Abstract
Purpose
Postmastectomy radiotherapy (PMRT) improves locoregional control (LRC) in patients with high-risk features after mastectomy. Young age continues to evolve as a potentially important risk factor. The objective of this study was to assess the benefits of PMRT in patients <35 years old treated with doxorubicin-based neoadjuvant chemotherapy for Stage II–III breast cancer.
Patients and Methods
We retrospectively analyzed 107 consecutive breast cancer patients <35 years old with Stage IIA–IIIC disease treated at our institution with doxorubicin-based neoadjuvant chemotherapy and mastectomy, with or without PMRT. The treatment groups were compared in terms of LRC and overall survival.
Results
Despite more advanced disease stages, the patients who received PMRT (n = 80) had greater rates of LRC (5-year rate, 88% vs. 63%, p = 0.001) and better overall survival (5-year rate, 67% vs. 48%, p = 0.03) than patients who did not receive PMRT (n = 27).
Conclusion
Among breast cancer patients <35 years old at diagnosis, the use of PMRT after doxorubicin-based neoadjuvant chemotherapy and mastectomy led to a statistically greater rate of LRC and overall survival compared with patients without PMRT. The benefit seen for PMRT in young patients provides valuable data to better tailor adjuvant, age-specific treatment decisions after mastectomy.
Keywords: Radiation therapy, mastectomy, young age, neoadjuvant chemotherapy
INTRODUCTION
Young age in breast cancer patients has been found in several large studies to predict for worse clinical outcomes (1–3). Compared with older patients, younger breast cancer patients exhibit a greater proportion of aggressive pathologic features such as lymphovascular space invasion, high nuclear grade, and a high proportion of estrogen receptor negativity (3–6). However, even with pathologic factors accounted for, young age remains an independent predictor for a worse outcome in patients treated with either breast-conserving therapy or mastectomy (1, 7, 8).
Recent updates of large randomized trials have highlighted the benefit of postmastectomy radiotherapy (PMRT) on outcome in high-risk breast cancer patients but have not reported age-specific results. A report of the Danish Breast Cancer Cooperative Group randomized studies that included >3,000 pre- and postmenopausal patients demonstrated that PMRT was associated with improved locoregional control (LRC) and a lower rate of distant metastases in high-risk patients treated with adjuvant chemotherapy (9). In addition, the British Colombia Trial (n = 318) demonstrated a benefit of PMRT on the 20-year rates of LRC and overall survival (OS) in premenopausal node-positive patients treated with adjuvant chemotherapy (10). Furthermore, the 15-year results from a meta-analysis of randomized trials by the Early Breast Cancer Trialists’ Collaborative Group confirmed that PMRT improves long-term LRC, regardless of nodal status, and improved 15-year OS in node-positive patients (11).
Data specific to patients treated with neoadjuvant chemotherapy are more limited. Our group previously examined the affect of PMRT in patients of all ages (n = 676, median age 49 years) treated with neoadjuvant chemotherapy and mastectomy and found that the addition of RT improved LRC and cause-specific survival for patients with locally advanced disease, such as those with four or more positive lymph nodes, T3 primary tumors, or Stage III disease (12). Despite the evidence demonstrating a benefit of PMRT in high-risk breast cancer patients, the evaluation of risk among patient <35 years old has not been adequately addressed. The purpose of this study was to examine the affect of PMRT in young breast cancer patients <35 years after neoadjuvant chemotherapy and mastectomy for Stage II–III disease.
PATIENTS AND METHODS
We retrospectively reviewed 107 patients <35 years old treated between 1975 and 2005 with Stage II–III breast cancer on protocols with neoadjuvant doxorubicin-based chemotherapy and mastectomy. All patients were clinically staged at diagnosis and retrospectively recategorized according to the 2003 American Joint Committee on Cancer Staging guidelines.
