Abstract
Preterm delivery (PTD) is one of the most significant contributors to neonatal mortality, morbidity, and long-term adverse consequences for health; with highest prevalence reported from India. The incidence of PTD is alarmingly very high in Northeast India. The objective of the present study is to evaluate the associative role of MTHFR gene polymorphism and progesterone receptor (PR) gene mutation (PROGINS) in susceptibility to PTD, negative pregnancy outcome and low birth weights (LBW) in Northeast Indian population.
Methods
A total of 209 PTD cases {extreme preterm (< 28 weeks of gestation, n = 22), very preterm (28–32 weeks of gestation, n = 43) and moderate preterm (32–37 weeks of gestation, n = 144) and 194 term delivery cases were studied for MTHFR C677T polymorphism and PR (PROGINS) gene mutation. Statistical analysis was performed using SPSS software.
Results
Distribution of MTHFR and PR mutation was higher in PTD cases. Presence of MTHFR C677T polymorphism was significantly associated and resulted in the increased risk of PTD (p < 0.001), negative pregnancy outcome (p < 0.001) and LBW (p = 0.001); more significantly in extreme and very preterm cases. Presence of PR mutation (PROGINS) also resulted in increased risk of PTD and negative pregnancy outcome; but importantly was found to increase the risk of LBW significantly in case of very preterm (p < 0.001) and moderately preterm (p < 0.001) delivery cases.
Conclusions
Both MTHFR C677T polymorphism and PR (PROGINS) mutation are evident genetic risk factors associated with the susceptibility of PTD, negative pregnancy outcome and LBW. MTHFR C677T may be used as a prognostic marker to stratify subpopulation of pregnancy cases predisposed to PTD; thereby controlling the risks associated with PTD.
Keywords: Preterm delivery, Negative pregnancy outcome, Low birth weight, MTHFR C677T polymorphism, PR (PROGINS) mutation, Northeast India
Highlights
-
•
This is the first study involving the analysis of genetic risk factors associated with preterm delivery in Northeast India.
-
•
MTHFR C677T polymorphism and PR (PROGINS) mutation in predisposition to preterm delivery, negative pregnancy outcome and low birth weight.
-
•
MTHFR C677T polymorphism may be used as a prognostic marker to stratify subpopulation of pregnancy cases predisposed to PTD; thereby controlling the risks associated with PTD.
Introduction
The period of gestation is one of the most important predictors of an infant's subsequent health and survival. The phenomenon of pregnancy can be compromised by a number of complications, such as threatened abortion, recurrent spontaneous miscarriage, preeclampsia, and preterm delivery ((Dekker et al., 1995, Lawn et al., 2006). Preterm delivery is defined as delivery occurring before 37 weeks of gestation, and is a major public health problem throughout the world (Beck et al., 2010 and Challis, 2000). It is one of the most significant contributors to neonatal mortality and morbidity (Norwitz and Robinson, 2001), with long-term adverse consequences for health, and cognitive outcome as well as financial implications for health care (Beck et al., 2010, Gissler et al., 1999, Wong and Edwards, in press). In almost all high and middle income countries of the world, preterm birth is the leading cause of child death (Liu et al., 2012). Based on gestation, preterm birth is subdivided into three different cohort as such, extremely preterm (< 28 weeks of gestation), very preterm (28 to < 32 weeks of gestation) and moderate or late preterm (32 to < 37 completed weeks of gestation) (Blencowe et al., 2013, Raisanen et al., 2013). Most premature babies (almost 80–85%) are born between 32 and 37 weeks of gestation (moderate/late preterm); whereas about 10% and 5% of preterm babies are born between 28 and < 32 weeks, and < 28 weeks of gestation respectively, and result in high number of fetal mortality cases, especially in low-income countries (Blencowe et al., 2012). At present, the global incidence of preterm birth is at an alarming rate (Blencowe et al., 2012). India occupies the topmost position in the prevalence of highest number of preterm birth cases, which is one of the leading causes of neonatal morbidity and mortality (Blencowe et al., 2012). Moreover, it is of serious concern that the North-eastern region of India is a zone with very high number of cases of preterm delivery (Gogoi and Prusty, 2013). Unfortunately, no scientific studies have been undertaken till date to elucidate the underlying molecular mechanism(s) or genetic predisposition associated with preterm delivery and related complications from this area which has a ethnically distinct population compared to other parts of Indian subcontinent, and is mostly tribal dominant.
The etiology of preterm delivery is unclear. It is thought to be a multifactorial, complex disorder with the involvement of physio-pathological, genetic and environmental factors (Beck et al., 2010, Goldenberg et al., 2008). There are several pathways which play a pivotal role for the maintenance of a successful pregnancy. The two most critical pathways associated with successful pregnancy are folate pathway and progesterone pathway. So any defect in these key pathways leads to the complication in pregnancy. Folate is a generic term for members of the vitamin B9 family, which plays a key role in cellular biosynthetic processes including cell division and cell growth, DNA synthesis and methylation (Solanky et al., 2010). Various pregnancy complications have been associated with folate deficiency, and folate dependent reactions have been shown to be essential for fetal growth and development, and for the maternal well-being (Van der Molen et al., 2000). 5-Methyl-enetetrahydrofolate reductase (MTHFR) is a key enzyme involved in folate metabolism which catalyses the conversion of 5,10-methylenetetrahydrofolate to 5-methylenetet-rahydrofolate, the circulatory form of folate. The common polymorphisms of the MTHFR gene which results in cytosine to thymine substitution at position 677 (677C → T) is responsible for the loss of enzyme activity (50–70% loss of activity in homozygotes and 30% loss in heterozygotes) which leads to a decreased pool of methyl-THF and hyperhomocysteinemia, particularly in folate deficiency (Frosst et al., 1995), which may influence common pregnancy outcomes (James, 1997) and can increase the risk of spontaneous abortion, preterm delivery, or intrauterine growth restriction (Committee on Nutritional Status During Pregnancy and Lactation, 1990). Another important pathway associate with the pregnancy is the progesterone pathway. Progesterone is essential in establishing and maintaining pregnancy (Karalis et al., 1996). Progesterone receptor (PR) mediates the physiologic effects of progesterone (Mesiano, 2004, Smith et al., 2002). Three linked single nucleotide polymorphisms (SNPs) (exon 1: G 1031 C; S344T, exon 4: G 1978T; L660V and exon 5: C 2310T; H770H) in the PR gene were found to be associated with Recurrent Spontaneous Abortion (Schweikert et al., 2004). The SNP in the exon 1 is reported to be apparently linked to the SNPs in exons 4 and 5 (Schweikert et al., 2004) which are in turn in linkage disequilibrium with PROGINS, a 306 base pairs (bp) insertion of PV/HS-1 Alu subfamily in intron G, between exons 7 and 8 in the codifying region of the hormone binding domain (Kieback et al., 1998 and Schweikert et al., 2004). Polymorphic variants of PR gene also have been implicated in implantation failure (Pisarska et al., 2003).
