Abstract
Mesenchymal stem cells (MSC) migrate to and proliferate within sites of inflammation and tumors as part of the tissue remodeling process. Radiation increases the expression of inflammatory mediators that could enhance the recruitment of MSC into the tumor microenvironment. To investigate this, bilateral murine 4T1 breast carcinomas (expressing renilla luciferase) were irradiated unilaterally (1 or 2 Gy). Twenty-four hours later, 2 × 105 MSC-expressing firefly luciferase were injected i.v. Mice were then monitored with bioluminescent imaging for expression of both renilla (tumor) and firefly (MSC) luciferase. Forty-eight hours postirradiation, levels of MSC engraftment were 34% higher in tumors receiving 2 Gy (P = 0.004) than in the contralateral unirradiated limb. Immunohistochemical staining of tumor sections from mice treated unilaterally with 2 Gy revealed higher levels of MSC in the parenchyma of radiated tumors, whereas a higher proportion of MSC remained vasculature-associated in unirradiated tumors. To discern the potential mediators involved in MSC attraction, in vitro migration assays showed a 50% to 80% increase in MSC migration towards conditioned media from 1 to 5 Gy-irradiated 4T1 cells compared with unirradiated 4T1 cells. Irradiated 4T1 cells had increased expression of the cytokines, transforming growth factor-β1, vascular endothelial growth factor, and platelet-derived growth factor-BB, and this up-regulation was confirmed by immunohistochemistry in tumors irradiated in vivo. Interestingly, the chemokine receptor CCR2 was found to be up-regulated in MSC exposed to irradiated tumor cells and inhibition of CCR2 led to a marked decrease of MSC migration in vitro. In conclusion, clinically relevant low doses of irradiation increase the tropism for and engraftment of MSC in the tumor microenvironment.
Introduction
Mesenchymal stem cells (MSC) are multipotent bone marrow–derived stem-like cells that have the capacity to differentiate into bone, cartilage, muscle, and connective tissues throughout the body (1, 2). This differentiation ability of MSC, combined with the fact that these cells are relatively easy to isolate and are genetically stabile when expanded in culture, has prompted great interest in using MSC for tissue regeneration. An important characteristic of MSC is that they migrate to sites of tissue injury such as the kidney (3), heart (4), and skin (5), primarily as a result of the local production of inflammatory mediators produced during tissue damage and remodeling. MSC delivered intravenously or intraarterially have also been shown to engraft within tumor micro-environments (6). This shared tropism of MSC for sites of injured tissue and for tumors is thought to result from similarities in the inflammatory milieu produced by healing wounds and tumors, evoking the notion that “tumors are wounds that never heal” (7). This tropism of MSC for tumors has sparked efforts to test it as a strategy for delivering antitumor agents directly into tumors. Indeed, the success seen in studies of MSC engineered to express IFN-β into breast carcinoma, melanoma, and glioma models (6, 8, 9) has now led to the initiation of clinical trials. Therefore, any process that could improve the engraftment of MSC in the tumor, and thereby enhance the delivery of the antitumor agent, could be desirable.
One potential method to enhance engraftment is the use of local tumor irradiation, a treatment modality frequently used in clinical cancer treatment. Adirect effect of irradiation is mitotic cell death and a subsequent increase in inflammation at the site of radiation. Indeed, total-body irradiation, which is used as part of a conditioning regimen, has been observed to modulate MSC migration to tissue injury and slightly increased whole body MSC engraftment, as determined by PCR (10). However, it is unclear if low-dose irradiation, which is more desirable due to lower toxicity on normal tissue would alter the tumor microenvironment sufficiently to elicit a similar effect.
As a consequence of the local inflammation postirradiation, a number of paracrine mediators are up-regulated. Inflammatory-related cytokines such as tumor necrosis factor-a (TNF-a) and platelet-derived growth factor (PDGF), as well as chemokines such as CCL2 and CCR8 (11, 12), which are involved in the trafficking of immune cells into inflammatory sites, have recently been shown to play a role in MSC-mediated chemotaxis to tumor-conditioned media in vitro (13).
Therefore, we tested whether low-dose irradiation would enhance the tropism for and engraftment of MSC in irradiated tumor microenvironments using a syngeneic murine model. Additionally, we investigated the role of inflammatory-related cytokines and chemokines in radiation-enhanced MSC migration, and identified the cytokines and chemokines implicated in this chemotaxis towards irradiated tumor microenvironments.
