Population Characteristics. [Accessed September 15, 2009]; Available at: http://seer.cancer.gov/registries/characteristics.html.
- 7.National Cancer Institute. Surveillance, Epidemiology and End Results (SEER) Program. [Accessed August 28, 2009]; Available at: http://seer.cancer.gov/
- 8.Surveillance, Epidemiology, and End Results (SEER) Program. SEER/Stat database: Incidence-SEER 17 Registries limited-use plus Hurricane Katrina impacted Louisiana cases, November 2008 submission (1973–2006 varying). Linked to county attributes-total US, 1969–2006 counties. Bethesda, Md: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; released April 2009, based on the November 2008 submission. [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Staging Manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 10.Housman DM, Decker RH, Wilson LD. Regarding adjuvant radiation therapy in Merkel cell carcinoma: selection bias and its affect on overall survival. J Clin Oncol. 2007;25:4503–4504. doi: 10.1200/JCO.2007.12.2895. author reply 4504–4505. [DOI] [PubMed] [Google Scholar]
- 11.Arriagada R, Le MG, Rochard F, Contesso G. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558–1564. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard DK, Farley DR. Retrospective study of women presenting with axillary metastases from occult breast carcinoma. World J Surg. 2004;28:535–539. doi: 10.1007/s00268-004-7290-y. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Redmond C, Poisson R, et al. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320:822–828. doi: 10.1056/NEJM198903303201302. [DOI] [PubMed] [Google Scholar]
- 15.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute randomized trial. Cancer. 2003;98:697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 16.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 17.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 18.Merson M, Andreola S, Galimberti V, Bufalino R, Marchini S, Veronesi U. Breast carcinoma presenting as axillary metastases without evidence of a primary tumor. Cancer. 1992;70:504–508. doi: 10.1002/1097-0142(19920715)70:2<504::aid-cncr2820700221>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Campana F, Fourquet A, Ashby MA, et al. Presentation of axillary lymphadenopathy without detectable breast primary (T0 N1b breast cancer): experience at Institut Curie. Radiother Oncol. 1989;15:321–325. doi: 10.1016/0167-8140(89)90077-7. [DOI] [PubMed] [Google Scholar]
- 20.Vilcoq JR, Calle R, Ferme F, Veith F. Conservative treatment of axillary adenopathy due to probable subclinical breast cancer. Arch Surg. 1982;117:1136–1138. doi: 10.1001/archsurg.1982.01380330004002. [DOI] [PubMed] [Google Scholar]
- 21.Varadarajan R, Edge SB, Yu J, Watroba N, Janarthanan BR. Prognosis of occult breast carcinoma presenting as isolated axillary nodal metastasis. Oncology. 2006;71:456–459. doi: 10.1159/000107111. [DOI] [PubMed] [Google Scholar]
- 22.Galimberti V, Bassani G, Monti S, et al. Clinical experience with axillary presentation breast cancer. Breast Cancer Res Treat. 2004;88:43–47. doi: 10.1007/s10549-004-9453-9. [DOI] [PubMed] [Google Scholar]
- 23.Olson JA, Jr, Morris EA, Van Zee KJ, Linehan DC, Borgen PI. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. 2000;7:411–415. doi: 10.1007/s10434-000-0411-4. [DOI] [PubMed] [Google Scholar]
- 24.Buchanan CL, Morris EA, Dorn PL, Borgen PI, Van Zee KJ. Utility of breast magnetic resonance imaging in patients with occult primary breast cancer. Ann Surg Oncol. 2005;12:1045–1053. doi: 10.1245/ASO.2005.03.520. [DOI] [PubMed] [Google Scholar]