Abstract
Major depressive disorder (MDD) is a prevalent and disabling condition, and many patients do not respond to available treatments. Deep transcranial magnetic stimulation (dTMS) is a new technology allowing non-surgical stimulation of relatively deep brain areas. This is the first double-blind randomized controlled multicenter study evaluating the efficacy and safety of dTMS in MDD. We recruited 212 MDD outpatients, aged 22–68 years, who had either failed one to four antidepressant trials or not tolerated at least two antidepressant treatments during the current episode. They were randomly assigned to monotherapy with active or sham dTMS. Twenty sessions of dTMS (18 Hz over the prefrontal cortex) were applied during 4 weeks acutely, and then biweekly for 12 weeks. Primary and secondary efficacy endpoints were the change in the Hamilton Depression Rating Scale (HDRS-21) score and response/remission rates at week 5, respectively. dTMS induced a 6.39 point improvement in HDRS-21 scores, while a 3.28 point improvement was observed in the sham group (p+0.008), resulting in a 0.76 effect size. Response and remission rates were higher in the dTMS than in the sham group (response: 38.4 vs. 21.4%, p+0.013; remission: 32.6 vs. 14.6%, p+0.005). These differences between active and sham treatment were stable during the 12-week maintenance phase. dTMS was associated with few and minor side effects apart from one seizure in a patient where a protocol violation occurred. These results suggest that dTMS constitutes a novel intervention in MDD, which is efficacious and safe in patients not responding to antidepressant medications, and whose effect remains stable over 3 months of maintenance treatment.
Keywords: Deep transcranial magnetic stimulation, major depressive disorder, treatment resistance, response, remission, maintenance treatment
Major depressive disorder (MDD) is a highly prevalent and disabling condition associated with significant morbidity and mortality (1,2). It has been estimated that 20-40% of patients do not benefit adequately from available interventions, including pharmacotherapy and psychotherapy (3). The lack of sufficient treatment response and the enormous impact of the disorder make the development of alternative treatment approaches a priority.
Repetitive transcranial magnetic stimulation (rTMS) has been proposed as one such novel treatment (4–6). TMS involves passing an electrical current through a coil placed against the scalp. The rapidly changing electrical current creates a time-varying magnetic field, which passes unimpeded through the scalp and skull and induces an electrical field in the cortex. This electrical field changes neuronal activity at the site of stimulation and within interconnected neuronal networks. TMS pulses applied in a repetitive train is referred to as rTMS.
The H-coil is a novel rTMS tool, which enables direct stimulation of deeper and larger brain volumes (7-10). This coil is designed to affect extensive neuronal pathways, including deeper cortical regions and fibers targeting subcortical regions, without a significant increase of the electric field induced in superficial cortical layers (7–10). Several open feasibility studies showed a clinically meaningful therapeutic action of H-coil deep TMS (dTMS), which was maintained by continuation of treatment for up to 18 weeks (11–15). These initial studies suggested that a stimulation intensity of 120% of the individual resting motor threshold (but not a more shallow stimulation induced by lower intensities) induces an antidepressant response.
Conventional rTMS procedures have been investigated in several psychiatric disorders, including unipolar depression, schizophrenia and bipolar disorder (16–18). A small number of acute (3-6 week) large scale randomized controlled multicenter trials have examined the antidepressant properties of conventional rTMS applied over the left dorsolateral prefrontal cortex (DLPFC) (19–21). Two of these studies showed significant antidepressant effects of rTMS, compared to placebo, in medication-free patients who had not responded to previous antidepressant treatment (19,21).
Though antidepressant properties of prefrontal rTMS have been clearly demonstrated in patients who did not respond to one antidepressant medication in the current episode, response and remission rates in these large controlled trials were small to moderate. Therefore, additional multicenter sham-controlled studies are needed to establish the short- and long-term efficacy of rTMS in patients suffering from therapy-resistant MDD (19,22).
In this study, we have addressed two key issues that may be critical for the antidepressant effect of rTMS. First, most clinical rTMS protocols have stimulated the left DLPFC. Recent studies have shown that different DLPFC subregions stimulated by standard protocols vary considerably in terms of their connectivity with medial prefrontal structures such as the subgenual cingulate gyrus (23,24), which appears to be an important region involved in the pathophysiology of MDD (25). Thus, it may be advantageous to stimulate less focally and more deeply to reach connecting fiber tracts. Second, conventional rTMS has been investigated over short acute treatment periods ranging from 3 to 6 weeks. The clinical durability of antidepressant effects over longer periods has not been studied before in randomized controlled trials.
We conducted a double-blind randomized placebo-controlled multicenter trial to investigate the efficacy and safety of H-coil dTMS applied daily as monotherapy in subjects with MDD who had either failed one to four antidepressant trials or not tolerated at least two antidepressant treatments in the current episode. The acute treatment phase of 4 weeks was followed by a maintenance treatment up to 12 weeks.
METHODS
Study overview
The study was conducted at 20 medical centers (13 in the U.S., four in Israel, two in Germany, and one in Canada), with active enrolment extending from October 2009 until January 2012. Institutional review board approval was obtained at all sites. The trial was carried out under an investigational device exemption from the U.S. Food and Drug Administration (FDA). An independent data and safety monitoring board reviewed participant safety and study progress.
The study design included three phases: a wash-out phase (1-2 weeks), during which patients were tapered off all antidepressants, mood stabilizers and antipsychotics; a 4-week acute treatment phase (daily treatment with dTMS or sham TMS), and a 12-week maintenance phase (two treatments per week of dTMS or sham TMS).