Materials and Methods
Cell isolation and culture
Mouse MSC (mMSC) from BALB/c mice were a generous gift from Dr. Brett Hall (Columbus Children's Research Institute, Columbus, OH). mMSC were cultured in alpha-minimal essential medium and 20% fetal bovine serum supplemented with L-glutamine and a penicillin-streptomycin mixture.
4T1 cells, a murine mammary carcinoma derived from BALB/c mice, were obtained from the American Type Culture Collection. Tumor cells were stably transduced using a lentivirus expressing green fluorescent protein and renilla luciferase (rLUC; ref. 14).
Irradiation of murine tumors
To test the effects of radiation on MSC engraftment in tumors, fiber-modified adenoviruses engineered to express firefly luciferase (Ad-ffLUC-F/K21; ref. 15) were first used to infect BALB/c mMSC, 1 day prior to injection. On the same day, animals were anesthetized with nembutal and their right hind limbs irradiated using a 60Co source; the rest of the animal was shielded. Specifically, the right hind limbs in 8 of the 10 mice were irradiated (total dose: 1 or 2 Gy in four animals each), with the contralateral tumor serving as an internal control. The remaining mice received no irradiation and served as control animals. On the next day, 2 × 105 mMSC-ffLUC were injected i.v. into the tail vein of the animals. One mouse from this cohort was treated with five daily fractions of 5 Gy to the irradiated limb.
in vivo analysis of MSC engraftment
BALB/c mice were housed according to institutional standards and treated with approved protocols. For the tumor growth experiment, animals were first anesthetized, after which 2 × 105 4T1 cells suspended in 200 μL of PBS were injected s.c. into both hind limbs of the animals. Thirteen days later, tumor growth was confirmed by bioluminescent imaging (IVIS-Xenogen 100 system; Caliper Lifesciences).
Firefly luciferase was used to detect MSC. The ffLUC substrate, D-luciferin (100 μL of 40 mg/mL in PBS; Xenogen) was injected i.p. ~10 min prior to imaging. For imaging tumors with rLUC, 100 μL of 200 ng/mL coelenterazine (Biotium, Inc.) in PBS was injected i.p., ~5 min prior to imaging. Bioluminescent images were acquired over 5 min to detect and quantify firefly luciferase expression (MSC) and 1 min for quantification of rLUC expression (tumors). Integrated counts were measured in regions of interest represented by red circles overlying the tumors (Fig. 1). Background counts, determined by identical measurements taken in an untreated mouse given substrate only, were subtracted from ffLUC measurements as shown in Fig. 1D.
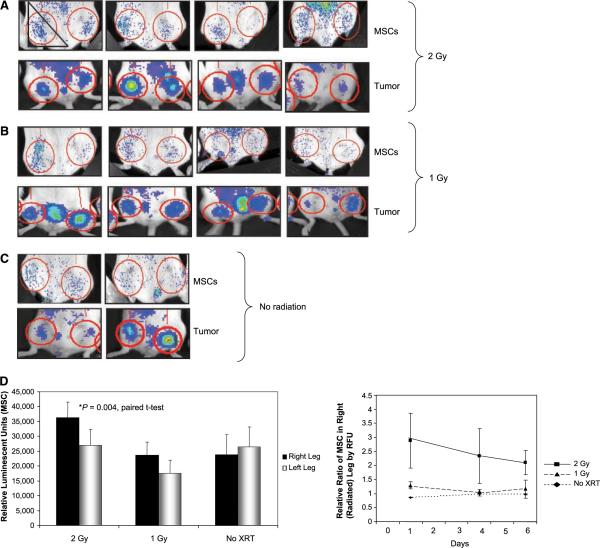
Figure 1.
MSC engraftment is enhanced in irradiated tumors. Mice with bilateral 4T1 hind limb tumors were radiated unilaterally to the right limb with 2 Gy (A) or 1 Gy (B). Representative radiated volume is represented with a triangle in the first image. All mice were injected i.v. with 2 × 105 of MSC-ffLUC the following day. Bioluminescent imaging of MSC (ffLUC) engraftment 1 day after i.v. injection (top). Tumor volume, represented by rLUC expression, prior to irradiation (bottom). D, quantification of absolute values of bioluminescent imaging of firefly luciferase within red circles in A to C (top). Relative ratio of MSC in the right leg at days 1, 4, and 6 (bottom).
Immunohistochemistry
For immunohistochemistry performed to detect MSC distribution, paraffin-embedded sections of 4T1 tumors were deparaffinized and rehydrated, with endogenous peroxidases quenched with 0.3% H2O2 in methanol. MSC were detected with goat anti-ffLUC (Promega) antibody at a final concentration of 20 μg/mL.