Considering the high rate of preterm delivery in northeast India, the lacunae regarding the availability of scientific data pertaining to preterm delivery and related complications, and available literature, the present study was undertaken with the aim to study the associative role of MTHFR gene polymorphism and progesterone receptor (PR) mutation (PROGINS) in susceptibility to preterm delivery, negative pregnancy outcome and baby birth weights.
Materials and methods
Patient enrolment and stratification
For the present study, pregnancy cases undergoing delivery (N = 403) were enrolled with all clinical details, from the Department of Obstetrics and Gynaecology of Gauhati Medical College and Hospital, and Down Town fertility clinic and IVF center, Down Town Hospital, under the supervision of a registered medical practitioner with informed consent. The cases were further stratified as term delivery (n = 194) or preterm delivery cases (delivery before 37 weeks of gestation) (n = 209) based on weeks of gestation. The study was approved by the Institutional Ethics Committee of Gauhati University. Pregnant females who were under 18 years of age and above 45 years of age, or cases having jaundice, tuberculosis, HPV infection, patients with urinary tract infection (UTI) were excluded from the study. Whole blood (2 ml) was collected from each patient with informed consent. Based on available literature (Blencowe et al., 2013, Raisanen et al., 2013) the 209 preterm delivery cases were further stratified into three cohorts in accordance to the gestation period as: extremely preterm (< 28 weeks, n = 22), very preterm (28 to < 32 weeks, n = 43) and moderately or late preterm (32 to < 37, n = 144). The genomic DNA was extracted from the collected whole blood using the standard phenol chloroform method.
PCR-RFLP analysis of MTHFR C677T polymorphism
The method described by Frosst et al. was used for detection of 677C → T polymorphism. Briefly, a length of 198 bp in exon 4 of MTHFR gene was amplified using the primers 5′ TGAAGGAGAAGGTGTCTGCGGGA3′ and 5′AGGACGGTGCGGTGAGAGTG3′, followed by restriction digestion using the HinfI enzyme. A single band of 198 bp characterized wild-type C allele for codon 677, while the presence of three bands at 198, 175 and 23 bp or 175 and 23 bp only characterized heterozygous and homozygous (characterizing presence of T allele for codon 677) variant status respectively. The genotyping analysis was performed by Diptika Tiwari. To improve the genotyping quality and validation, 20% of samples were re-genotyped by other laboratory personnel (Somdatta Das) blinded to the term-preterm delivery status of the subjects, and results were reproducible with no discrepancy in genotyping.
Mutation analysis of progesterone receptor (PR) gene or PROGINS detection
PROGINS, a haplotype of progesterone receptor consisting of 306-bp insertion in intron G together with point mutations in exon 4 and 5 is associated with reduced amounts of gene transcript and lesser response to progesterone. Since these three mutations always occur together and are in complete linkage disequilibrium, detection of 306 bp insertion in intron G is used to identify PROGINS. PCR was done using the primers designed by Agoulnik et al. (2004) {F:5′GCCTCTAAAATGAAAGGCAGAAAGC3′ and R:5′GTATTTTCTTGCTAA ATGTCTC3′}, to amplify intron G of PR gene. Mutation in intron G was detected by the presence of 476 bp fragments while the presence of only 170 bp fragments represented wild type allele. After completion of genotyping analysis by Diptika Tiwari, 20% of samples were randomly re-genotyped by Dr. PD Bose to validate the results (blinded to the case status of the subjects), and results were reproducible with no discrepancy in genotyping.
Statistical analysis
All statistical analyses were performed by the standard methods using SPSS computer software (Version 13, SPSS Inc., Chicago, IL, USA). Results were expressed as means ± standard deviations (SD). The χ2 goodness of fit test was used for any deviation from Hardy–Weinberg equilibrium. The significance was described as Pearson p-value. Associations between MTHFR polymorphism, PR mutation and preterm delivery and negative pregnancy outcome risk were calculated as odds ratio (OR) with 95% confidence intervals (CIs); a two tailed p-value less than 0.05 was considered statistically significant.
Results
Demographical profile of the enrolled cases
For the present prospective study we enrolled patients undergoing delivery from the Obstetrics and Gynaecology Department of GMCH under the supervision of a registered medical practitioner with all clinical data and informed consent. Patients were stratified on the basis of gestational period as term (n = 194) and preterm (n = 209); preterm cases were further classified into three cohorts viz.; extremely preterm (< 28 weeks, n = 22), very preterm (28 to < 32 weeks, n = 43) and moderately or late preterm (32 to < 37 weeks, n = 144). The cases were enrolled with all clinical details of the mother and the baby weight at birth (Table 1). The baby birth weight was significantly lower in all the preterm delivery case groups compared to the term delivery cases (p < 0.001); as well as in extremely preterm cases compared to very (p < 0.001) and moderately preterm delivery cases (p < 0.001). The baby birth weight was also significantly lower to very preterm cases compared to the moderately preterm cases (p < 0.001).
Table 1.
Demographical profile of cohorts.
| Cases | Maternal age | Parity |
Birth weight |
||
|---|---|---|---|---|---|
| Median | Range | Alive | Death | ||
| Term | 24.180 ± 4.126 | 0 | 0–3 | 2.823 ± 0.4520 | N/Aa |
| Extremely preterm | 22.545 ± 3.180 | 0 | 0–2 | 1.107 ± 0.3889 | 0.8458 ± 0.517 |
| Very preterm | 25.139 ± 4.427 | 1 | 0–3 | 1.542 ± 0.4094 | 1.4 ± 0.707 |
| Moderately preterm | 24.853 ± 4.416 | 0 | 0–4 | 1.953 ± 0.4820 | 1.813 ± 0.571 |
No cases of mortality were reported in term cases.