Subjects
Patients were recruited via public media advertisements and physician referrals. Site personnel phone-screened potential participants, and those meeting inclusion and exclusion criteria underwent additional on-site screening. All subjects signed an informed consent document before undergoing any study procedures.
Eligible subjects were antidepressant medication-free (following the wash-out period) outpatients, aged 22-68 years, with a DSM-IV diagnosis of MDD, single or recurrent episode. The duration of current episode was at least one month but no more than 7 years. Subjects were required to have a Clinical Global Impression Severity of Illness (CGI-S) score of at least 4 and a total score of at least 20 on the 21-item Hamilton Depression Rating Scale (HDRS-21) at screening visit. Symptom stability was required during the 2-week wash-out period. Instability was defined as a change of ±30% or more from the total HDRS-21 score that was observed at the screening assessment.
Antidepressant treatment resistance during the current episode was assessed using the Antidepressant Treatment History Form (ATHF, 26). Subjects were required to have failed at least one but no more than four adequate antidepressant treatments, or to have had intolerance to at least two antidepressants in the current episode.
Subjects were excluded if they had a lifetime history of psychosis, bipolar disorder, obsessive-compulsive disorder (current or within the past year), post-traumatic stress disorder or eating disorders. Subjects suffering from anxiety or personality disorders were eligible only if this was not their primary diagnosis. Additional exclusion criteria were any significant neurological disorder or insult; increased risk of seizure for any reason or familial or personal history of epilepsy; lifetime lack of response to an adequate trial of electroconvulsive therapy; prior treatment with rTMS, dTMS or a vagus nerve stimulator implant; pregnancy; presence of intracranial implants or any other metal object within or near the head, excluding the mouth, that could not be safely removed; a present risk of suicide or a history of suicide attempt in the last 3 years.
Study design
Patients were randomly assigned to either active dTMS or sham TMS (1:1 ratio) by an interactive web response system based on the random allocation sequence generated by the study statisticians. They were stratified per center by severity of disease as determined by baseline HDRS-21 scores (<26 vs. ≥26) and ATHF treatment resistance levels (ATHF 1, level 3 and ATHF ≥2, level 1–2 vs. ATHF 2–4, level 3).
During the acute treatment phase, TMS sessions were performed daily in a 5-day sequence (5 days per week) for 4 weeks. During the maintenance phase, subjects were treated twice a week (with at least 48 hours between sessions) for 12 weeks. Subjects were discontinued from the study at any point if they were considered by the investigators to be at an elevated risk for suicide. Subjects were also discontinued if they did not experience a sufficient improvement in depressive symptoms after 5 weeks of treatment in two consecutive assessments. A sufficient improvement was defined as a decrease of at least one point on CGI-S from baseline.
Antidepressants, mood stabilizers and antipsychotics were not permitted during the study. Sedatives/hypnotics which were prescribed prior to commencement of treatment were allowed to be continued during the study as appropriate. Anxiolytics, sedatives and hypnotics were allowed to be prescribed during the study in a pre-defined dosage range.
Device description
The TMS sessions were delivered using a Brainsway dTMS system with the H1-coil investigational device (Brainsway Ltd., Jerusalem, Israel). The H1-coil has been designed to stimulate deep prefrontal cortex areas that include neuronal pathways associated with the brain reward system (8,14). The coil is placed in a helmet to allow effective cooling during stimulation, and the frame of the inner rim of the coil is flexible in order to accommodate the variability in human skull shape. In addition to the active H1-coil, a sham coil was included in the same helmet. The sham coil mimics scalp sensations and acoustic artifact of the real H1-coil, without inducing neuronal activation, as most of the elements of the sham coil are located far above the patient's head and the electric field induced by the sham coil is negligible and insufficient to induce neuronal activation in the patient's brain (27).
The combined coil was connected to a magnetic card reader, which was, in turn, connected to an electrical switch designed to alternate between the sham and active coils. The card reader was designed to read both operator cards and patient cards that encode the patients' treatment group assignments. When the operator card was swiped by the card reader, the system was set to an active stimulation mode in which the system operator determined the subjects' motor threshold. After completion of this stage, the assigned patient card was swiped by the card reader, and the treatment was administered according to the group to which the subject had been randomized. In this manner, all study personnel were blinded to the treatment assignment.
dTMS protocol
Before starting each treatment, subjects were instructed to insert earplugs to lessen any possible adverse effects on hearing. The individual motor threshold at rest was measured at the beginning of each treatment (with the operator card) by delivering single stimulation pulses to the respective “hot spot” of the motor cortex (27). The left DLPFC was chosen as the treatment target site, and was targeted by placing the coil 6 cm anterior to the “hot spot” according to a ruler attached on the subject's cap. During the first three treatments, sites were allowed to titrate stimulation intensity up from 100% to 120% of the individual motor threshold in order to improve subjects' tolerability to the treatment.
The treatment group received dTMS doses of 18 Hz, at stimulator power output of 120% of the measured individual motor threshold. Each dTMS repetition included 2-sec pulse trains separated by 20-sec inter-train intervals. Subjects received 55 trains in each treatment session, for a total of 1980 pulses per session. Each session lasted about 30 min, of which the dTMS delivery lasted 20 min. The control group received sham (inactive) treatment with identical parameters. Subjects were told that face and hand twitching may occur due to either sham or active treatment.
Efficacy and safety assessments
All efficacy outcome measures were performed by a blinded study rater who was not permitted access to the treatment sessions. Raters were required to pass the study rater certification program, which was developed to ensure adequate scoring reliability and rating skills. Patients were instructed not to disclose any details of the treatment session to the study raters during rating sessions. Furthermore, patients were instructed to report all adverse events only to the device operator. Efficacy ratings were administered at baseline and once weekly until the end of the study (week 16).