For immunohistochemistry performed to detect the secreted factors in irradiated cells, tumor tissue was processed as described above. Cytokines that increase MSC migration (9), which have also been shown to be up-regulated following irradiation, were candidates for eliciting this effect. These include PDGF-ββ, vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGF-β). For this study, mouse anti-VEGF, rabbit anti-PDGF, anti-SDF-1, and anti-CCL2 antibodies were used at a 1:50 dilution (Abcam). The anti-rabbit TGF-β antibody (R&D Systems) was used at a concentration of 20 μ/mL. Antibodies were detected with a species-appropriate peroxidase kit (Vector Laboratories).
Peroxidase was detected with a 3-amino-9-ethylcarbazole substrate kit (Vector Laboratories). Slides were counterstained with hematoxylin QS (Vector Laboratories). Image quantification and cell counts were performed by two independent, blinded reviewers.
To determine if MSC were localized in the tumor parenchyma or associated with tumor vasculature, immunohistochemistry was performed with mouse anti-CD31 (Abcam) and goat anti-ffLUC as described above. MSC were then counted in six high-power fields for three sections and scored as either parenchymal or vascular-associated. The cells were counted by two independent blinded reviewers.
In vitro cell migration assays
In vitro cell migration assays were performed to determine if MSC migration is mediated in part by a factor(s) secreted by irradiated tumor cells. For this experiment, 2 × 104 BALB/c mMSCs were plated on transwell plates with 8 mm pores (Fisher Scientific). 4T1 cells in serum-free media were irradiated with a cesium source to 1, 2, 5, or 10 Gy. The supernatants of the cells was transferred to the lower chamber of migration plates 8 h after irradiation, and the migration of mMSC was assessed 24 h later. The migrated cells were then fixed, stained, and counted. Subsequently, stained cells were eluted from membranes and absorbance measurements were performed.
ELISA for cytokines
4T1 cells in serum-free media were treated with 0, 2, or 5 Gy using a cesium irradiator. Media was removed at intervals from 0 to 48 h, centrifuged and frozen. ELISA assays for TGF-β1, PDGF-BB, and VEGF were then performed on supernatants (R&D Systems).
Real-time PCR arrays
BALB/c MSC were cocultured with 4T1 or irradiated 4T1 cells for 48 h. RNAwas extracted from MSC and reverse transcribed. Quantitative real-time PCR was used to profile the expression of 84 genes encoding inflammatory chemokines and their receptors according to the manufacturer's instructions (Superarray). A two-group t test was used to compare normalized expression values.
Results
Low-dose tumor irradiation increases MSC engraftment in vivo
The bioluminescence imaging findings reflecting MSC engraftment in mice are shown in Fig. 1. At 48 h postirradiation, more MSC were detected in the irradiated than in the unirradiated limbs of all treated animals. In addition, tumors treated with 2 Gy showed significantly greater ffLUC luminescence (average integrated counts of 3.6 × 104 versus 2.7 × 104, P = 0.004, paired t test) than did the tumors treated with 1 Gy. The four mice treated with 1 Gy had modestly higher numbers of MSC in the irradiated limb but the difference was not statistically significant (average integrated counts of 2.4 × 104 versus 1.8 × 104, P = 0.17; Fig. 1A and B). In contrast, we detected an equal distribution of MSC in the right and left hind limbs of the control (unirradiated) mice (2.4 × 104 average counts in the right leg versus 2.6 × 104 average counts in the left leg, P = 0.12; Fig. 1C). Quantitation of integrated counts is shown in Fig. 1D.
We also imaged MSC on days 4 and 6 after irradiation, but there was no difference in the absolute levels of MSC engraftment at these later times. We also observed moderate tumor reduction in response to low-dose irradiation. To control for equal tumor volume, the MSC counts were normalized to the ratio of the right and left leg tumors. With normalization to the tumor counts, the relative numbers of MSC showing bioluminescence were significantly higher in tumors irradiated with 2 Gy than in unirradiated tumors at all time points. Although tumors irradiated with 1 Gy showed a slightly higher proportion of MSC 1 day after injection, 6 days later, the distribution was the same in the right and left hind limbs, as was also observed in the unirradiated control mice (Fig. 1D). The results shown here are representative of two independent experiments. In an additional experiment, five mice were treated unilaterally to 2 Gy and had significantly higher levels of MSC engraftment in the irradiated limb (data not shown).
