Abstract
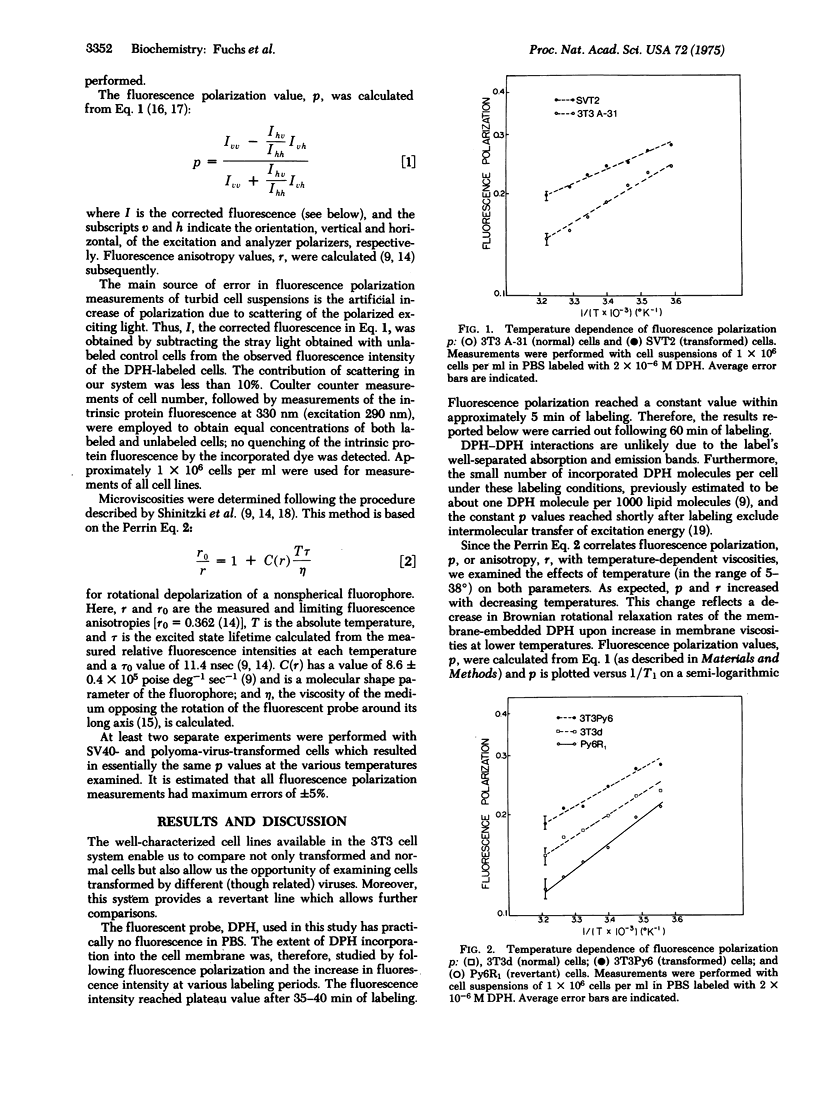
Studies of the fluorescence polarization of 1,6-diphenyl-1,3,5-hexatriene in membranes of normal, transformed, and revertant 3T3 cells allowed estimation of microviscosities (eta) of the lipid bilayers of these cells. Fluorescence polarization values observed with normal cells were significantly lower than those observed with cells transformed either by polyoma virus or by simian virus 40. The values of fluorescence polarization obtained with revertant Py6R1 cells were lower than those obtained with the normal cells. The calculated microviscosities (eta) show a 50% increase upon transformation. Possible correlations between the bilayer viscosity and the mobility of the receptors in the membrane are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Bühler G., Solomon F. Properties of particle movement in the plasma membrane of 3T3 mouse fibroblasts. Exp Cell Res. 1974 Apr;85(2):225–233. doi: 10.1016/0014-4827(74)90121-9. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L., Burger M. M. Absence of a cell membrane alteration function in non-transforming mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Oct;67(2):929–934. doi: 10.1073/pnas.67.2.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L., Goldman E. Indirect complementation of a nontransforming mutant of polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):41–44. doi: 10.1101/sqb.1974.039.01.008. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN R. F., BOWMAN R. L. FLUORESCENCE POLARIZATION: MEASUREMENT WITH ULTRAVIOLET-POLARIZING FILTERS IN A SPECTROPHOTOFLUOROMETER. Science. 1965 Feb 12;147(3659):729–732. doi: 10.1126/science.147.3659.729. [DOI] [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Gaffney B. J. Fatty acid chain flexibility in the membranes of normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1975 Feb;72(2):664–668. doi: 10.1073/pnas.72.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M. Cholesterol as a bioregulator in the development and inhibition of leukemia. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4229–4231. doi: 10.1073/pnas.71.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M., Sachs L. Rotational relaxation time of concanavalin A bound to the surface membrane of normal and malignant transformed cells. J Mol Biol. 1973 Dec 5;81(2):245–253. doi: 10.1016/0022-2836(73)90192-7. [DOI] [PubMed] [Google Scholar]
- Kury P. G., Ramwell P. W., McConnell H. M. The effect of prostaglandins E1 and E2 on the human erythrocyte as monitored by spin labels. Biochem Biophys Res Commun. 1974 Jan 23;56(2):478–483. doi: 10.1016/0006-291x(74)90867-5. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. The relationship of concanavalin A binding to lectin-initiated cell agglutination. J Cell Biol. 1973 Oct;59(1):134–142. doi: 10.1083/jcb.59.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit V. A., Edidin M. Lateral phase separation of lipids in plasma membranes: effect of temperature on the mobility of membrane antigens. Science. 1974 Jun 14;184(4142):1183–1185. doi: 10.1126/science.184.4142.1183. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B., Gitler C. Microviscosity of the cell membrane. Biochim Biophys Acta. 1972 Oct 23;288(1):231–236. doi: 10.1016/0005-2736(72)90242-8. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem. 1974 Apr 25;249(8):2652–2657. [PubMed] [Google Scholar]
- Shinitzky M., Dianoux A. C., Gitler C., Weber G. Microviscosity and order in the hydrocarbon region of micelles and membranes determined with fluorescent probes. I. Synthetic micelles. Biochemistry. 1971 May 25;10(11):2106–2113. doi: 10.1021/bi00787a023. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974 Jan 5;85(4):603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]