The primary endpoint was the change in the total score on the HDRS-21 from baseline to week 5. The secondary efficacy endpoints were response and remission rates at week 5. Response was defined as a reduction of at least 50% in the total HDRS-21 score compared to baseline, and remission was defined by a total HDRS-21 score <10.
Safety was assessed at every treatment visit by the operator. Patients were asked to report any adverse events since their previous visit. Adverse events were coded using the current version of the Medical Dictionary for Regulatory Activities. Additional safety evaluations included auditory threshold tests performed at baseline, week 6, and at end-of-study visit. Subjects were also evaluated for cognitive changes at baseline, week 5 and at end-of-study visit.
Statistical analysis
We determined that 85 subjects per group (170 in total) would provide 90% power at a significance level of 5% (two-tailed) in detecting a difference of 3.75 points in the mean change from baseline HDRS-21 scores between treatment and sham groups, considering a standard deviation of 7.5 points (data from pilot study), and assuming an effect size of 0.5. Allowing for a 15% dropout from the study irrespective of study arm and success of treatment, 200 randomized subjects were required. Approximately 20% of the subjects were expected to leave the study between screening and randomization, thus it was necessary to screen approximately 250 subjects in order to arrive at the point of randomization with at least 200 subjects.
The study results were analyzed for two patient populations: the intention-to-treat (ITT) and the per-protocol (PP) analysis set. The ITT set included all subjects who met the study eligibility criteria and received at least one dTMS/sham treatment. Patients who were not administered stimulation at the protocol-specified intensity (i.e., 120% of their individual motor thresholds) were excluded from the PP cohort. The PP population thus included all subjects from the ITT set who received the protocol-specified treatment and completed the 16-week treatment regimen or withdrew before completion per the study protocol. Comparisons of baseline demographic and clinical characteristics and safety assessments were performed on the ITT analysis set. The primary efficacy analysis was performed using the PP analysis set.
Comparisons of baseline demographic and clinical characteristics between the study groups were performed to ensure that the groups were balanced at baseline and that the randomization was successful. For comparison of means (continuous variables), the two-sample t-test or a non-parametric equivalent was used. For comparison of proportions (categorical variables), the chi-square test or Fisher's exact test was used, as appropriate. The change in HDRS-21 total score from baseline to week 5 (primary endpoint) was compared between the treatment groups using a repeated measures analysis (RMA) of covariance (SAS® MIXED procedure). The analysis, which aims to compare the slopes of the changes in HDRS-21 scores between study arms, included the following fixed effects: time from randomization (in weeks), treatment group, time x treatment group interaction, center (site of study), baseline HDRS-21 score, and ATHF category at baseline. Baseline HDRS-21 score was entered as a continuous variable so as to minimize the potential for co-linearity problems.
Individual subjects' intercepts and time effects were also included in the model as random effects (random intercept and slope model). The principal statistical analysis was a comparison between the slopes of the treatment groups, derived from the time x treatment interaction term from the RMA model described above. The adjusted mean slope of change from baseline in HDRS-21 scores at week 5 post-randomization is estimated from the model (least square means, LS-means) for each study group as well as for the difference between the groups' adjusted mean slopes, and these are presented together with 95% confidence intervals.
Secondary outcome measures were the response and remission rates at week 5. Tertiary endpoints were the change from baseline to week 16, as well as response and remission rates at week 16. The change in HDRS-21 total score from baseline to week 16 was compared between the treatment groups using analysis of covariance of the change from baseline to the last observed value (LOV). Baseline HDRS-21 score, ATHF category at baseline, and site were entered as covariates. The LOV is defined as the last available post-baseline visit data up to and including the last treatment visit or termination visit. The adjusted mean change (LS-means) from baseline in HDRS-21 score at week 16 (LOV) is estimated from the model (LS-means) for each study group, as well as the difference between the adjusted means. These are presented together with 95% confidence intervals.
The overall significance level for this study was 0.05 using two-tailed tests, except for treatment x site interaction that was tested at a significance level of 0.01. Nominal p values are presented. Statistical analyses were performed using SAS® V9.3 (SAS Institute, Cary NC, USA). Effect size was the difference between slopes/pooled standard deviation of baseline HDRS-21 score.
RESULTS
Subjects
Following phone screening of over 900 potential participants, of which 428 were invited for additional on-site screening, 233 subjects were enrolled, of which the ITT set included 212 subjects (excluding subjects who did not comply with the inclusion/exclusion criteria or left the study before receiving a single treatment). Thirty-one subjects in the ITT set who did not receive the adequate TMS regimen as specified in the protocol were excluded to form the PP analysis set (N+181). The PP analysis set thus included only subjects who completed the study without any major protocol violations. For this reason, it was considered the most appropriate for the purpose of assessing the efficacy of dTMS.
Eligible and consenting subjects were randomized to either the dTMS group (N+111; ITT+101, PP+89) or the sham control group (N+122; ITT+111, PP+92). The numbers of patients who dropped out of the study later on as well as the reasons for dropouts are presented in the CONSORT diagram (Figure 1). The two study groups were statistically similar at baseline with respect to demographic parameters, clinical characteristics and HDRS-21 mean scores (Table1).
Figure 1.
CONSORT diagram. dTMS – deep transcranial magnetic stimulation, ITT – intention-to-treat analysis, PP – per-protocol analysis, MT – motor threshold.
Table 1.