The median number of recovered axillary lymph nodes after mastectomy was 17.5 (range, 1–54). A total of 80 patients were treated with PMRT and 27 were not. Of these 27 patients, 21 had zero to three positive lymph nodes at surgery and were not referred for PMRT, and 6 patients had four or more lymph nodes (1 patient refused RT and 5 were not referred for unknown reasons). For the 80 patients treated with PMRT, the treatment volumes during this period typically included the chest wall and draining lymphatics, including the supraclavicular and internal mammary nodal regions (median dose 50 Gy), followed by a chest wall boost (median dose 10 Gy). All patients underwent computed tomography simulation and planning for optimal target coverage with minimal exposure to the lung and heart. The chest wall was usually treated with medial and lateral tangents using photons designed to include the entire chest wall. A separate supraclavicular anterior photon field was matched at the nondivergent superior border of the tangential fields designed to encompass the undissected Level III axilla and axillary apex. An electron field was often matched medially to the medial tangential field, with particular care to cover the internal mammary nodal region while respecting critical structures, including the heart and lung. Finally, the chest wall was typically boosted with electrons designed to include the mastectomy scar with an adequate margin.
Statistical analysis
The distributions of the clinical and pathologic factors between groups of patients were compared using the chi-square test. Locoregional recurrence (LRR) was defined as disease recurrence on the ipsilateral chest wall or in the ipsilateral axillary, supraclavicular, infraclavicular, or internal mammary lymph nodes. Any other site of recurrence was scored as distant metastasis. All LRRs were considered independent events, regardless of whether they occurred before or after distant metastasis. The 5-year actuarial rates of LRR and OS were calculated according to the Kaplan-Meier method, and comparisons between the two patient groups were made using the log–rank test. All survival statistics were measured from the date of diagnosis. All p values are two-sided, and p ≤0.05 was considered significant.
RESULTS
Patient characteristics
The median follow-up for irradiated (n = 80) and nonirradiated (n = 27) patients was 75 and 63 months, respectively (72 months for all patients combined, range 2–238). The median follow-up for the 61 surviving patients was 92 months. Table 1 outlines a comparison of the disease characteristics between the treatment groups. Patients in the PMRT group had a statistically greater percentage of Stage III tumors (83% vs. 41%; p <0.05), greater percentage of lymphovascular space invasion (49% vs. 33%; p = 0.04), and Stage T4 disease (50% vs. 26%; p = 0.002). Also, a nonsignificant trend toward a more advanced nodal stage was found in the PMRT treatment group because of N2 and N3 disease. No difference was found between the groups with respect to nuclear grade, hormone receptor status, tamoxifen use, margin status, presence of extracapsular extension, or number of nodes sampled. No relationship was found between RT volume and outcome.