Distribution of MTHFR polymorphism and PR mutation and its association with preterm delivery
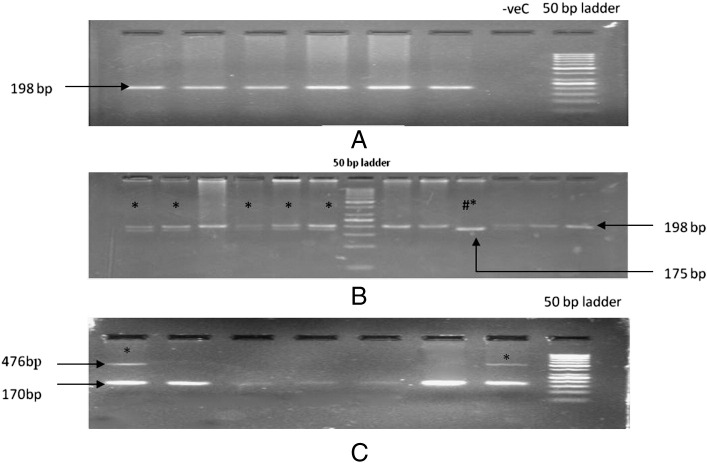
The MTHFR C677T polymorphism is important with regards to the enzyme activity of the gene product, and was screened by PCR-RFLP method using HinfI restriction digestion. The MTHFR PCR amplification showed a presence of 198 bp band (Fig. 1A), which then generated fragments of single uncut band of 198 bp (wild-type), three bands of 198 + 175 + 23 bp (heterozygote) or two bands of 175 + 23 bp (homozygote) (Fig. 1B) as a RFLP product after restriction digestion. PROGINS mutation is congenital. PR mutation is characterized by the presence 306 bp of ALU insertion. Therefore, allele specific PCR was employed to study the presence of any mutation in this gene. The wild type genotype shows a band sized 170 bp, whereas the mutated form shows bands at 476 bp due to the presence of the 306 bp ALU insertion (Fig. 1C).
Fig. 1.
A: Representative photograph of agarose gel electrophoresis for PCR amplification of MTHFR gene fragment. B: Representative photograph of agarose gel electrophoresis of the restriction digestion products of MTHFR gene showing the presence of either wild type or heterozygous (characterized by presence of 198 + 175 + 23 bp and marked by *) or homozygous condition (characterized by the presence of 175 + 23 bp marked by #*) for term and preterm cases. C: Representative photograph of agarose gel electrophoresis showing allele specific PCR amplification of PR gene showing the presence of either wild type (characterized by 170 bp) or heterozygous (characterized by presence of 170 + 306 bp and marked by *) for preterm cases.
The distribution of MTHFR polymorphism was significantly higher (p < 0.001) in preterm delivery cases (29.18%) compared to term delivery cases (12.37%). The results didn't show any deviation from Hardy–Weinberg equilibrium. The presence of MTHFR polymorphism was found to significantly increase the risk of preterm pregnancy by more than two folds {OR = 2.872 (1.704–4.841) at 95%CI, p < 0.001}. Although, the distribution of PR mutation was higher in preterm delivery cases (5.26%) compared to term delivery cases (2.06%), it didn't result in significant increase (p = 0.088) in the risk of preterm delivery compared to controls {OR = 2.652 (0.830–8.474) at 95%CI, p = 0.115} (Table 2).
Table 2.
MTHFR and PR genotype distribution between term and preterm.
| Cases | N |
MTHFR genotype |
MTHFR variant allele | P value | Odds ratio | ||
|---|---|---|---|---|---|---|---|
| Wildtype | Heterozygote | Homozygote | |||||
| Term | 194 | 170 [87.62] | 20 [10.30] | 4 [2.06] | 24 [12.37] | Ref | 2.872 {1.704–4.841} |
| Preterm | 209 | 148 [70.81] | 49 [23.44] | 12 [5.74] | 61 [29.18] | < 0.001⁎ | |
| Cases | N | PR mutation (PROGINS) | PROGIN variant allele | P value | Odd ratio | ||
| Wildtype | Heterozygote | Homozygote | |||||
| Term | 194 | 190 [97.93] | 4 [2.06] | 0 [0.00] | 4 [2.06] | Ref | 2.652 {0.830–8.474} |
| Preterm | 209 | 198 [94.73] | 11 [5.26] | 0 [0.00] | 11 [5.26] | 0.115 | |
Cases defined as number [%age].
Statistically significant.
Risk factor analysis for preterm delivery between term and different stratified cohorts of preterm based on MTHFR polymorphism
When distribution of MTHFR polymorphism in term delivery cases was analyzed against three different cohorts of preterm cases viz., extremely, very and moderately preterm delivery groups; it was found that the presence of MTHFR polymorphism is significantly associated with extremely (p < 0.001), very (p = 0.001) as well as in moderately preterm delivery (p = 0.002). Moreover, the presence of MTHFR polymorphism significantly increased the risk of extremely {OR = 5.903 (2.302–15.138) at 95% CI, p < 0.001}, very {OR = 3.420 (1.587–7.369) at 95% CI, p = 0.002} and moderately preterm delivery {OR = 2.449 (1.388–4.322) at 95% CI, p = 0.002} compared to term delivery cases (Table 3).
Table 3.
MTHFR genotype distribution between different stratified cohorts.
| Cases | N |
MTHFR genotype |
Less common allele | P value | Odds ratio | ||
|---|---|---|---|---|---|---|---|
| Wildtype | Heterozygote | Homozygote | |||||
| Term | 194 | 170 [87.62] | 20 [10.30] | 4 [2.06] | 12.37 | Ref | 5.903 {2.302–15.138} |
| Extremely preterm | 22 | 12 [54.54] | 10 [45.45] | 0 [0.00] | 45.45 | < 0.001⁎ | |
| Term | 194 | 170 [87.62] | 20 [10.30] | 4 [2.06] | 12.37 | Ref | 3.420 {1.587–7.369} |
| Very preterm | 43 | 29 [67.44] | 11 [25.58] | 3 [6.97] | 32.55 | .002⁎ | |
| Term | 194 | 170 [87.62] | 20 [10.30] | 4 [2.06] | 12.37 | Ref | 2.449 {1.388–4.322} |
| Moderately preterm | 144 | 107 [74.30] | 28 [19.44] | 9 [6.25] | 25.69 | .002⁎ | |
Cases defined as number [%age]].