Demographic data and baseline characteristics of subjects by treatment group (intention-to-treat analysis set)
| dTMS (N+101) | Sham (N+111) | p | |
|---|---|---|---|
| Age (years, mean±SD) | 45.1±11.7 | 47.6±11.6 | 0.1241 |
| Gender (% male) | 52.5 | 52.3 | 1.000 |
| Ethnicity (% Caucasian) | 94.1 | 87.4 | 0.6866 |
| Body mass index (mean±SD) | 28.1±7.1 | 27.8±7.0 | 0.7837 |
| Age at first episode (years, mean±SD) | 25.3±11.5 | 26.9±12.7 | 0.3357 |
| Duration of current episode (months, mean±SD) | 21.7±16.3 | 19.5±15.2 | 0.3217 |
| History of suicide attempts (% without any) | 88.1 | 92.8 | 0.3471 |
| Antidepressants in current episode (%) | |||
| None | - | 0.9 | 0.1880 |
| One | 24.8 | 24.3 | |
| Two | 33.7 | 31.5 | |
| Three | 15.8 | 17.1 | |
| Four | 10.9 | 19.8 | |
| Five or more | 14.9 | 6.3 | |
| Number of failed medications at ATHF level ≥3 (%) | |||
| None | 6.9 | 12.6 | 0.3838 |
| One or two | 71.3 | 66.7 | |
| Three or more | 21.8 | 20.7 | |
| Baseline HDRS-21 score (mean±SD) | 23.5±4.3 | 23.4±3.7 | 0.7641 |
| Motor threshold at first treatment (mean ±SD) | 59.8±8.3 | 61.1±8.9 | 0.2745 |
dTMS – deep transcranial brain stimulation, ATHF – Antidepressant Treatment History Form, HDRS – Hamilton Depression Rating Scale
Efficacy measures
The primary efficacy endpoint was the change in HDRS-21 total score from baseline to end of week 5, i.e. after subjects had completed 4 weeks of acute dTMS treatment and were one week into the maintenance phase.
In the PP analysis set, the estimated slope in the dTMS group was -6.39 compared with -3.28 in the sham group. The difference of -3.11 (95% CI: -5.40, -0.83) points between the slopes was statistically significant (p+0.008), with an effect size of 0.76 (Table2, Figure 2). In the ITT analysis set, the difference of -2.23 points (95% CI: -4.54, 0.07) between the slopes across 5 weeks fell just short of reaching statistical significance (p+0.0578), with a corresponding effect size of 0.58 (Table2).
Table 2.
Primary, secondary and tertiary efficacy measures
| ITT | p | PP | p | |||
|---|---|---|---|---|---|---|
| dTMS (n+101) | Sham (n+111) | dTMS (n+89) | Sham (n+92) | |||
| Primary efficacy measure | ||||||
| Slope of change, 5 weeks (95% CI) | −6.17 (−7.78, −4.55) | −3.94 (−5.58, −2.29) | 0.0578 | −6.39 (−7.97, −4.79) | −3.28 (−4.91, −1.63) | 0.0080 |
| Secondary efficacy measures | ||||||
| Response rate, week 5 (%) | 37.0 | 27.8 | 0.0310 | 38.4 | 21.4 | 0.0138 |
| Remission rate, week 5 (%) | 30.4 | 15.8 | 0.0158 | 32.6 | 14.6 | 0.0051 |
| Tertiary efficacy measures | ||||||
| LS-mean of change, 16 weeks (95% CI) | −8.04 (−9.91, −6.16) | −6.31 (−7.99, −4.62) | 0.1040 | −8.55 (−10.51, −6.57) | −6.07 (−7.87, −4.27) | 0.0259 |
| Response rate, 16 weeks (%) | 40.6 | 26.0 | 0.0276 | 44.3 | 25.6 | 0.0086 |
| Remission rate, 16 weeks (%) | 29.2 | 22.1 | 0.2530 | 31.8 | 22.2 | 0.1492 |
dTMS – deep transcranial brain stimulation, ITT – intention-to-treat analysis, PP – per-protocol analysis, LS – mean-least square mean
Figure 2.
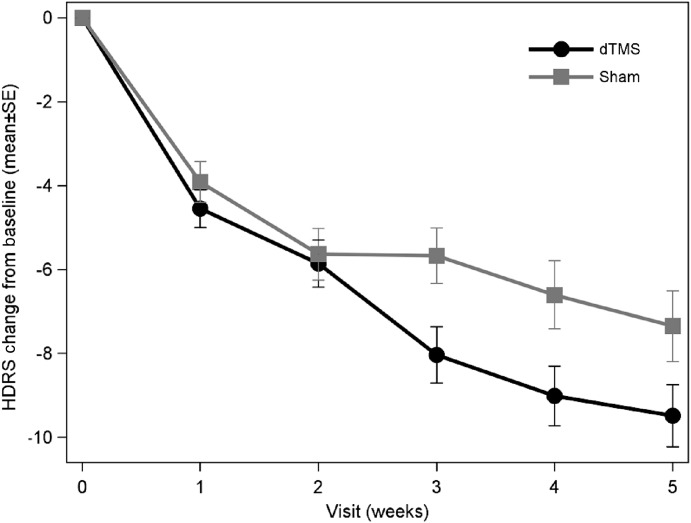
Change in Hamilton Depression Rating Scale (HDRS-21) total score from baseline over time to the primary time point (end of week 5) for deep transcranial magnetic stimulation (dTMS) and sham groups in the per-protocol analysis
The secondary efficacy measures were response and remission rates at week 5. Response rates (PP set) were 38.4% for dTMS versus 21.4% for sham treatment (chi-square test, p+0.0138). Remission rates (PP set) were 32.6% and 14.6% for dTMS and sham TMS, respectively (chi-square test, p+0.0051) (Table2, Figure 3).
Figure 3.