Table 1.
Patient and tumor characteristics
| Characteristic | No RT (n) | RT(n) | p |
|---|---|---|---|
| Clinical stage | 0.002 | ||
| IIA | 3 (11) | 1 (1) | |
| IIB | 12 (46) | 12 (15) | |
| IIIA | 4 (15) | 23 (29) | |
| IIIB | 6 (23) | 37 (47) | |
| IIIC | 1 (4) | 6 (8) | |
| Clinical T stage | 0.001 | ||
| T1 | 2 (7) | 0 (0) | |
| T2 | 11 (41) | 11 (14) | |
| T3 | 7 (26) | 29 (36) | |
| T4 | 7 (26) | 40 (50) | |
| Clinical N stage | 0.163 | ||
| N0 | 5 (19) | 9 (11) | |
| N1 | 17 (63) | 37 (46) | |
| N2 | 4 (15) | 28 (35) | |
| N3 | 1 (4) | 6 (8) | |
| Pathologic tumor size (cm) | 0.113 | ||
| 0–2 | 12 (44) | 38 (48) | |
| 2.1–5 | 9 (33) | 28 (35) | |
| ≥5.1 | 1 (4) | 10 (13) | |
| Unknown | 5 (19) | 4 (5) | |
| No. of positive nodes | 0.130 | ||
| 0 | 9 (33) | 19 (24) | |
| 1–3 | 11 (41) | 22 (28) | |
| ≥4 | 6 (22) | 38 (47) | |
| Unknown | 1 (4) | 1 (1) | |
| Percentage of positive nodes | 0.031 | ||
| <20 | 20 (74) | 36 (45) | |
| ≥20 | 7 (26) | 43 (54) | |
| Unknown | 0 (0) | 1 (1) | |
| Nodes sampled (n) | 0.183 | ||
| <10 | 2 (7) | 11 (14) | |
| ≥10 | 23 (85) | 68 (85) | |
| Unknown | 2 (7) | 1 (1) | |
| LVSI | 0.039 | ||
| Present | 9 (33) | 39 (49) | |
| Absent | 18 (67) | 33 (41) | |
| Unknown | 0 (0) | 8 (10) | |
| Tumor grade | 0.798 | ||
| 1 | 12 (44) | 36 (45) | |
| 2 | 9 (33) | 20 (25) | |
| 3 | 5 (19) | 21 (26) | |
| Unknown | 1 (4) | 3 (4) | |
| Margin status | 0.273 | ||
| Free/negative | 26 (96) | 66 (83) | |
| Involved | 0 (0) | 5 (6) | |
| Close (<2 mm) | 0 (0) | 5 (6) | |
| Unknown | 1 (4) | 4 (5) | |
| Estrogen receptor | 0.435 | ||
| Positive | 13 (48) | 32 (40) | |
| Negative | 8 (30) | 36 (45) | |
| Unknown | 6 (22) | 12 (15) | |
| Progesterone receptor | 0.320 | ||
| Positive | 9 (33) | 22 (28) | |
| Negative | 8 (30) | 39 (49) | |
| Unknown | 10 (37) | 19 (24) | |
| Hormonal treatment | 0.227 | ||
| Yes | 6 (22) | 25 (31) | |
| No | 21 (78) | 50 (63) | |
| Unknown | 0 (0) | 5 (6) | |
| Response to neoadjuvant chemotherapy | 0.552 | ||
| CR | 4 (15) | 15 (19) | |
| PR | 22 (81) | 53 (66) | |
| NR | 1 (4) | 2 (3) | |
| PD | 0 (0) | 4 (5) | |
| Unknown | 0 (0) | 6 (7) | |
| Adjuvant chemotherapy | 0.165 | ||
| Yes | 19 (70) | 66 (84) | |
| No | 8 (30) | 13 (16) |
Abbreviations: RT = radiotherapy; LVSI = lymphovascular space invasion; CR = complete response; PR = partial response; NR = no response; PD = progressive disease.
Data in parentheses are percentages.
Locoregional recurrence
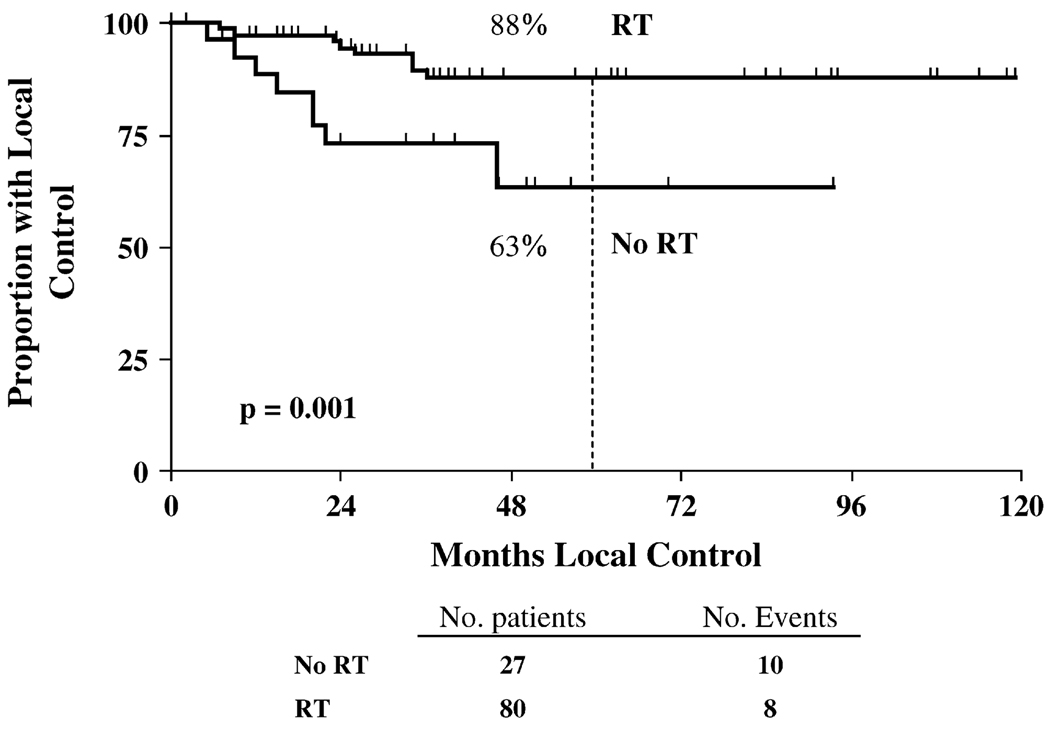
Of the 80 patients in the PMRT group, 18 had locoregional failure, and of the 27 patients in the no RT group, 10 had LRR. The 5-year LRR rate was 12% for the 80 patients who received PMRT compared with 37% in the 27 patients who did not receive PMRT (p = 0.001; Fig. 1). The sites of locoregional failure included the isolated chest wall in 7 patients, isolated supraclavicular region in 6 patients, simultaneous chest wall and supraclavicular region in 3 patients, simultaneous chest wall and infraclavicular node in 1 patient, and simultaneous chest wall and axilla, infraclavicular, and supraclavicular nodes in 1 patient.