Statistically significant.
When the distribution of MTHFR polymorphism was compared between the extremely, very and moderately preterm cohorts, the difference in distribution was found to be statistically non-significant (p = NS); but importantly, the presence of MTHFR polymorphism increased the risk of extremely preterm delivery compared to very {OR = 1.726 (0.602–4.593) at 95% CI, p = 0.416} and moderately preterm delivery {OR = 2.410 (0.962–6.039) at 95% CI, p = 0.074}, whereas there was no prominent fold change in the increased risk between very and moderately preterm delivery groups {1.396 {.667–2.924} at 95% CI, p = 0.436} (Table 3).
Risk factor analysis for preterm delivery between term and different stratified cohorts of preterm based on PR (PROGIN) mutation
When distribution of PR mutation (PROGINS) was analyzed between term delivery cases against three different cohorts of preterm cases viz., extremely, very and moderately preterm delivery groups; it was found that the presence of PR mutation was not significantly associated with extremely (p = 0.058), very (p = 0.329) or moderately preterm delivery (p = 0.152). But importantly, the presence of PR mutation increased the risk of extremely {OR = 4.750 (0.818–27.576) at 95% CI, p = 0.116}, very {OR = 2.317 (0.411–13.078) at 95% CI, p = 0.299} and in moderately preterm delivery {OR = 2.427 (0.697–8.454) at 95% CI, p = 0.215} compared to term delivery (Table 4).
Table 4.
PR mutation distribution between different stratified cohorts.
| Cases | N |
PR genotype |
Less common allele | P value | Odds ratio | ||
|---|---|---|---|---|---|---|---|
| Wild-type | Heteroz-ygote | Homozygote | |||||
| Term | 194 | 190 [97.93] | 4 [2.06] | 0 [0.00] | 2.06 | Ref | 4.750 {.818–27.576} |
| Extremely preterm | 22 | 20 [90.90] | 2 [9.09] | 0 [0.00] | 9.09 | .116 | |
| Term | 194 | 190 [97.93] | 4 [2.06] | 0 [0.00] | 2.06 | Ref | 2.317 {.411–13.078} |
| Very preterm | 43 | 41 [95.34] | 2 [4.65] | 0 [0.00] | 4.65 | .299 | |
| Term | 194 | 190 [97.93] | 4 [2.06] | 0 [0.00] | 2.06 | Ref | 2.427 {.697–8.454} |
| Moderately preterm | 144 | 137 [95.13] | 7 [4.86] | 0 [0.00] | 4.86 | .215 | |
Cases defined as number [%age].
When the distribution of PR mutation was analyzed between the different stratified preterm cohorts, it was found that there was no significant difference in distribution of PR mutations between extremely and moderately preterm (p = 0.416), extremely and very preterm (p = 0.484) and very and moderately preterm delivery cases (p = 0.955). Although there was no significant correlation between the preterm groups, the presence of PR variant genotype was associated with increase in the risk of extremely preterm delivery compared to very {OR = 2.050 (0.269–15.633) at 95% CI, p = 0.599} and moderately preterm delivery {OR = 1.957 (0.380–10.089) at 95% CI, p = 0.340}; whereas there was no prominent fold change in the risk of very preterm delivery compared to moderately preterm delivery (p = NS) (Table 4).
Association of MTHFR polymorphism and PR mutation with pregnancy outcome
The distribution of MTHFR polymorphism and PR mutation was also studied in detail with respect to pregnancy outcome (Table 5). When all the term and preterm cases were considered, Wilcoxon signed rank test based correlation analysis showed that negative pregnancy outcome (IUD/fetal death) significantly correlated with MTHFR (p < 0.001) and PR (p = 0.005) variant genotype. The presence of MTHFR polymorphism significantly increased the risk of negative pregnancy outcome by more than three folds {OR = 3.421(1.656–7.069) at 95%CI, p = 0.001}; whereas presence of mutant PR genotype increased the risk of negative pregnancy non-significantly by 1.7 folds {OR = 1.707(0.369–7.898) at 95%CI, p = 0.367}. Within the preterm group, only MTHFR polymorphism (p = 0.032) but not PR mutation (p = 0.866) was found to significantly increase the risk of negative pregnancy outcome {OR = 2.263 (1.061–4.826) at 95%CI, p = 0.039}.
Table 5.
Distribution of MTHFR polymorphism and PR mutation in different cohorts based on pregnancy outcome.
| (Outcome with MTHFR variant) | |||||||
|---|---|---|---|---|---|---|---|
|
MTHFR variant |
Wild (n = 148) |
Heterozygotes (n = 49) |
Homozygotes (n = 12) |
||||
| Baby outcome | N | Alive | Dead | Alive | Dead | Alive | Dead |
| Term | 194 | 170 [87.62] | 0[0.00] | 20 [10.30] | 0[0.00] | 4 [2.06] | 0[0.00] |
| Extremely preterm | 22 | 8[36.36] | 4[18.18] | 2[9.09] | 8[36.36] | 0[0.00] | 0[0.00] |
| Very preterm | 43 | 22[51.16] | 7[16.27] | 7[16.27] | 4[9.30] | 3[6.97] | 0[0.00] |
| Moderately preterm | 144 | 99[68.75] | 8[5.55] | 26[18.05] | 2[1.38] | 8[5.55] | 1[0.69] |
| (Outcome with PR variant) | |||||||
| PR variant | Wild (n = 198) |
Heterozygotes (n = 11) |
Homozygotes (n = 0) |
||||
| Baby outcome | N | Alive | Dead | Alive | Dead | Alive | Dead |
| Term | 194 | 190[97.93] | 0[0.00] | 4[2.06] | 0[0.00] | 0[0.00] | 0[0.00] |
| Extremely preterm | 22 | 8 [36.36] | 12[54.54] | 2[9.09] | 0[0.00] | 0[0.00] | 0[0.00] |
| Very preterm | 43 | 30 [69.76] | 11 [25.58] | 2[4.65] | 0[0.00] | 0[0.00] | 0[0.00] |
| Moderately preterm | 144 | 128 [88.88] | 9 [6.25] | 5[3.47] | 2[1.38] | 0[0.00] | 0[0.00] |
Significance of MTHFR polymorphism and PR mutation in deciding the outcome in preterm cases
Within the extremely (< 28 weeks) preterm group
In the extremely preterm group, the presence of MTHFR polymorphism was not significantly associated with negative pregnancy outcome (p = 0.069), but it significantly resulted in an eight fold increase in the risk of negative pregnancy outcome {OR = 8.000 (1.127–56.793) at 95%CI, p = 0.043}. Comparatively, the presence of PR mutation was not found to be associated with negative pregnancy outcome (p = 0.456).