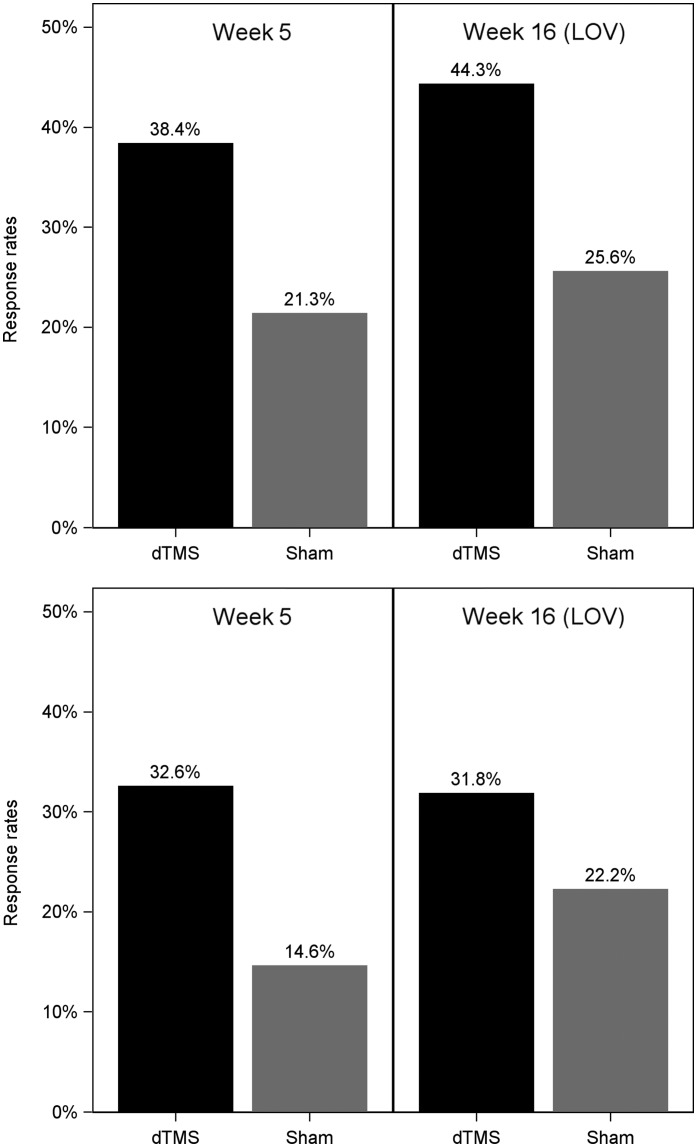
Response and remission rates for deep transcranial magnetic stimulation (dTMS) and sham groups at the end of week 5 and of week 16 (last observed value, LOV) in the per-protocol analysis. Response: p+0.0138 (5 weeks), p+0.0086 (16 weeks). Remission: p+0.0051 (5 weeks), p+0.1492 (16 weeks)
The tertiary efficacy measures were change in HDRS-21 total score from baseline to week 16 and response and remission rates at week 16. The difference of 2.47 points between the LS-means of the active and sham groups at week 16 was statistically significant (p+0.0259). Additionally, the response rates of the PP set at week 16 (LOV) were 44.3% after dTMS versus 25.6% after sham treatment (chi-squared test, p+0.0086). The week 16 remission rates (LOV) were 31.8% and 22.2% in the dTMS and sham groups, respectively (p+0.1492, chi-squared test) (Table2, Figure 3).
A subset analysis was performed to assess if there was a different response to treatment in subjects who failed one or two medications versus subjects who failed three or more medications in the current episode. The primary and secondary outcome measures were estimated in each subset. The change from baseline over time until the primary endpoint (5 weeks) for the active and sham groups was compared by repeated measures ANOVA models as described for the primary measure above. The difference between the estimated slopes of the dTMS and sham groups was -3.23 points (95% CI: -6.19, -0.27, p+0.0327) in the first stratum (failed one or two medications), and -3.10 (95% CI: -6.76, 0.56, p+0.0958) in the second stratum (failed three or more medications). Remission rates in the first stratum were 36.6% (N+15/41) for the dTMS group and 16.7% (N+8/48) for the sham group (p+0.032, chi-square test). Remission rates in the second stratum were 28.9% (N+13/45) for dTMS group and 12.2% (N+5/41) for the sham group (p+0.057, chi-square test). So, patients with higher resistance to medications tended to be somewhat less responsive to dTMS, but the effect of treatment was still significant in patients who failed one or two medications and marginally significant relative to the sham group in patients who failed three or more medications (Figure 4).
Figure 4.