Distribution of MSC within irradiated tumors
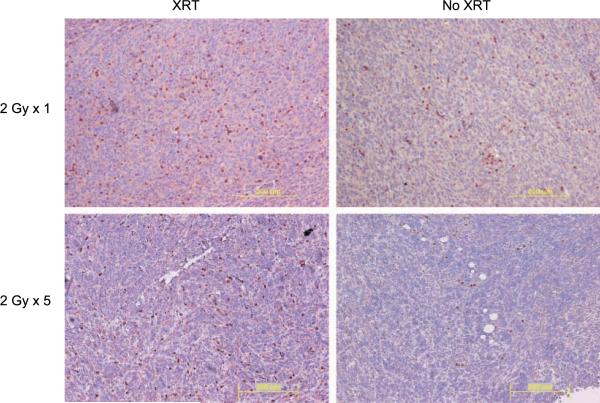
The mice treated above were sacrificed on days 3, 4, and 5 after MSC injection for immunohistochemical confirmation of enhanced MSC engraftment in irradiated tumors. MSC identified with anti-ffLUC antibody were detected in the peripheral tumor stroma, the lining of the necrotic regions, intravascular structures, and the tumor parenchyma for both radiated and unirradiated tumors. Higher numbers of MSC were detected in irradiated tumors at all time points, confirming the bioluminescent imaging (Fig. 2). Further enrichment of MSC was observed in mice treated with five daily fractions of 2 Gy as compared with the enrichment seen in the untreated limb (Fig. 2).
Figure 2.
Immunohistochemical analysis of MSC migration to radiated 4T1 tumors. Mice with bilateral 4T1 hind limb tumors were radiated to their right hind limbs. The following day, 3 × 105 MSC were injected i.v. Mice were sacrificed 3, 4, and 5 d after MSC injection and immunohistochemistry was performed with anti-firefly luciferase antibody on tumor sections to detect MSC. Representative images of tumor regions with MSC were taken from 4 d after MSC injection from radiated (XRT) and unirradiated (No XRT) tumors. Immunohistochemistry was performed with anti-firefly luciferase antibody from 4T1 tumors treated with five daily fractions of 2 Gy (2C) and the 4T1 tumor in the untreated control limb.
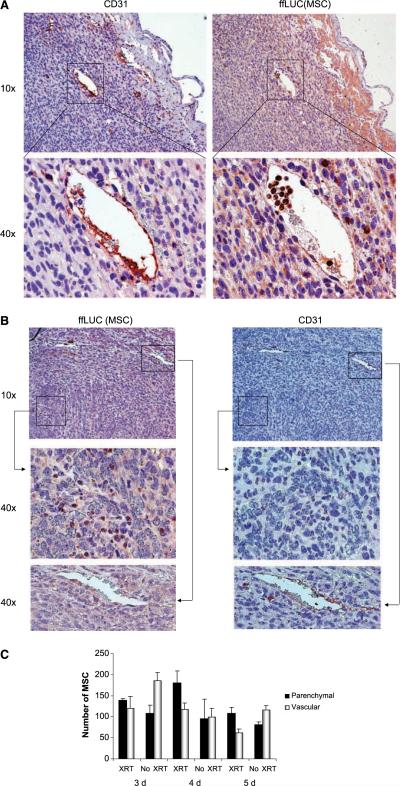
However, we observed different patterns of MSC distribution in irradiated versus unirradiated tumors. In particular, MSC were more commonly associated with intravascular or perivascular structures (as determined by CD31 staining) in unirradiated tumors (Fig. 3A), whereas MSC were present in higher proportions in the parenchyma of irradiated tumors (Fig. 3B). The proportions of MSC in the parenchyma of irradiated tumors were higher on days 3, 4, and 5 after irradiation (Fig. 3C), at 55%, 61%, and 63% of cells, respectively, compared with 35%, 50%, and 42%, respectively, in the parenchyma of the unirradiated tumors.
Figure 3.
MSC distribution within unirradiated (A) and irradiated (B) 4T1 tumors. Immunohistochemically stained 4T1 tumors taken 4 days after i.v. injection of MSC. CD31 staining is used to identify the vasculature and ffLUC was used to detect MSC in serial tissue sections. A, MSC are localized primarily to CD31-stained vessels in unirradiated tumors. B, MSC which infiltrated into the parenchyma of an irradiated tumor (top). Middle and bottom, high-power images of designated regions from the top panels: 40× images of a region of high MSC infiltration without CD31-positive vessels (middle); a CD31-positive vessel distant from the region of MSC infiltration into tumor parenchyma (bottom). C, cells in the viable tumor mass were counted and scored as vascular-associated or intraparenchymal. Columns, mean; bars, SE.
