Abstract
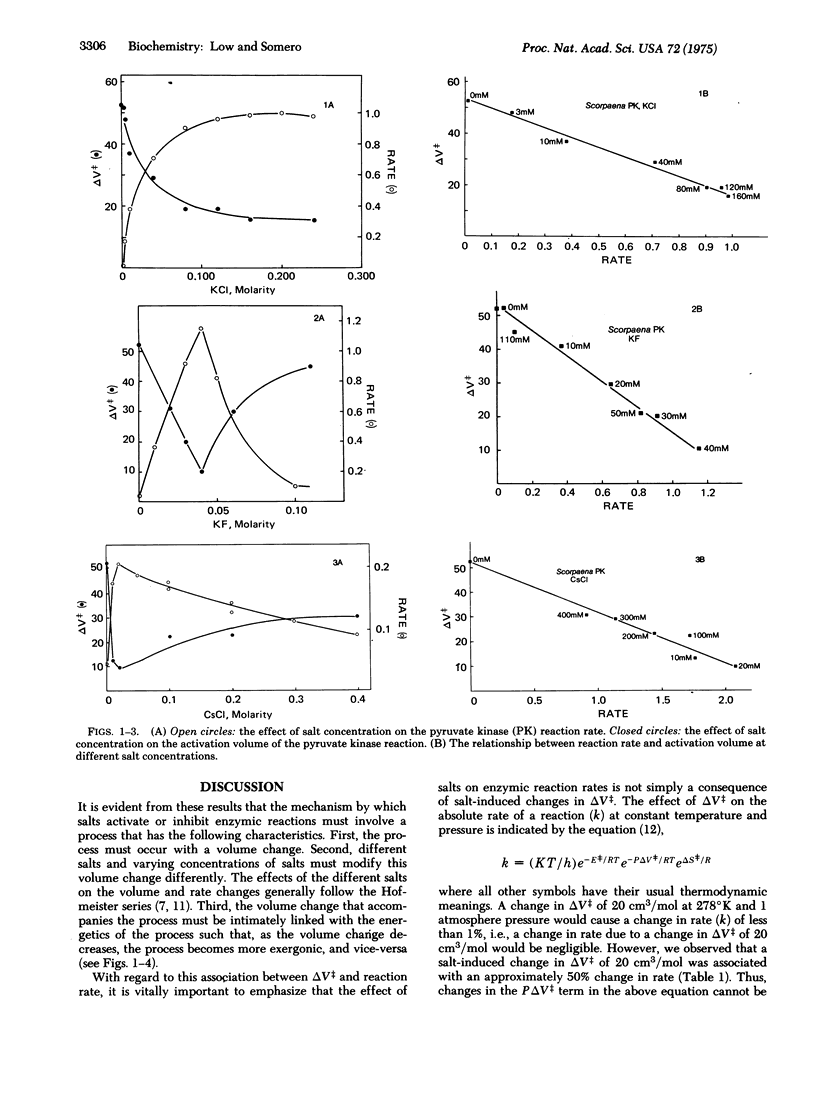
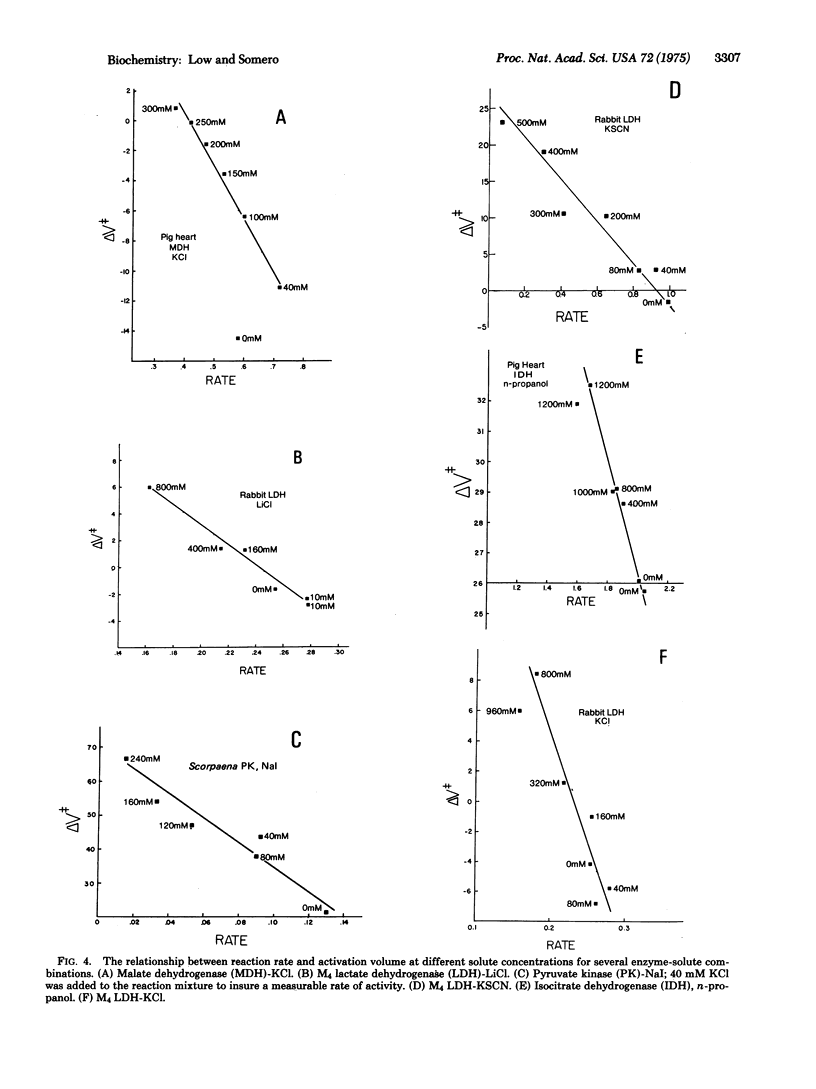
There exists a linear correlation between the effect of a salt on the rate of an enzymic reaction and its effect on the activation volume (delta V++) of the reaction. Salts that increase delta V++ invariably decrease the rate of the reaction, and vice versa. The salt effects on reaction rate are, however, much larger than would be predicted solely on the basis of pressure-volume work changes deriving from the observed alterations in delta V++. Different inorganic salts affect reaction rates and activation volumes in a manner that reflects the salts' positions in the Hofmeister series. These observations, taken in conjunction with data on the effects of salts on protein functional group (aminoacid side-chains and peptide linkages) hydration, lead us to propose the following hypothesis to account for salt activation and inhibition of catalysis. Aminoacid side-chains and peptide linkages located on or near the protein surface change their exposure to water during conformational events in catalysis. These protein group transfers are accompanied by large volume and energy changes that are due largely to changes in the organization of water around these groups. When these transfer processes occur during the rate-limiting step in catalysis, these energy and volume changes can contribute to the free energy of activation (delta G++) and the activation volume of the reaction. By influencing the degree to which water can organize around transferred protein groups, salts can modify both the delta G++ (rate) and the delta V++ of a reaction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRATTON C. B., HOPKINS A. L., WEINBERG J. W. NUCLEAR MAGNETIC RESONANCE STUDIES OF LIVING MUSCLE. Science. 1965 Feb 12;147(3659):738–739. doi: 10.1126/science.147.3659.738. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Oliveira R. J., Westort C. Thermodynamics of protein denaturation. Effect of pressu on the denaturation of ribonuclease A. Biochemistry. 1970 Feb 17;9(4):1038–1047. doi: 10.1021/bi00806a045. [DOI] [PubMed] [Google Scholar]