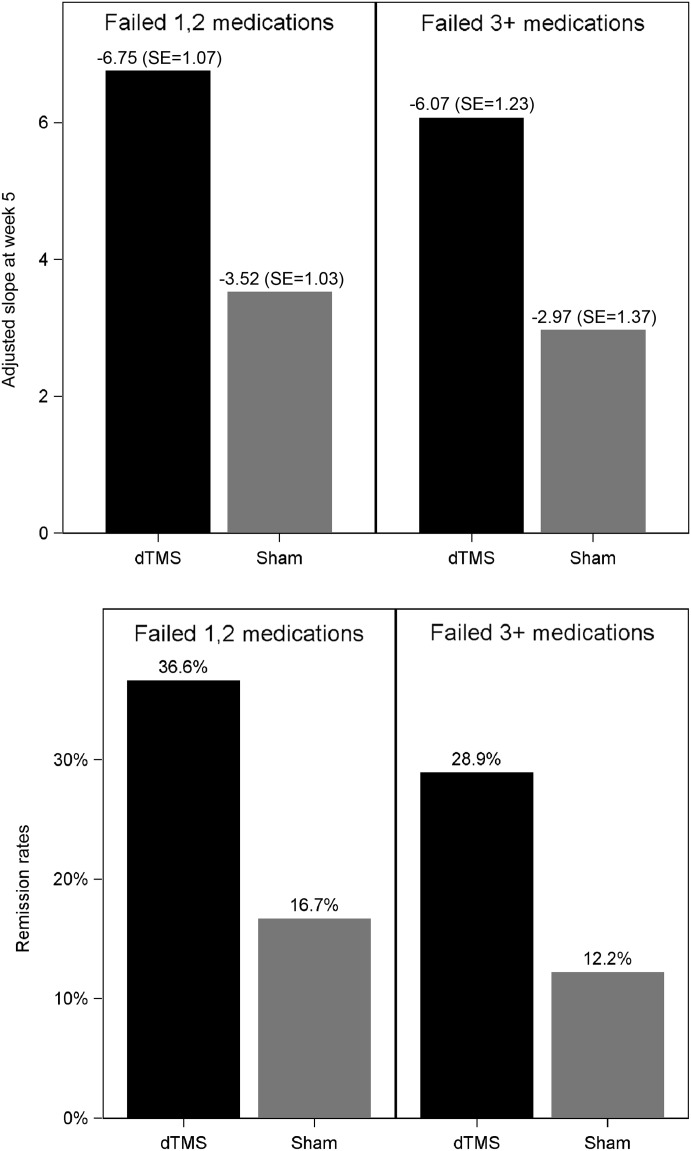
Antidepressant effect of deep transcranial magnetic stimulation (dTMS) in relation to the number of failed pharmacotherapy trials
As an additional measure of clinical efficacy, we calculated the total amount of time (in weeks) during which subjects satisfied HDRS-21 criteria for response and remission. Subjects had to complete a minimum of two weeks of treatment sessions in order to be included in this analysis. The highest obtainable result was 16 (for patients who remitted or responded already in the first week of treatment and remained in remission or response until the end of the study, without leaving the study) and the lowest was 0 (for patients who did not achieve remission or response at all). The mean time in response in the dTMS group was 4.9 weeks versus 2.8 weeks in the sham group (p+0.001, Wilcoxon two-sample test). The mean time in remission in the dTMS group was 3.7 weeks versus 2.1 weeks in the sham group (p+0.003, Wilcoxon two-sample test). The distributions of percentage of time in response and in remission out of the total time in the study for the dTMS and sham groups are shown in Figure 5. The mean percentage of time in response in the dTMS group was 36±4% versus 22±3% in the sham group (p+0.002, Wilcoxon two-sample test). The mean percentage of time in remission in the dTMS group was 26±3% versus 16±3% in the sham group (p+0.005, Wilcoxon two-sample test).
Figure 5.
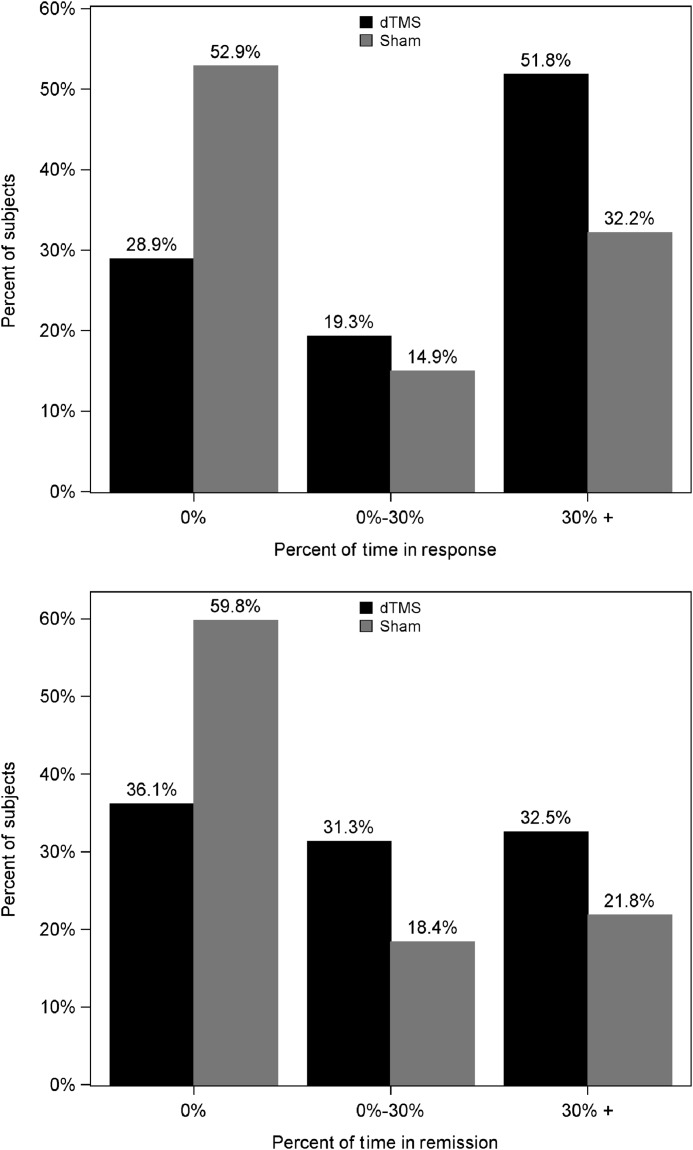
Percentage of patients achieving response or remission for 0%, 0–30% and >30% of the total time in the study in the deep transcranial magnetic stimulation (dTMS) and sham groups
Center bias was evaluated by entering the group x site x time interaction into the model for the primary endpoint and assessing statistical significance at the 0.01 level. No statistically significant differences were found in slopes of change from baseline HDRS-21 score between the study groups stratified by center (F+1.10, df+18,151, p+0.36).