Irradiation-induced expression of cytokines implicated in MSC engraftment
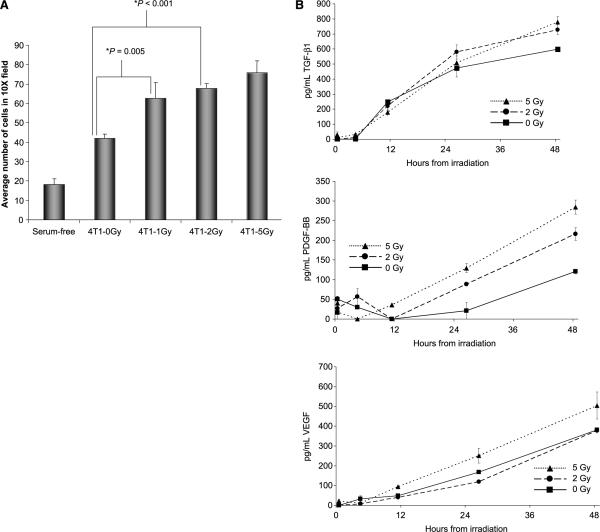
As an in vitro correlate to confirm the observed enhanced MSC migration towards irradiated tumors, we performed in vitro migration assays. In the transwell migration assay (Fig. 4A), MSC exposure to conditioned supernatant from 1 Gy–treated 4T1 cells resulted in a 50% increase in the number of migrated MSC ( from an average of 42 to 63 migrated cells/high-power field, P = 0.005; two-group t test). MSC migration was further increased by 81% in response to exposure to conditioned media from 4T1 cells treated with 5 Gy of radiation ( from an average of 42 to 76 migrated cells/high-power field, P < 0.001; two-group t test).
Figure 4.
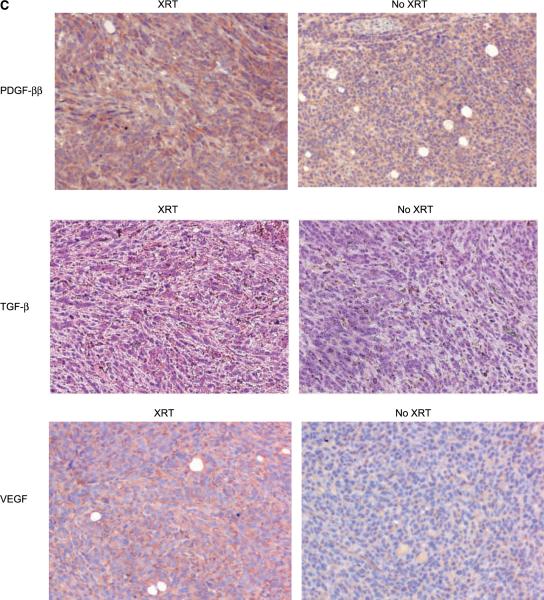
Irradiation-induced secreted cytokines enhance MSC engraftment. A, MSC were exposed to conditioned supernatant from 4T1 cells treated with 1, 2, or 5 Gy in a transwell migration assay. Migrated cells were fixed, stained, and counted. Columns, mean of triplicate experiments; bars, SE. B, ELISA assays for TGF-β, VEGF, and PDGF-BB were performed on conditioned media from cultured 4T1 cells taken over 48 h. Points, mean taken and tested in triplicate; bars, SE. C, representative tumor sections from murine 4T1 tumors were stained for chemoattractant mediators. The expressions of PDGF-BB and TGF-β after five fractions of 2 Gy, and VEGF expression after a single 2 Gy fraction.
These results suggest that secreted factors produced by irradiated tumor cells facilitate MSC chemotaxis. We hypothesized that cytokines such as TGF-β1, VEGF, and PDGF-BB, which have been implicated in MSC migration in vitro (8, 16–18), were expressed at higher levels in the supernatants of irradiated 4T1 cells. To test this, 4T1 cells were irradiated to 0, 2, or 5 Gy and levels of TGF-β1, VEGF, and PDGF-BB were quantified with ELISA assay (Fig. 4B). Forty-eight hours after a 5 Gy dose of irradiation, levels of TGF-β1, VEGF, and PDGF-BB were higher (30%, 32%, and 135%, respectively). Interestingly, levels of PDGF-BB and TGF-β1, but not VEGF, were increased following 2 Gy irradiation. We confirmed the up-regulation of these same cytokines in tumors irradiated in vivo with immunohistochemical staining (Fig. 4C).