Fig. 1.
Rate of 5-year actuarial locoregional control for all 107 patients treated with radiotherapy (RT) and without RT (No RT).
The mean and median time to local recurrence in all patients was 29 and 23 months, respectively. Table 2 shows the relationship between selected clinical and pathologic characteristics of patients in each treatment group and LRR. Four patients had clinical Stage IIA disease and only 1 received PMRT (this patient also had perineural invasion and extracapsular extension in a positive axillary lymph node). All 4 of these patients were alive and disease free at the last follow-up visit. The addition of PMRT improved LRR in both those with clinical Stage IIB disease (0% vs. 44%, p = 0.003) and those with clinical Stage IIIA–IIIC (15% vs. 36%, p = 0.023). Of the 24 patients with clinical Stage IIB disease, 10 presented with T3N0M0 disease and 14 had T2N1M0 disease. Of these 14 patients, 10 had pathologic nodal disease at surgery that was more advanced than was apparent from the initial clinical examination and radiographic studies (6 patients with four or more lymph nodes). Of the 10 patients with tumors refractory to chemotherapy, 7 received PMRT and 3 did not (2 declined and 1 was lost to follow-up). Of the 7 patients who underwent PMRT, none experienced locoregional failure. However, of the 3 remaining patients with disease progression during chemotherapy who did not receive PMRT, 2 had LRR. Only 7 patients had clinical Stage T2 disease with one to three positive lymph nodes after surgery; therefore, no meaningful analyses could be conducted in this small subset.
Table 2.
Five-year actuarial rates of LRR according to clinical and pathologic factors
| 5-y LRR rate | |||
|---|---|---|---|
| Characteristic | No RT (%) | RT (%) | p |
| Clinical stage | |||
| IIA | 0 | 0 | NA |
| IIB | 44 | 0 | 0.003 |
| IIIA | 25 | 16 | 0.435 |
| IIIB | 33 | 13 | 0.1062 |
| IIIC | 100 | 17 | 0.276 |
| Clinical T stage | |||
| T1 | 0 | 0 | NA |
| T2 | 32 | 0 | 0.028 |
| T3 | 43 | 16 | 0.051 |
| T4 | 43 | 13 | 0.015 |
| Clinical N stage | |||
| N0 | 40 | 0 | 0.045 |
| N1 | 28 | 13 | 0.170 |
| N2 | 50 | 13 | 0.028 |
| N3 | 100 | 17 | 0.276 |
| Positive nodes (n) | |||
| 0 | 35 | 15 | 0.079 |
| 1–3 | 30 | 11 | 0.164 |
| ≥4 | 37 | 12 | 0.001 |
Abbreviations: LRR = locoregional recurrence; RT = radiotherapy; NA = not applicable.
Radiotherapy also correlated with reduced LRR in patients stratified according to T stage (T2, p = 0.028; T3, p = 0.051; and T4, p = 0.015) and N stage (N0, p = 0.045 and N2, p = 0.028).