- Caserta G., Cervigni T. Piezoelectric theory of enzymic catalysis as inferred from the electromechanochemical principles of bioenergetics. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4421–4424. doi: 10.1073/pnas.71.11.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovich S., Somogyi B. Letter: A molecular enzyme model based on oriented energy transfer. J Theor Biol. 1973 Oct;41(3):567–569. doi: 10.1016/0022-5193(73)90063-5. [DOI] [PubMed] [Google Scholar]
- George P., Witonsky R. J., Trachtman M., Wu C., Dorwart W., Richman L., Richman W., Shurayh F., Lentz B. "Squiggle-H2O". An enquiry into the importance of solvation effects in phosphate ester and anhydride reactions. Biochim Biophys Acta. 1970 Nov 3;223(1):1–15. doi: 10.1016/0005-2728(70)90126-x. [DOI] [PubMed] [Google Scholar]
- HAMMES G. G. MECHANISM OF ENZYME CATALYSIS. Nature. 1964 Oct 24;204:342–343. doi: 10.1038/204342a0. [DOI] [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Klotz I. M. Comparison of molecular structures of proteins: helix content; distribution of apolar residues. Arch Biochem Biophys. 1970 Jun;138(2):704–706. doi: 10.1016/0003-9861(70)90401-7. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Neet K. E. The catalytic and regulatory properties of enzymes. Annu Rev Biochem. 1968;37:359–410. doi: 10.1146/annurev.bi.37.070168.002043. [DOI] [PubMed] [Google Scholar]
- Low P. S., Somero G. N. Activation volumes in enzymic catalysis: their sources and modification by low-molecular-weight solutes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3014–3018. doi: 10.1073/pnas.72.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumry R. Conformational mechanisms for free energy transduction in protein systems: old ideas and new facts. Ann N Y Acad Sci. 1974 Feb 18;227:46–73. doi: 10.1111/j.1749-6632.1974.tb14373.x. [DOI] [PubMed] [Google Scholar]