MSC exposed to irradiated tumor cells up-regulate CCR2
Chemotaxis involves both the release of homing signals presented via chemokines and expression of specific receptors on the migrating effector cells. Having established that tumor cells up-regulate chemotactic cytokines in response to radiation, we sought to identify changes in MSC that may facilitate recruitment towards irradiated tumors. The chemokines and chemokine receptors, a group of secreted factors and cognate ligands that have well-described functions in immune cell chemotaxis, were interrogated. Recent literature suggest a role for one such chemokine receptor (CCR8) in the migration of MSC migration toward tumor-conditioned media in vitro (13).
With the goal of identifying ligands and/or receptors that were involved specifically in the response to irradiated tumors, gene expression in MSC following 48 h of coculture with irradiated 4T1 cells was compared with MSC exposed to untreated 4T1 cells. The expression of 84 chemokine and chemokine receptors in these MSC were quantitated with real-time PCR.
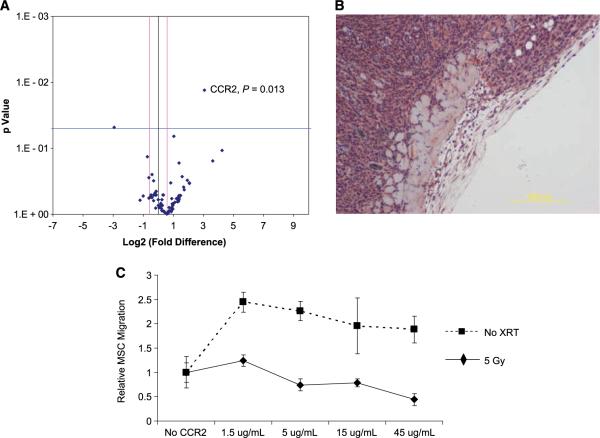
After analysis, CCR2 was the only significantly up-regulated gene, with an 8.48-fold higher expression in MSC exposed to irradiated 4T1 cells as compared with MSC exposed to untreated 4T1 cells (P = 0.013; Fig. 5A). The CCR2 ligand, CCL2, was highly expressed in the parenchyma of murine 4T1 tumors in vivo (Fig. 5B). No difference in CCL2 expression levels was appreciated with immunohistochemistry in the irradiated tumor.
Figure 5.
CCR2 is up-regulated in MSC exposed to irradiated 4T1 cells and blocking CCR2 inhibits MSC migration. Quantitative real-time PCR was performed on MSC exposed to 4T1 cells (eight replicates) and irradiated 4T1 cells (two replicates of 2 Gy and two replicates of 5 Gy). Gene expression was compared for 84 chemokines and chemokine receptors. A, individual genes are plotted as a function of P value and fold expression. B, the CCR2 ligand, CCL2 or MCP-1 is expressed in murine 4T1 tumors. C, in vitro transwell migration assays were performed with MSC incubated with anti-CCR2 antibody. Points, mean; bars, SE.
Anti-CCR2 antibody suppresses MSC migration in response to irradiation
To investigate the contribution of CCR2 in MSC-mediated chemotaxis to irradiated tumors, in vitro transwell migration assays were performed following preincubation with increasing concentrations of a neutralizing anti-CCR2 antibody (Fig. 5C). A dose-dependent decrease in migration was observed in MSC exposed to irradiated 4T1, whereas MSC exposed to untreated 4T1 cells did not show a decrease in migration. Additionally, we observed no decrease in MSC migration in wells with MSC pretreated with a control IgG antibody (data not shown). At the highest concentration of neutralizing antibody (45 μg/mL), the number of migrated MSC decreased by 44% (by absorbance measurements of eluted migrated cells) and 49% by cell count (P = 0.056, two-group t test).
Discussion
We have shown on the basis of both bioluminescent imaging and immunohistochemical findings that the low-dose irradiation of murine tumors in a syngeneic model enhances the tropism for and engraftment of MSC in irradiated tumor environments. We further showed that MSCs show a particular tendency to migrate into the parenchyma of irradiated tumors. Furthermore, this enhanced MSC tropism and engraftment seems to be mediated, at least in part, by two mechanisms. First, the tumor cells secrete higher levels of paracrine factors after irradiation. Second, specific chemokine receptors on the MSC are up-regulated in response to irradiated tumor cells.
The local irradiation of normal tissue in addition to total-body irradiation have previously been shown to increase the local engraftment of MSC, as well as to enhance engraftment out of the irradiation field (10). However, this effect has only been observed in the setting of high doses of irradiation. That is, Francois et al. (10) used 26.5 Gy for local leg irradiation, as compared with 2 Gy used in this study. This 10-fold higher dose would be expected to cause considerable tissue damage that could attract MSC by a secondary effect not associated with local inflammation. The data herein is the first report of tumor irradiation directly increasing MSC engraftment into the tumor microenvironment, and this engraftment occurs rapidly, within 24 h after MSC injection, and at clinically relevant doses of irradiation. Repeated fractions of low-dose radiation, such as those used clinically, should only amplify this engraftment of MSC in human tumors, making this a very attractive strategy for augmenting the tumor-killing effect of the antitumor agents delivered by MSC to the tumor.