Within the very (28 to < 32 weeks) preterm group
In the very preterm group, the MTHFR polymorphism (p = 0.758) or presence of PR mutation (p = 0.401) distribution was not found to be statistically significantly associated with negative pregnancy outcome. The presence of MTHFR polymorphism {OR = 1.257 (0.298–5.296) at 95%CI, p = 1.000} or PR mutation also didn't result in a significant fold increase in the risk of negative pregnancy outcome.
Within the moderately (32 to < 37 weeks) preterm group
Within the moderately preterm group, the presence of MTHFR polymorphism was not associated (p = 0.901) with any significant increase in negative pregnancy outcome {OR = 1.092 (0.274–4.353) at 95%CI, p = 1.000}. When the importance of the presence of PR mutation was analyzed with respect to pregnancy outcome, it was found that the presence of PR mutation was significantly associated with negative pregnancy outcome in the moderately preterm group (p = 0.033). Moreover, the presence of PR mutation resulted in increased risk of negative pregnancy outcome in this group {OR = 5.689 (0.965–33.521) at 95%CI, p = 0.090}.
Association of MTHFR polymorphism and PR mutation with baby birth weight
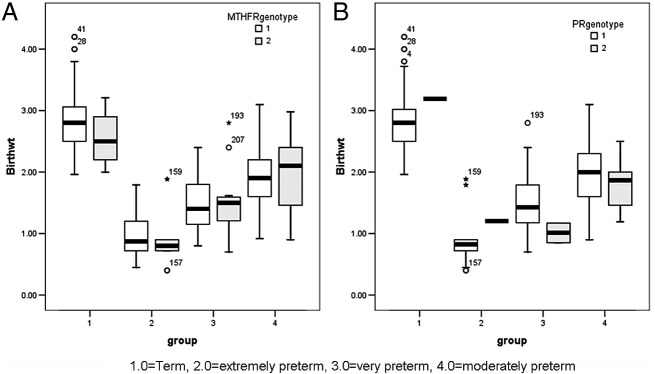
When all the term and preterm cases was considered, results showed that MTHFR polymorphism (p = 0.001) and PR mutation (p = 0.035) were significantly associated with lower baby birth weight. The distribution of MTHFR polymorphism and PR mutation was also studied in detail with respect to low birth weight in all the pregnancy cases. The MTHFR polymorphism was found to be significantly associated with low birth weight in term delivery cases (p = 0.007) but not in any of the preterm delivery groups (p = NS). When all the term and preterm cases were considered, the presence of PR mutation was not found to have any significant association with low birth weight in either term or preterm delivery cases (Fig. 2A,B; Table 6).
Fig. 2.
[A]: Box plot analysis showing significant association of MTHFR polymorphism with low birth weight in term (p < 0.001) and extremely preterm (p = 0.006) delivery cases. [B]: Box plot analysis showing significant association of PR mutation with low birth weight in very preterm (p < 0.001) and moderately preterm (p < 0.001) delivery cases.
Table 6.
Difference in baby birth weight based on presence of MTHFR polymorphism and PR mutation in different cohorts.
| Cases |
MTHFR |
P value |
PR |
P value | ||
|---|---|---|---|---|---|---|
| Wild | Mutant | Wild | Mutant | |||
| Term | 2.866 ± 0.4496 | 2.563 ± 0.3803 | 0.007 | 2.818 ± 0.4530 | 3.19 ± 0.002 | 0.143 |
| Preterm | 1.759 ± 0.5513 | 1.712 ± 0.686 | 0.722 | 1.7623 ± 0.5869 | 1.535 ± 0.5084 | 0.166 |
Further within all the groups Wilcoxon signed rank test based correlation analysis showed that the presence of MTHFR polymorphism significantly increases the risk of low birth weight in pregnancy, in case of term (p < 0.001) and extremely (p = 0.006) preterm delivery cases, whereas the presence of PR mutation was found to increase the risk of low birth weight significantly in case of very preterm (p < 0.001) and moderately preterm (p < 0.001) delivery cases (Fig. 2B).
Discussion
Preterm birth is defined as birth occurring before 37 weeks of gestation and is one of the most significant contributors to neonatal mortality and morbidity, with long-term adverse consequences for health (Beck et al., 2010, Gissler et al., 1999, Wong and Edwards, in press). Highest global prevalence of preterm delivery has been reported from India [WHO report, November 2013, (Blencowe et al., 2012). Anthropological studies suggest that Northeast India also depicts an alarming picture of preterm delivery resulting in neonatal mortality and morbidity (Gogoi and Prusty, 2013); but till date, no scientific study has been undertaken to elucidate the underlying the molecular risk factors associated with the susceptibility to preterm delivery in the NE Indian population, which is ethnically distinct from the other parts of India and its majorly mostly tribal dominated. Well-established observations support a genetic influence on the risk of preterm delivery and premature birth. Since folate and progesterone receptor pathway and the associated genes have been shown to influence the maintenance and outcome of pregnancy (Bose et al., 2011, Solanky et al., 2010); therefore we have undertaken this study to delineate the associative role of MTHFR polymorphism and PR gene mutation in predisposition to preterm delivery in Northeast India. For the present study, pregnancy cases were stratified based to gestation period as term delivery cases (> 37 weeks), extremely preterm (< 28 weeks), very preterm (28 to < 32 weeks) and moderate or late preterm cases (32 to < 37 completed weeks of gestation) (Blencowe et al., 2013, Raisanen et al., 2013). The majority of the enrolled preterm cases belonged to the moderate or late preterm cohort (68.90%), while the lowest number of preterm cases belonged to the extremely preterm cohort (10.53%). Similar observations of distribution of preterm cases have been reported by Blencowe et al. (2012), whereby it has been reported that most premature babies (> 80%) are born between 32 and 37 weeks of gestation (moderately preterm), about 10% of preterm babies are born between 28 and < 32 week and about 5% of premature babies are born before 28 wk of gestation. (Blencowe et al., 2012).