- Neville M. C., Ling G. N. Synergistic activation of beta-galactosidase by Na and Cs. Arch Biochem Biophys. 1967 Mar 20;118(3):596–610. doi: 10.1016/0003-9861(67)90395-5. [DOI] [PubMed] [Google Scholar]
- Nowak T. Monomethylammonium ion as a magnetic resonance probe for monovalent cation activators. The monovalent cation in pyruvate kinase catalysis. J Biol Chem. 1973 Oct 25;248(20):7191–7196. [PubMed] [Google Scholar]
- STEVENS C. L., LAUFFER M. A. POLYMERIZATION-DEPOLYMERIZATION OF TOBACCO MOSAIC VIRUS PROTEIN. IV. THE ROLE OF WATER. Biochemistry. 1965 Jan;4:31–37. doi: 10.1021/bi00877a007. [DOI] [PubMed] [Google Scholar]
- Storm D. R., Koshland D. E. A source for the special catalytic power of enzymes: orbital steering. Proc Natl Acad Sci U S A. 1970 Jun;66(2):445–452. doi: 10.1073/pnas.66.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suelter C. H. Enzymes activated by monovalent cations. Science. 1970 May 15;168(3933):789–795. doi: 10.1126/science.168.3933.789. [DOI] [PubMed] [Google Scholar]
- Swift T. J., Fritz O. G., Jr A proton spin-echo study of the state of water in frog nerves. Biophys J. 1969 Jan;9(1):54–59. doi: 10.1016/S0006-3495(69)86368-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Györgyi A. The mechanism of muscle contraction. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3343–3344. doi: 10.1073/pnas.71.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. C., Stowring L., Morales M. F. The effect of structure-disrupting ions on the activity of myosin and other enzymes. J Biol Chem. 1966 Jan 25;241(2):309–316. [PubMed] [Google Scholar]
- White J. P., Kuntz I. D., Cantor C. R. Studies on the hydration of Escherichia coli ribosomes by nuclear magnetic resonance. J Mol Biol. 1972 Mar 14;64(2):511–514. doi: 10.1016/0022-2836(72)90514-1. [DOI] [PubMed] [Google Scholar]
- Zipp A., Kauzmann W. Pressure denaturation of metmyoglobin. Biochemistry. 1973 Oct 9;12(21):4217–4228. doi: 10.1021/bi00745a028. [DOI] [PubMed] [Google Scholar]