Survival
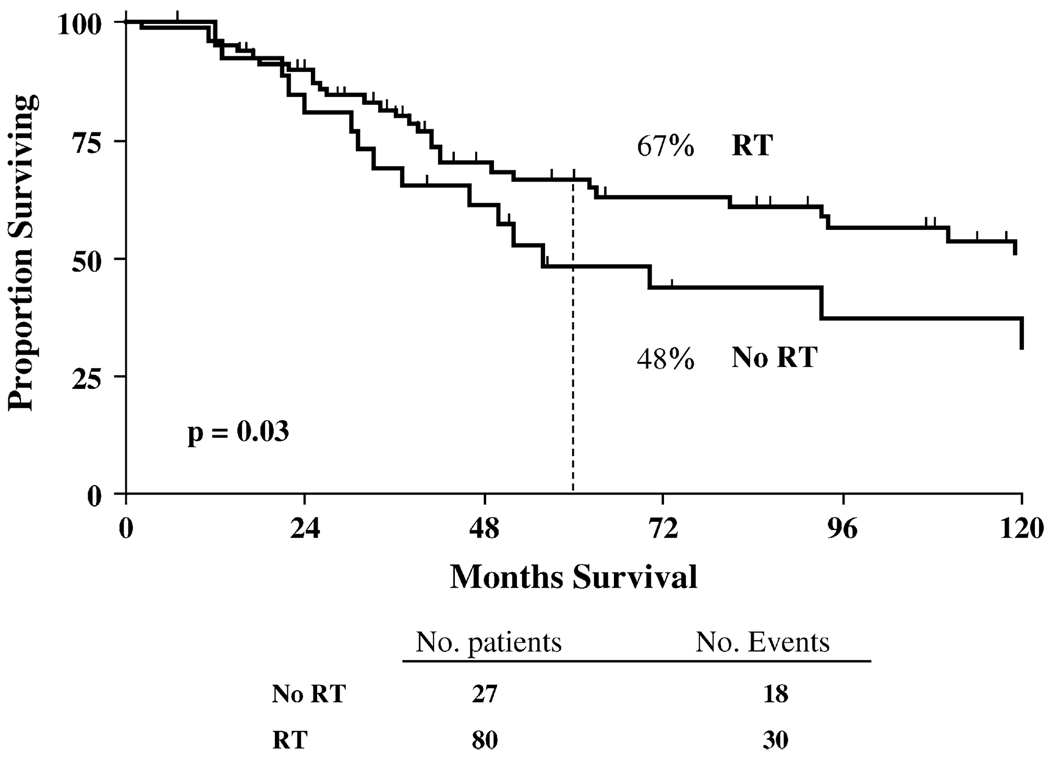
Of the 18 patients with locoregional failure, only 2 were alive at last follow-up. The 5-year actuarial rate of OS for all patients (Stage II–III) was 67% in the PMRT group and 48% in the non-RT group (p = 0.031; Fig. 2). Subset analyses of survival by clinical stage (Table 3) found that for Stage IIB patients, PMRT significantly improved OS compared with that for those who did not receive PMRT (92% vs. 56%, p = 0.033). In Stage IIIA–IIIC patients, those who received PMRT had significantly improved OS compared with patients who did not receive PMRT (60% vs. 27%, p = 0.014). Additionally, RT improved survival in patients with four or more positive nodes at surgery (67% vs. 48%, p = 0.031) and in patients with lymphovascular space invasion (83% vs. 57%, p = 0.01).
Fig. 2.
Rate of 5-year actuarial survival for all 107 patients treated with radiotherapy (RT) and without RT (No RT).
Table 3.
Five-year actuarial survival rates according to clinical and pathologic factors
| 5-y Survival rate (%) | |||
|---|---|---|---|
| Characteristic | No RT | RT | p |
| Clinical Stage | |||
| IIA | 100 | 100 | NA |
| IIB | 56 | 92 | 0.033 |
| IIIA | 50 | 66 | 0.154 |
| IIIB | 17 | 60 | 0.064 |
| IIIC | 0 | 33 | 0.56 |
| Clinical T stage | |||
| T1 | 100 | 100 | NA |
| T2 | 76 | 89 | 0.364 |
| T3 | 29 | 70 | 0.009 |
| T4 | 14 | 58 | 0.045 |
| Clinical N stage | |||
| N0 | 0 | 89 | 0.012 |
| N1 | 68 | 60 | 0.689 |
| N2 | 25 | 73 | 0.115 |
| N3 | 0 | 33 | 0.56 |
| Positive nodes (n) | |||
| 0 | 56 | 67 | 0.076 |
| 1–3 | 47 | 86 | 0.435 |
| >4 | 48 | 67 | 0.031 |
| LVSI | |||
| Present | 57 | 83 | 0.01 |
| Absent | 33 | 49 | 0.377 |
Abbreviations: NA = not applicable; other abbreviations as in Table 1.