Integrity of blinding in patients was assessed using a forced choice questionnaire. Of the 198 subjects who answered the questionnaire (one subject did not respond at all to the forced choice question, one subject discontinued treatment and did not respond, and 12 subjects could not decide what to answer), 138 (69.7%) thought they were receiving the active treatment. Of these, 78 (56.5%) were actually in the dTMS group and 60 (43.5%) in the sham group. This difference was not statistically significant.
Safety measures
Adverse events were defined and reported in the study according to system organ class and preferred term based on the Medical Dictionary for Regulatory Activities classification. Within the dTMS group, three subjects (3.0%) reported application site discomfort, five (5.0%) application site pain, 27 (26.7%) headache, two (2.0%) muscle twitching, two (2.0%) back pain, and two (2.0%) insomnia. Within the sham group, two subjects (1.8%) reported application site discomfort, none application site pain, 21 (18.9%) headache, none muscle twitching, three (2.7%) back pain, two (1.8%) anxiety, and four (3.6%) insomnia. Only one adverse event category showed a significant difference between study groups: application site pain (p+0.02). This effect is commonly reported with TMS treatment.
Eight serious adverse events were reported in seven subjects. Four of them were reported in the sham group (two cases of suicidal ideation, one of nausea and vomiting, and one of nephrolithiasis); three were reported in two subjects in the dTMS group (one case of elbow fracture, one of cluster headache, and one of seizure); and one was reported in a subject not randomized to the study (a suicide attempt). Only one out of the eight serious adverse events was considered device-related: one subject (female, 26 years old) experienced a generalized seizure which lasted about 2 min. The seizure occurred towards the end of her ninth dTMS treatment session. The patient entered a post-ictal state after the seizure. Following a full neurological examination and several hours of observation in the emergency room, the patient was released with no additional medical intervention. The subject was withdrawn from the study and there were no reported sequelae as a result of the event. The seizure occurred following excessive consumption of alcohol on the night before treatment that was not reported to the treating physician or operator at the time of treatment. The event was reported to the FDA. This serious adverse event was considered device-related, albeit with the caveat that withdrawal from alcohol may have led to a reduction of seizure threshold and consequently to this seizure during dTMS.
DISCUSSION
This double-blind placebo-controlled multicenter study demonstrates the efficacy and safety of dTMS in MDD patients who did not benefit from previous antidepressant treatment. The therapeutic effect was essentially stable during a maintenance phase up to 16 weeks, and a clinically meaningful improvement was seen also in patients who had not responded to three or more previous antidepressant medications.
dTMS is a novel type of rTMS which differs from standard rTMS by custom made coils (H-coils) with a greater depth of effective stimulation (7–10). The H1-coil has been specially developed for deeper and non-focal stimulation of dorsolateral and ventrolateral prefrontal areas that also project into other areas of brain reward system. These anatomical targets have been suggested to be particularly relevant for therapeutic effects of non-invasive brain stimulation in MDD (5).
The therapeutic effects of dTMS observed here were clinically relevant and maintained up to week 16. Although this study was not designed to compare the effect of dTMS with that of standard TMS, we hypothesize that the marked antidepressant efficacy of dTMS is related to the novel design of the H-coil, which enables stimulation of deeper prefrontal cortical areas that project into subcortical networks. Recent studies suggest that stimulation of prefrontal cortical regions with extensive connections to the subgenual cingulate gyrus may be crucial for the antidepressant action of standard rTMS (24). Since the exact location of these cortex regions varies greatly between individuals (28), and standard TMS coils exert a more focal and superficial stimulation, optimal stimulation targets may be easily missed with standard coils. Nevertheless, a study directly comparing dTMS and standard TMS is needed to prove the superiority of dTMS. In addition, further studies are needed to clarify which anatomical structures and pathways exactly mediate the therapeutic action of dTMS.
The efficacy of dTMS and standard TMS cannot be compared at the moment because previous studies investigating rTMS not only vary by TMS parameters, but also differ by inclusion criteria, patient characteristics and efficacy criteria. O'Reardon et al (21) reported HDRS-17 response and remission rates of 24.5% and 15.5% in subjects treated with active rTMS for 6 weeks compared to 13.7% and 8.9% in sham-stimulated control subjects. In a duration-adaptive study (3-week acute treatment phase with a 3-week extension for clinical improvers), George et al (19) reported remission rates of 14.1% following active rTMS compared to 5.1% with placebo rTMS. Thus, the results of the current and previous studies not only vary for active treatment groups, but also in relation to the outcome of sham TMS treatment. In the current study, a rather higher response (PP: 21.4%) and remission rate (PP: 14.6%) was observed after sham treatment in comparison to both previous trials (19,21). This could be due to patient selection and to an improved sham condition in which the sham coil was built in the same helmet as the active coil. In this sham condition, most of the elements are located far above the patient's head, generating an electric field that stimulates skin and scalp muscles but is insufficient to produce neuronal activation. Moreover, the operator neither needs to apply electrical stimulation in conjunction with sham TMS, nor to exchange active and sham coils manually as in earlier multicenter studies (19,21).
dTMS was well tolerated by the majority of patients and the main side effect was pain during application, usually not requiring any special care. There was one seizure induced by dTMS in this study, which may have been related to alcohol consumption the night before treatment. To date, out of over 3,500 patients treated with dTMS across studies, there have been five seizures. This risk of seizure with dTMS is quite similar to that of standard TMS and is likely related to the total energy induced by either coil and not the larger distribution and less concentrated electric field induced by the dTMS coil. Notably, the seizure was self-limited and with no persistent medical sequelae.