In this study, we first evaluated the alteration in MTHFR gene and its association in pre-disposition to preterm delivery. Low folate intake and unfavorable MTHFR genotype have been proved to contribute to chronic decidual vasculopathy and ultimately preterm birth (Kramer et al., 2001). Multiple variants of the MTHFR gene are known, with the most studied being polymorphism C677T, which produces a thermolabile enzyme associated with hyperhomocysteinemia (Frosst et al., 1995) in relation to multiple pathological entities, including obstetric complications and preterm delivery (Alfirevic et al., 2002, Lauszus et al., 2001, Scholl and Johnson, 2000). Similar to these reported observations, the data from our study also showed that the distribution of MTHFR variant genotype was significantly higher in preterm delivery cases compared to term delivery cases (p < 0.001). Within three different cohorts the distribution of MTHFR variant genotype was significantly associated with extremely (p < 0.001), very (p = 0.001) as well as moderately preterm delivery (p = 0.002). Moreover, the presence of MTHFR variant genotype significantly increased the risk of extremely {OR = 5.903, p < 0.001}, very {OR = 3.420, p = 0.002} and moderately preterm delivery {OR = 2.449, p = 0.002} compared to term delivery cases; extremely preterm delivery compared to very {OR = 1.726, p = 0.416} and moderately preterm delivery {OR = 2.410, p = 0.074}. Our data is contrary to the reports published by Resch et al. (2004) who documented that there is no distinct association between methylenetetrahydrofolate reductase C677T mutations and preterm birth Caucasian women, but correlates with the study of Valdez et al. (2004) which showed a significant increase in the frequency of polymorphisms for MTHFR C677T and among Mexican women who had a history of preterm delivery compared with controls.
Next, when MTHFR polymorphism was studied with respect to the negative pregnancy outcome (IUD/fetal death) between term and preterm cases, it was observed that in preterm cases the presence of MTHFR polymorphism, was significantly associated with fetal death (p < 0.001) and also it increased the risk of negative pregnancy outcome by more than three folds {OR = 3.421, p = 0.001}. Also within the preterm group it was found that MTHFR polymorphism (p = 0.032) significantly increases the risk of negative pregnancy outcome {OR = 2.263 (1.061–4.826) at 95%CI, p = 0.039}. Analysis of the role of MTHFR polymorphism in negative pregnancy outcome within three different cohorts: extremely, very and moderately preterm showed that, the presence of MTHFR polymorphism was associated with negative pregnancy outcome in the case of extremely preterm delivery (p = 0.069) and resulted in a significant increase in the risk of negative pregnancy outcome {OR = 8.000, p = 0.043}; whereas in very (p = 0.758) and moderately (p = 0.901) preterm group, the MTHFR polymorphism was not found to be statistically significantly associated with negative pregnancy outcome. Therefore, our results indicate the associative role of MTHFR polymorphism in negative pregnancy outcome in preterm delivery cases, and is similar to reports published by Isotalo et al. (2000), who have also reported that existence of MTHFR polymorphism has a potential role in compromised fetal viability.
Further, we studied the association of MTHFR polymorphism with low birth weight of a baby within term and all the three cohorts of preterm cases. Our study showed that the MTHFR polymorphism was associated with low birth weight significantly between all the groups significantly (p < 0.001). Also based on Wilcoxon signed rank test it was found that MTHFR polymorphism was significantly associated with low birth weight in term (p < 0.001) and extremely preterm cases (p = 0.006). The results showing association of MTHFR polymorphism and low birth weight correlate with studies from other groups (Sukla et al., 2013) showing that the presence of MTHFR 677T polymorphism is a potential risk factor for low birth weight (LBW) amongst which 16% were delivered prematurely in North Indian population.
Another important pathway associated with pregnancy is progesterone receptor pathway. Progesterone is essential in establishing and maintaining pregnancy by stimulating and regulating various functions like implantation of the embryo, promoting uterine growth and suppressing myometrical contractility (Clarke and Sutherland, 1990, Graham and Clarke, 1997, Pepe and Albrecht, 1995) and also acting as an anti-inflammatory agent and regulator of immune response (Szekeres-Bartho et al., 2001). Progesterone receptor (PR) mediates the physiologic effects of progesterone. Several mutations have been described for the PR gene, among which one stands out: a mutation named PROGINS, which finally results in reduction in final activity mediated by progesterone (Gomes et al., 2006). We evaluated the PROGINS mutation using allele-specific PCR and correlated these results with preterm delivery, pregnancy outcome and baby birth weight. Our result depicts that the distribution of the PR mutation was higher in preterm delivery cases (5.26%) compared to term delivery cases (2.06%), but it didn't result in a significant increase (p = 0.088) in the risk of preterm delivery compared to controls {OR = 2.652, p = 0.115}. When distribution of PR mutation (PROGINS) was analyzed between term delivery cases against three different cohort of preterm cases viz., extremely, very and moderately preterm delivery groups; it was found that the presence of PR variant genotype was not significantly associated with extremely, very or moderately preterm delivery (p = NS). But importantly, the presence of PR mutation increased the risk of extremely {OR = 4.750, p = 0.116}, very {OR = 2.317, p = 0.299} and in moderately preterm delivery {OR = 2.427, p = 0.215} compared to term delivery. Our results show that PR mutation (PROGINS) is not significantly associated with preterm delivery risk, and is similar to reports from other research groups (Diaz-Cueto et al., 2008, Oliveira et al., 2011). Our data on PROGINS is contrary to the reports published by Ehn et al. (2007) who documented that genetic variation in the PGR gene of either the mother or the fetus may trigger preterm labour.
Next, when the presence of PR mutation was studied for association with change in the risk of negative pregnancy outcome (IUD/fetal death), we found that PR mutation was non-significantly associated with the increase in negative pregnancy outcome {OR = 1.707, p = 0.367}. Importantly, the presence of PR mutation was significantly associated with negative pregnancy outcome in the moderately preterm group (p = 0.033) and resulted in increased risk of negative pregnancy outcome in this group {OR = 5.689, p = 0.090}. Similar to our result the finding reported by Aruna et al. (2010) and Kurz et al. (2001) for Indian and Australian populations did not support the association between PROGINS Alu-Insertion and idiopathic repeated spontaneous abortions. Further, although overall considering all the term and preterm delivery cases, PR mutation was found to be significantly associated with lower birth weight (p = 0.035); but the presence of PR mutation was not found to have any significant association with low birth weight in either term or preterm delivery cases. Wilcoxon signed rank test based analysis showed that PR mutation was found to increase the risk of low birth weight significantly in the case of very preterm (p < 0.001) and moderately preterm (p < 0.001) delivery cases.