DISCUSSION
This is the first report evaluating the affect of PMRT in breast cancer patients <35 years old at diagnosis and treated with anthracycline-based neoadjuvant chemotherapy and mastectomy. Our data found that the addition of PMRT in patients with Stage IIB-III disease led to superior rates of 5-year LRC and OS. When patients with Stage IIB disease were analyzed separately, an improvement in LRC and survival with the addition of PMRT remained statistically significant. The large magnitude of benefit seen from PMRT in young patients provides valuable data to better tailor adjuvant age-specific treatment decisions in difficult clinical circumstances. Our findings provide evidence supporting the recommendation for adjuvant RT for these patients and should guide physicians in their counseling of young patients.
Young age has previously been suggested as a predictive factor for worse outcome in breast cancer patients treated with mastectomy without RT. A recent large retrospective study from Canada that analyzed >800 patients with T1–T2 disease and one to three positive lymph nodes treated with mastectomy and chemotherapy found age <45 years to be an independent risk factor for LRR after mastectomy, with a hazard ratio of 2.5 (13). Furthermore, a large meta-analysis of five National Surgical Adjuvant Breast Project trials with >5,700 patients with all disease stages treated with mastectomy and adjuvant chemotherapy found that younger patients had greater rates of LRR with or without distant failure (26.1% among the 20–39-year-old patients) (14). Finally, retrospective reports, including one from our institution that included patients with Stage II–III disease treated with neoadjuvant chemotherapy and mastectomy, have suggested that young age is a risk factor for LRR (15, 16). Although these studies suggest that young age might be an adverse prognostic factor for outcome, it is important to note that not all reports have shown similar results (17–19). Furthermore, the results from subset analyses should be interpreted cautiously.
With large randomized trials such as the British Colombia trial and Danish 82b trial (both studies included premenopausal patients) and recent meta-analyses showing an improvement in LRC and survival with the use of PMRT in breast cancer (9–11), an emphasis has been placed on identifying subsets of patients who might be at high risk of local recurrence, particularly those for whom the recommendation of PMRT is not routine. Consensus panels, including those from the American Society of Therapeutic Radiology and Oncology, American Society of Clinical Oncology, and the National Institutes of Health, have recommended the use of PMRT in patients with four or more positive axillary nodes and those with T3 or T4 primary tumors (20–22). However, these groups have cited insufficient evidence to recommend PMRT for those with smaller primary tumors and one to three positive lymph nodes after surgery or patients with potentially high-risk features such as young age. Furthermore, recommendations regarding patients who receive neoadjuvant chemotherapy are not yet concrete. For example, the American Society of Clinical Oncology panel concluded that “there is insufficient evidence to make recommendations or suggestions on whether all patients treated with preoperative systemic therapy should be given PMRT after surgery.”
To date, this is the largest series of breast cancer patients <35 years treated with neoadjuvant chemotherapy and mastectomy with or without PMRT. Our results suggest that young patients with Stage IIB-III disease derive a large benefit in LRC and OS with the addition of PMRT, with a two-thirds reduction in LRR (12% vs. 37% at 5 years with and without PMRT, respectively) and 40% improvement in OS (67% vs. 48% at 5 years with and without PMRT, respectively). RT improved survival for young patients with Stage IIB disease, a result not seen in a previous report of similarly treated patients of all ages (12). A significant proportion of the patients with clinical Stage IIB had either Stage T3 disease (42%) or four or more positive lymph nodes after surgery (25%) because of disease progression, two factors independently prognostic for local recurrence after mastectomy. Despite this, our results suggest that young age might be a powerful prognostic factor to gauge the benefit of PMRT in patients with Stage IIB or greater disease. Relatively few of our patients had Stage II disease and one to three positive lymph nodes. Therefore, the benefit of PMRT for young patients in this group remains unclear.