The present study was the first multicenter TMS study assessing the effects of maintenance therapy. The 12-week period of bi-weekly treatments proved the therapeutic effect of dTMS to be durable long after the acute daily treatment phase, even without concomitant antidepressant medications. Deep TMS at a bi-weekly schedule may be an acceptable alternative to antidepressant therapy for the long term as well.
There are several limitations to this study. First, 14.6% of the ITT analysis set were not treated at the stimulation intensity defined by the protocol and had to be excluded from the PP analysis. This was presumably due to the flexibility of the operator in titrating stimulation intensity from 100% up to 120% of individual motor threshold in order to improve tolerability. Thus, patients were more likely to stay at an intensity below the optimal level compared to trials where rTMS was defined at a fixed intensity after a brief lead-in period (19,21). The importance of adequate intensity (120% of individual motor threshold) should be highly emphasized when training operators to use this system for antidepressant treatment, as lower intensity does not allow stimulation of deep prefrontal cortex areas and is therefore less likely to produce the desired clinical response (14).
Second, patients with psychotic depression were excluded from the study. This decision was based on a previous trial that demonstrated the superiority of electroconvulsive therapy to rTMS in this patient group (29). However, it cannot be ruled out that psychotic patients may benefit from dTMS treatment, particularly if it is administered concomitantly with antipsychotic medication. Third, in the present study patients were withdrawn from antidepressant medications prior to dTMS as required by regulatory authorities. However, in a real-life clinical setting, antidepressant medication that leads to a partial response might be augmented with dTMS treatment. The safety and efficacy of such a strategy was demonstrated in a previous study (13).
In conclusion, the present randomized and placebo-controlled trial demonstrates that dTMS is an effective and tolerable treatment for patients with MDD who have not successfully responded to treatment with antidepressant medications in the current episode. The effects appear durable, with maintenance of efficacy up to 16 weeks. A clinically significant improvement was seen in even the higher treatment-resistant patients.
Acknowledgments
This study was supported by Brainsway, which produces the dTMS H-coil systems.
References
- Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M. Global burden of disease and risk factors. Washington: World Bank; 2006. [PubMed] [Google Scholar]
- Greden JF, et al. The burden of disease for treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl. 16):26–31. [PubMed] [Google Scholar]
- Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. Is it time to introduce repetitive transcranial magnetic stimulation into standard clinical practice for the treatment of depressive disorders? Aust N Zeal J Psychiatry. 2003;37:5–11. doi: 10.1046/j.1440-1614.2003.01115.x. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM. Rapid-rate transcranial magnetic stimulation and ECT. Convuls Ther. 1994;10:251–4. [PubMed] [Google Scholar]
- Roth Y, Amir A, Levkovitz Y, et al. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J Clin Neurophysiol. 2007;24:31–8. doi: 10.1097/WNP.0b013e31802fa393. [DOI] [PubMed] [Google Scholar]
- Zangen A, Roth Y, Voller B, et al. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. 2005;116:775–9. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. 2002;19:361–70. doi: 10.1097/00004691-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Roth Y, Pell GS, Chistyakov AV, et al. Motor cortex activation by H-coil and figure-8 coil at different depths. Combined motor threshold and electric field distribution study. Clin Neurophysiol. 2014;125:336–43. doi: 10.1016/j.clinph.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Bersani FS, Girardi N, Sanna L, et al. Deep transcranial magnetic stimulation for treatment-resistant bipolar depression: a case report of acute and maintenance efficacy. Neurocase. 2013;19:451–7. doi: 10.1080/13554794.2012.690429. [DOI] [PubMed] [Google Scholar]
- Harel EV, Rabany L, Deutsch L, et al. H-coil repetitive transcranial magnetic stimulation for treatment resistant major depressive disorder: an 18-week continuation safety and feasibility study. World J Biol Psychiatry. 2014;15:298–306. doi: 10.3109/15622975.2011.639802. [DOI] [PubMed] [Google Scholar]
- Isserles M, Rosenberg O, Dannon P, et al. Cognitive-emotional reactivation during deep transcranial magnetic stimulation over the prefrontal cortex of depressive patients affects antidepressant outcome. J Affect Disord. 2011;128:235–42. doi: 10.1016/j.jad.2010.06.038. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Harel EV, Roth Y, et al. Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimul. 2009;2:188–200. doi: 10.1016/j.brs.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Roth Y, Harel EV, et al. A randomized controlled feasibility and safety study of deep transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:2730–44. doi: 10.1016/j.clinph.2007.09.061. [DOI] [PubMed] [Google Scholar]
- Dell'Osso B, D'Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27:141–4. doi: 10.1097/YCT.0b013e3181f66601. [DOI] [PubMed] [Google Scholar]
- Dlabac-de Lange JJ, Knegtering R, Aleman A. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry. 2010;71:411–8. doi: 10.4088/JCP.08r04808yel. [DOI] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–84. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–16. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- Herwig U, Fallgatter AJ, Höppner J, et al. Antidepressant effects of augmentative transcranial magnetic stimulation: randomised multicentre trial. Br J Psychiatry. 2007;191:441–8. doi: 10.1192/bjp.bp.106.034371. [DOI] [PubMed] [Google Scholar]
- O'Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48:962–70. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, et al. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012;62:2232–43. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, et al. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl.16):10–7. [PubMed] [Google Scholar]
- Isserles M, Shalev AY, Roth Y, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder – a pilot study. Brain Stimul. 2013;6:377–83. doi: 10.1016/j.brs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2012;66:151–60. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry. 2000;47:314–24. doi: 10.1016/s0006-3223(99)00254-1. [DOI] [PubMed] [Google Scholar]