To conclude, the present study strongly highlights the association of genetic factors in predisposition to preterm delivery and related complications. The presence of MTHFR C677T polymorphism is a risk factor for preterm delivery, negative pregnancy outcome and low birth weight. Moreover screening of MTHFR C677T polymorphism of the pregnant women during the first trimester can be used as a prognostic marker in the stratification of pregnancy cases who are at the risk of for extreme, very and moderate preterm delivery; and appropriate need based clinical interventions and required precautions can be taken for the prevention of premature delivery, also negating the risk of negative pregnancy outcome and low birth weight, thereby reducing the neonatal mortality and morbidity rates. Although PR mutation (PROGINS) was not significantly associated with preterm delivery in our cohort, but PROGINS mutation is an associative risk factor for negative pregnancy outcome in some of the preterm delivery cases, and is also associated with increased risk of low birth weight in a subpopulation of preterm delivery cases; and hence may also be treated as a risk factor independently or in combination of MTHFR in the prognosis of preterm delivery and related complications.
Acknowledgments
The authors would like to thank DBT, Govt. of India for providing the necessary infrastructure to carry out all the experimental work smoothly. The authors are thankful to Dr. Bidisha Hazarika and Dr. Devender Mittal for their support during sample collection for the study.
References
- Agoulnik I.U., Tong X.-W., Fischer D.-C., Körner K., Atkinson N.E., Edwards D.P., Headon D.R., Weigel N.L., Kieback D.G. A germline variation in the progesterone receptor gene increases transcriptional activity and may modify ovarian cancer risk. J. Clin. Endocrinol. Metab. 2004;89:6340–6347. doi: 10.1210/jc.2004-0114. [DOI] [PubMed] [Google Scholar]
- Alfirevic Z., Roberts D., Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002;10:6–14. doi: 10.1016/s0301-2115(01)00496-1. [DOI] [PubMed] [Google Scholar]
- Aruna M., Nagaraja T., Andal S., Tarakeswari S., Sirisha P.V.S., Reddy A.G., Thangaraj K., Singh L., Reddy B.M. 5(1) 2010. Role of Progesterone Receptor Polymorphisms in the Recurrent Spontaneous Abortions: Indian Case; p. e8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S., Wojdyla D., Say L., Betran A.P., Merialdi M. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010;88(1):31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Oestergaard M.Z., Chou D., Moller A.B., Narwal R., Adler A., Vera Garcia C., Rohde S., Say L., Lawn J.E. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Chou D., Oestergaard M., Say L., Moller A.B., Kinney M., Lawn J. Born too soon: the global epidemiology of 15 million preterm births. Reproductive. 2013;10(Suppl. 1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose P.D., Das B.C., Kumar A., Gondal R., Kumar D., Kar P. High viral load and deregulation of the progesterone receptor signaling pathway: association with hepatitis E-related poor pregnancy outcome. J. Hepatol. 2011;54(6):1107–1113. doi: 10.1016/j.jhep.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Challis J.R.G., Jr. Mechanism of parturition and preterm labor. Obstet. Gynecol. Surv. 2000;55:650–660. doi: 10.1097/00006254-200010000-00025. [DOI] [PubMed] [Google Scholar]
- Clarke C.L., Sutherland R.L. Progestin regulation of cellular proliferation. Endocr. Rev. 1990;11(2):266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- Committee on Nutritional Status During Pregnancyand Lactation . National Academy Press; Washington, DC: 1990. Institute of Medicine. Nutrition during Pregnancy. [Google Scholar]
- Dekker G.A., de Vries J.I., Doelitzsch P.M. Underlying disorders associated with severe early-onset preeclampsia. Am. J. Obstet. Gynecol. 1995;173:1042–1048. doi: 10.1016/0002-9378(95)91324-6. [DOI] [PubMed] [Google Scholar]
- Diaz-Cueto L., Dominguez-Lopez P., Cantillo-Cabarcas J., Perez-Figueroa G., Arechavaleta-Velasco M., Arechavaleta-Velasco F. Progesterone receptor gene polymorphisms are not associated with preterm birth in a Hispanic population. Int. J. Gynaecol. Obstet. 2008;103(2):153–157. doi: 10.1016/j.ijgo.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Ehn N., Cooper M.E., Orr K., Shi M., Johnson M.K., Caprau D., Dagle J., Steffen K., Johnson K., Marazita M., Merrill D., Jeffrey C. Murray evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatr. Res. 2007;62(5):630–635. doi: 10.1203/PDR.0b013e3181567bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosst P., Blom H.J., Milos R., Goyette P., Sheppard C.A., Matthews R.G., Boers G.J., den Heijer M., Kluijtmans L.A., van den Heuvel L.P., Rozen R. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- Gissler M., Jarvelin M.R., Louhiala P., Rahkonen O., Hemminki E. Can children's health be predicted by perinatal health? Int. J. Epidemiol. 1999;28(2):276–280. doi: 10.1093/ije/28.2.276. [DOI] [PubMed] [Google Scholar]
- Gogoi M., Prusty R.K. Maternal anaemia, pregnancy complications and birth outcome: evidences from north-east India. J. N. East India Stud. 2013;3(1):74–85. [Google Scholar]
- Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. (PMID:18177778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M.T.V., Castro R.A., Villanova F.E., Silva I.D.C.G. Relação entre polimorfismo do gene do receptor de progesterona, raça, paridade e ocorrência de leiomioma uterino. Rev. Bras. Ginecol. Obstet. 2006;28:278–284. [Google Scholar]
- Graham J.D., Clarke C.L. Physiological action of progesterone in target tissues. Endocr. Rev. 1997;18(4):502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- Isotalo P.A., Wells G.A., Donnelly J.G. Neonatal and fetal methylenetetrahdrofolate reductase genetic polymorphisms: an examination of C677T and A1298C mutations. Am. J. Hum. Genet. 2000;67(4):986–990. doi: 10.1086/303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W.P. Long term fetal programming of body composition and longevity. Nutr. Rev. 1997;55:S31–S43. doi: 10.1111/j.1753-4887.1997.tb06097.x. [DOI] [PubMed] [Google Scholar]