One limitation of this analysis was its retrospective nature. All retrospective studies inherently risk an imbalance of patient and tumor characteristics. The two cohorts (PMRT vs. no PMRT) in our study had differences in several factors, but the more advanced tumor characteristics were in the PMRT group and yet they had the improved locoregional and survival benefit. This emphasizes the advantage with PMRT to overcome negative pathologic features in this cohort. A second limitation of this study was the modest number of patients with Stage IIB disease, for whom the results need to be confirmed in a larger study. Although this is the largest series of patients <35 years treated with neoadjuvant chemotherapy and mastectomy with or without RT, multivariate analysis was not possible because of relatively limited number of events.
CONCLUSION
The addition of PMRT was associated with significant improvement in LRC and OS in young patients <35 years old after neoadjuvant chemotherapy and mastectomy for clinical Stage IIB-III breast cancer and should help to guide difficult treatment decisions.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.de la Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 2.Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869–1874. doi: 10.1016/s0140-6736(00)02292-3. [DOI] [PubMed] [Google Scholar]
- 3.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: Are there age differentials? J Natl Cancer Inst Monogr. 1994;16:35–42. [PubMed] [Google Scholar]
- 5.Agrup M, Stal O, Olsen K, et al. C-erbB-2 overexpression and survival in early onset breast cancer. Breast Cancer Res Treat. 2000;63:23–29. doi: 10.1023/a:1006498721508. [DOI] [PubMed] [Google Scholar]
- 6.Marcus JN, Watson P, Page DL, et al. Pathology and heredity of breast cancer in younger women. J Natl Cancer Inst Monogr. 1994;16:23–34. [PubMed] [Google Scholar]
- 7.Matthews RH, McNeese MD, Montague ED, et al. Prognostic implications of age in breast cancer patients treated with tumorectomy and irradiation or with mastectomy. Int J Radiat Oncol Biol Phys. 1988;14:659–663. doi: 10.1016/0360-3016(88)90086-7. [DOI] [PubMed] [Google Scholar]
- 8.Oh JL, Bonnen M, Outlaw ED, et al. The impact of young age on locoregional recurrence after doxorubicin-based breast conservation therapy in patients 40 years old or younger: How young is “young”? Int J Radiat Oncol Biol Phys. 2006;65:1345–1352. doi: 10.1016/j.ijrobp.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen HM, Overgaard M, Grau C, et al. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: Long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24:2268–2275. doi: 10.1200/JCO.2005.02.8738. [DOI] [PubMed] [Google Scholar]
- 10.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiotherapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 11.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 12.Huang EH, Tucker SL, Strom EA, et al. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol. 2004;22:4691–4699. doi: 10.1200/JCO.2004.11.129. [DOI] [PubMed] [Google Scholar]
- 13.Truong PT, Olivotto IA, Kader HA, et al. Selecting breast cancer patients with T1–T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1337–1347. doi: 10.1016/j.ijrobp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: Results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247–4254. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Fodor J, Polgar C, Major T, et al. Locoregional failure 15 years after mastectomy in women with one to three positive axillary nodes with or without irradiation the significance of tumor size. Strahlenther Onkol. 2003;179:197–202. doi: 10.1007/s00066-003-1010-7. [DOI] [PubMed] [Google Scholar]
- 16.Garg AK, Strom EA, McNeese MD, et al. T3 disease at presentation or pathologic involvement of four or more lymph nodes predict for locoregional recurrence in stage II breast cancer treated with neoadjuvant chemotherapy and mastectomy without radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:138–145. doi: 10.1016/j.ijrobp.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: Experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- 18.Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 19.Arriagada R, Le MG, Contesso G, et al. Predictive factors for local recurrence in 2006 patients with surgically resected small breast cancer. Ann Oncol. 2002;13:1404–1413. doi: 10.1093/annonc/mdf227. [DOI] [PubMed] [Google Scholar]
- 20.Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst. 2001;93:979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 21.Harris JR, Halpin-Murphy P, McNeese M, et al. Consensus statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:989–990. doi: 10.1016/s0360-3016(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 22.Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–1569. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]