The enhanced biodistribution of MSC observed within irradiated tumors could be explained in part by properties of the tumor vasculature. That is, irradiation has been shown to enhance vascular permeability (19), which could facilitate the passage of MSC through the endothelial cell barrier, an observation that may explain why we observed a higher proportion of MSC in the parenchyma of irradiated tumors than that in unirradiated tumors. However, the fact that we found few perivascular MSC suggests that the change in vascular permeability is not the dominant mechanism responsible for the increased tropism of MSC to irradiated tumors.
We also observed that within 8 h of irradiation, tumor cells increased the production and secretion of cytokines that enhance the migratory properties of MSC. VEGF, PDGF-BB, and TGF-β were up-regulated after tumor cell irradiation. ELISAassays showed that these cytokines increased in a dose-dependent manner over 48 h. These same cytokines were confirmed to be up-regulated in the irradiated murine tumors in vivo that attracted increased numbers of MSC. A number of other inflammatory cytokines, including SDF-1, basic fibroblast growth factor, interleukin-1β, and TNF-α have also been shown to be both involved in MSC migration (8, 20, 21) and up-regulated by irradiation (19, 22, 23), which may also contribute to our observed tumor-based enhanced chemotactic effect.
In addition to tumor cells secreting factors that enhance MSC engraftment, we asked whether changes occur in the MSC in response to irradiated tumor cells that contribute to the observed increase in migration. We found that the chemokine receptor, CCR2, is up-regulated in MSC exposed to irradiated tumor cells. CCR2 is best described by its role in monocyte mobilization and recruitment into inflammatory sites (11, 24). CCR2 seems to be expressed on MSC in an inducible fashion, as it is undetectable or expressed at low levels (25) on untreated cells but could be up-regulated by inflammatory cytokines such as TNF-α (13, 26) or by other factors secreted by irradiated tumor cells as shown in this study. CCL2, a CCR2 ligand, is expressed in many tumor types and has been associated with tumor progression. This tumor promotion effect has been proposed to occur via the attraction of tumor-associated macrophages or myeloid suppressor cells that support tumor growth (27). In our study, CCL2 expression in tumors was present but not appreciably modulated in response to irradiation. These findings suggests that MSC migration to irradiated tumors may result from a dynamic interplay in which tumor cells secrete cytokines in response to radiation, leading to chemokine receptor up-regulation on MSC, and ultimately resulting in enhanced chemotaxis towards the chemokine ligand-bearing tumor. In support of this, we found that anti-CCR2 suppressed MSC migration markedly in response to radiated tumor cells, possibly due in part to the higher levels of CCR2 on MSC exposed to irradiated tumor cells. Manipulation of the CCL2/CCR2 axis may decrease the enhanced MSC engraftment in irradiated tumors which could be of therapeutic use if MSC are found to enhance radiation resistance. Interestingly, exposure of MSC to a low dose of anti-CCR2 (1.5 μg/mL) resulted in an increase in migration to unirradiated 4T1 cells and to a lesser extent to irradiated 4T1 cells, whereas migration to irradiated 4T1 cells was inhibited at higher concentrations. The reason for this concentration-dependent effect is not clear but it may be due to a partial agonist effect of the antibody, which is a phenomenon that has been previously described for other antireceptor antibodies (28, 29).
The use of tumor-tropic MSC that can be isolated, expanded, and modified ex vivo for the delivery of antitumor agents is a promising approach. However, achieving a sufficient number of tumor-targeted MSC may be a limitation of this strategy. Our findings indicate that radiation therapy might be an important means of achieving this aim. Another potential hurdle to overcome for the full benefit of MSC-based cancer therapy is the adequate exposure of all tumor cells to MSC-secreted proteins. The wider parenchymal distribution of MSC observed in irradiated tumors may provide a solution to this. An alternative approach might be to use fractionated local irradiation followed by the delivery of MSC expressing radio-sensitizing cytokines, such as interleukin-24 or TNF-α, which potentiate the effects of low-dose irradiation.
In regards to the biological significance of MSC engrafting in radiated microenvironments, one could speculate that MSC contribute to the fibrotic response, which is a major contributor to radiation-induced toxicity. Alternatively, MSC could be involved in tissue repair—providing revascularization structures. Evidence for both scenarios exists with migrated bone marrow cells being implicated in parotid gland repair from radiation damage (30) as well as radiation-induced lung fibrosis (16).