- Karalis K., Goodwin G., Majzoub J.A. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat. Med. 1996;2:556–560. doi: 10.1038/nm0596-556. [DOI] [PubMed] [Google Scholar]
- Kieback D.G., Tong X.-W., Weigel N.L., Agoulnick I.U. A genetic mutation in the progesterone receptor (PROGINS) leads to an increased risk of nonfamilial breast and ovarian cancer causing inadequate control of estrogen driven proliferation. J. Soc. Gynecol. Investig. 1998;5:40. [Google Scholar]
- Kramer M.S., Goulet L., Lydon J., Seguin L., Namara H.M., Dassa C., Platt R.W., Chen M.F., Gauthier H., Genest J., Kahn S., Libman M., Rozen R., Masse A., Miner L., Asselin G., Benjamin A., Klein J., Koren G. Socio-economic disparities in preterm birth: causal pathways and mechanisms. Paediatr. Perinat. Epidemiol. 2001;15(Suppl. 2):104–123. doi: 10.1046/j.1365-3016.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Kurz C., Tempfer C.B., Boecskoer S., Unfried G., Nagele F. The PROGINS progesterone receptor gene polymorphism and idiopathic recurrent miscarriage. J. Soc. Gynecol. Investig. 2001;8:295–298. doi: 10.1016/s1071-5576(01)00123-x. [DOI] [PubMed] [Google Scholar]
- Lauszus F.F., Gron P.L., Klebe J.G. Association of polymorphism of methylene tetra hydrofolate reductase with urinary albumin excretion rate in type 1 diabetes mellitus but not with pre-eclampsia, retinopathy, and preterm delivery. Acta Obstet. Gynecol. Scand. 2001;80:803–806. doi: 10.1034/j.1600-0412.2001.080009803.x. [DOI] [PubMed] [Google Scholar]
- Lawn J.E., Wilczynska-Ketende K., Cousens S.N. Estimating the causes of 4 million neonatal deaths in the year 2000. Int. J. Epidemiol. 2006;35(3):706–718. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- Liu L., Johnson H., Cousens S., Perin J., Scott S., Lawn J., Ruden I., Campbell H., Cibulskis R., Mengying L. Global, regional and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- Mesiano S. Myometrial progesterone responsiveness and the control of human parturition. J. Soc. Gynecol. Investig. 2004;11:193–202. doi: 10.1016/j.jsgi.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Norwitz E.R., Robinson J.N. A systematic approach to the management of preterm labor. Semin. Perinatol. 2001;25:223–235. doi: 10.1053/sper.2001.26417. [DOI] [PubMed] [Google Scholar]
- Oliveira T.A., Cunha D.R., Policastro A., Traina E., Gomes M.T., Cordioli E. The progesterone receptor gene polymorphism as factor of risk for the preterm delivery. Rev. Bras. Ginecol. Obstet. 2011;33(6):271–275. [PubMed] [Google Scholar]
- Pepe G.J., Albrecht E.D. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr. Rev. 1995;16(5):608–648. doi: 10.1210/edrv-16-5-608. [DOI] [PubMed] [Google Scholar]
- Pisarska M.D., Carson S.A., Casson P.R., Tong X., Buster J.E. A mutated progesterone receptor allele is more prevalent in unexplained infertility. Fertil. Steril. 2003;80:651–653. doi: 10.1016/s0015-0282(03)00755-6. [DOI] [PubMed] [Google Scholar]
- Raisanen S., Gissler M., Saari J., Kramer M., Heinonen S. Contribution of risk factors to extremely, very and moderately preterm births—register-based analysis of 1,390,742 singleton births. PLoS ONE. 2013;8(4):e60660. doi: 10.1371/journal.pone.0060660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch B., Gallistl S., Kutschera J., Mannhalter C., Muntean W., Mueller W.D. Thrombophilic polymorphisms—factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations—and preterm birth. Wien. Klin. Wochenschr. 2004;116:622–626. doi: 10.1007/s00508-004-0223-9. [DOI] [PubMed] [Google Scholar]
- Scholl T., Johnson W.G. Folic acid: influence on the outcome of pregnancy. Am. J. Clin. Nutr. 2000;71:1295S–1303S. doi: 10.1093/ajcn/71.5.1295s. [DOI] [PubMed] [Google Scholar]
- Schweikert A., Rau T., Berkholz A., Allera A., Daufeldt S. Association of progesterone receptor polymorphism with recurrent abortions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004;113:67–72. doi: 10.1016/j.ejogrb.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Smith R., Mesiano S., McGrath S. Hormone trajectories leading to human birth. Regul. Pept. 2002;108:159–164. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Solanky N., Jimenez R.A., D'Souza S.W., Sibley C.P., Glazier J.D. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010;31:134–143. doi: 10.1016/j.placenta.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Sukla K.K., Tiwari P.K., Kumar A.K., Raman R. Low birthweight (LBW) and neonatal hyperbilirubinemia (NNH) in an Indian cohort: association of homocysteine, its metabolic pathway genes and micronutrients as risk factors. PLoS ONE. 2013;8(8):e71587. doi: 10.1371/journal.pone.0071587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres-Bartho J., Barakonyi A., Miko E., Polgar B., Palkovics T. The role of gamma/delta T cells in the feto-maternal relationship. Semin. Immunol. 2001;13:229–233. doi: 10.1006/smim.2000.0318. [DOI] [PubMed] [Google Scholar]
- Valdez L.L., Quintero A., Garcia E., Olivares N., Celis A., Rivas F., Jr. Thrombophilic polymorphisms in preterm delivery. Blood Cells Mol. Dis. 2004;33:51–56. doi: 10.1016/j.bcmd.2004.04.011. [DOI] [PubMed] [Google Scholar]
- van der Molen E.F., Verbruggen B., Novakova I. Hyperhomocysteinemia and other thrombotic risk factors in women with placental vasculopathy. BJOG. 2000;107:785–791. doi: 10.1111/j.1471-0528.2000.tb13341.x. [DOI] [PubMed] [Google Scholar]
- Wong H.S., Edwards P. Nature or nurture: a systematic review of the effect of socio-economic status on the developmental and cognitive outcomes of children born preterm. Matern. Child Health J. 2013;17(9):1689. doi: 10.1007/s10995-012-1183-8. [DOI] [PubMed] [Google Scholar]