In summary, we found that low-dose irradiation enhances MSC engraftment in the tumor microenvironment and increases MSC infiltration into the tumor parenchyma. Further studies are warranted to determine the best method of irradiation delivery to optimize this effect, to investigate the clinical utility of this observation, and to further study the effect of this strategy on the tumor response to irradiation.
Acknowledgments
Grant support: National Cancer Institute grants CA-1094551 and CA-116199 (F.C. Marini); CA-55164, CA-16672, and CA-49639 (M. Andreeff) and by the Stringer Professorship for Cancer Treatment and Research (M. Andreeff). Supported in part by grants from the Susan G. Komen Breast Cancer Foundation, the Ladies Leukemia League, and the W.M. Keck Foundation (A. Klopp, J.L. Dembinski, and F.C. Marini).
Footnotes
Conflict of interest statement: No authors have any conflict of interest with regard to the work submitted in this manuscript.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mosca JD, McIntosh KR. Human mesenchymal stem cells: progenitor cells for cartilage, bone, fat and stroma. Curr Top Microbiol Immunol. 2000;251:3–11. doi: 10.1007/978-3-642-57276-0_1. [DOI] [PubMed] [Google Scholar]
- 3.Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 4.Wu GD, Nolta JA, Jin YS, et al. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation. 2003;75:679–85. doi: 10.1097/01.TP.0000048488.35010.95. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006;326:725–36. doi: 10.1007/s00441-006-0270-9. [DOI] [PubMed] [Google Scholar]
- 6.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 7.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 8.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 9.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 10.Francois S, Bensidhoum M, Mouiseddine M, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–9. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 11.Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–61. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu C, Edwards EW, Tacke F, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–41. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringe J, Strassburg S, Neumann K, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–46. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 14.Ling X, Wang Y, Dietrich MF, Andreeff M, Arlinghaus RB. Vaccination with leukemia cells expressing cell-surface-associated GM-CSF blocks leukemia induction in immunocompetent mice. Oncogene. 2006;25:4483–90. doi: 10.1038/sj.onc.1209477. [DOI] [PubMed] [Google Scholar]
- 15.Yotnda P, Zompeta C, Heslop HE, Andreeff M, Brenner MK, Marini F. Comparison of the efficiency of transduction of leukemic cells by fiber-modified adeno-viruses. Hum Gene Ther. 2004;15:1229–42. doi: 10.1089/hum.2004.15.1229. [DOI] [PubMed] [Google Scholar]
- 16.Epperly MW, Cao S, Goff J, et al. Increased longevity of hematopoiesis in continuous bone marrow cultures and adipocytogenesis in marrow stromal cells derived from Smad3(−/−) mice. Exp Hematol. 2005;33:353–62. doi: 10.1016/j.exphem.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Birnbaum T, Roider J, Schankin CJ, et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–7. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 18.Schichor C, Birnbaum T, Etminan N, et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–10. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8:1765–80. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt A, Ladage D, Schinkothe T, et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24:1750–8. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- 21.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–63. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Jendrossek V, Belka C. The role of PDGF in radiation oncology. Radiat Oncol. 2007;2:5. doi: 10.1186/1748-717X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–8. [PubMed] [Google Scholar]
- 24.Charo IF. CCR2: from cloning to the creation of knockout mice. Chem Immunol. 1999;72:30–41. doi: 10.1159/000058724. [DOI] [PubMed] [Google Scholar]
- 25.Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 26.Lopez Ponte A, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–45. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 27.Huang B, Lei Z, Zhao J, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Mijares A, Lebesgue D, Wallukat G, Hoebeke J. From agonist to antagonist: Fab fragments of an agonist-like monoclonal anti-β(2)-adrenoceptor antibody behave as antagonists. Mol Pharmacol. 2000;58:373–9. doi: 10.1124/mol.58.2.373. [DOI] [PubMed] [Google Scholar]
- 29.Shiao SL, McNiff JM, Masunaga T, Tamura K, Kubo K, Pober JS. Immunomodulatory properties of FK734, a humanized anti-CD28 monoclonal antibody with agonistic and antagonistic activities. Transplantation. 2007;83:304–13. doi: 10.1097/01.tp.0000251426.46312.d5. [DOI] [PubMed] [Google Scholar]
- 30.Lombaert IM, Wierenga PK, Kok T, Kampinga HH, deHaan G, Coppes RP. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res. 2006;12:1804–12. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]