Abstract
Background
Prophylactic defibrillator implantation is recommended in dilated, nonischemic heart disease and left ventricular ejection fraction of ≤0.30 to 0.35. Noninvasive testing should improve accuracy in decision making of prophylactic defibrillator implantation.
Methods and Results
We enrolled 60 patients (median age 57 years) with dilated cardiomyopathy and LVEF ≤0·50, and 30 control subjects (median age 59) with LVEF>0·50. The protocol included an initial assessment, a second assessment after 3 years, and a final follow-up: pharmacological baroreflex testing (BRS), short-term spectral analysis of heart rate variability (LF/HF), and long-term time domain analysis (SDNN), exercise Microvolt T-wave alternans (MTWA) and signal-averaged ECG, and corrected QT-time. The median follow-up was 7 years. Endpoints were cardiac death, resuscitated cardiac arrest and arrhythmic death.
Cardiac death was observed in 21 patients. Resuscitated cardiac arrest and arrhythmic death due to ventricular tachyarrhythmias (VTs) ≥ 240/min was observed in 7 and 10 patients, respectively. In the single time point analysis, MTWA, BRS and SDNN at initial testing added significant information regarding cardiac death. MTWA added information on resuscitated cardiac arrest or arrhythmic death at multiple time points (P<0·001). False negative MTWA results were seen in 8% of patients.
Conclusions
Non-invasive testing and LVEF could not reliably identify those DCM patients at risk of fatal VTs. Therefore, the strategy to confine prophylactic ICD implantation to DCM patients with severely reduced LV function should be reconsidered.
Keywords: Arrhythmic death, risk stratification, dilated cardiomyopathy
Introduction
Non-ischemic, dilated cardiomyopathy (DCM) is a common cause of congestive heart failure and is a risk factor for sudden cardiac death and especially arrhythmic death (AD).1 Current guidelines for the primary prevention with implantable cardioverter-defibrillators (ICDs) in patients with DCM are based on a few landmark trials.2–4 Risk stratification is based on virtual borders of left ventricular ejection fraction (0·30–0·35 or less) and non-sustained ventricular tachycardia as arrhythmia risk predictors.5 Holter monitoring has demonstrated a prevalence of episodes of non-sustained ventricular tachycardia of 49–60%. In contrast, the annual incidence of sustained ventricular tachycardia is remarkably low, namely 3–4%.6, 7 The high proportion of the remaining patients without cardiac events8 raises the question how to discriminate high- and low-risk patients. In patients with ischemic and non-ischemic cardiomyopathy noninvasive assessments of autonomic tone and cardiac electrical substrate have been developed to identify high-risk patients.9–13 Combining different assessments of autonomic tone10, 13 or combining different assessments of electrical substrate14 have given conflicting results in DCM. The objective of this paper is to evaluate the clinical relevance of a combined assessment of autonomic tone plus cardiac electrical substrate at multiple time points for the prediction of fatal arrhythmias in patients with DCM.
Methods
This prospective observational, observer-blind study was approved and monitored by the local ethics committee. All participants gave written consent. Screening, enrollment (2002–2003), clinical testing and follow-up (2003–2013) were conducted at the Medical University of Vienna, Austria. Test scoring, interpretation and statistical data processing (2009–2013) underwent a blinded assessment at the Vanderbilt Autonomic Dysfunction Center, Nashville, USA. After enrollment, optimal medical treatment was established. Test results were not disclosed to participants or their physicians. Antiarrhythmic drug therapy and ICD implantation were not guided by the study.
Population
A total of 60 patients with non-ischemic, dilated cardiomyopathy (DCM) and 30 control subjects (CSU) without DCM were studied. Patients were eligible to participate if they were at least 18 years of age, had a LVEF of 0·50 or less (DCM) or more than 0·50 (CSU), had recently undergone coronary angiography with ventriculography as standard use of care independently from study participation, echocardiography, magnetic resonance imaging at the physician’s discretion, had no history of sustained ventricular arrhythmia or permanent atrial fibrillation and were non-dependent on ventricular pacing.
Cardiac Electrical Substrates
Corrected QT-times with Bazett´s formula (QTC) were obtained using automated analysis systems. Non-sustained ventricular tachycardia (nsVT) and signal-averaged ECG (SAECG) were obtained using 24-hour Holter monitoring devices. NsVT was defined as a run of ≥ 3 ventricular beats lasting no longer than 30 seconds without syncope. SAECG were obtained by time-domain analysis. Late potentials were present if 2 of the following parameters were fulfilled: QRSf >114 ms, LAS40 >38 ms, and RMS40 <20 AV.15 Microvolt T-wave alternans (MTWA) was assessed during submaximal bicycle exercise (CH 2000®, Cambridge Heart Inc., Bedford, MA, USA). Patients were taking their regular cardiovascular medications, including beta-blockers. The latter were withheld on the day of the investigation. The MTWA test was automatically interpreted (Version D10) and verified.16
Autonomic Function
For baroreflex testing, continuous finger blood pressure by the vascular unloading technique, beat-to-beat stroke volume by impedance cardiography, and 4 lead ECG was recorded with the Task Force Monitor_system (TFM®, CNSystems, Graz, Austria).17 After a resting period in supine position of at least 30 min, three bolus injections of Phenylephrine (2–4 µg/kg) were given intravenously at intervals of 10 min to raise systolic blood pressure by 15–40 mmHg.18 A regression line was fit between data pairs of blood pressure and R-R intervals. Only regression lines with a R2 coefficient either more than 0·80 or statistical significant linear regression (F-test on the regression model, p<0·05) were accepted. Baroreceptor reflex sensitivity (BRS, msec/mmHg) was obtained by calculating the mean from at least three slope determinations. Short-time spectral measurement of heart rate variability (HRV) was obtained during a 10-minutes resting period in supine position before pharmacological baroreflex testing.19 Time domain measures and long-term spectral parameters of HRV were obtained from 24 hour Holter recordings. The standard deviation of all normal-to-normal R–R intervals (SDNN) was used to calculate time domain parameter for HRV.19
End-points, classification of death
The primary end-point was time to arrhythmic death (AD) or resuscitated cardiac arrest (RCA). Cardiac death (CD) and all-cause mortality were secondary endpoints. Deaths were categorized utilizing an adapted form of the Hinkle classification.20 Appropriate ICD therapy without VT acceleration that failed to save the patient’s life at the time of arrhythmias was classified as AD.21 An RCA was ventricular fibrillation or VT >240 bpm leading to syncope before ICD therapy, and multiple slower VT episodes (electrical storm) leading to syncope and ICD discharge without ICD therapy related acceleration. All other ICD therapies due to VT <240 bpm were not taken as surrogate for AD.
Sample size justification and statistical analysis
The accrual interval will be one year with additional follow-up of 10 years. Based on our prior knowledge and literature review, the median survival time for the control patients was 15 years. If the true ratio (hazard ratio) of mortality for control subjects relative to dilated cardiomyopathy patients is 2.5, we will need to study 56 dilated cardiomyopathy patients and 28 control patients to be able to reject the null hypothesis that the dilated cardiomyopathy and control survival curves are equal with 80% power. The Type I error probability (two-sided) associated with this test of this null hypothesis is 0.05. Data were summarized as median with 1st and 3rd Quartiles or percentage (N) for continuous and categorical variables. Wilcoxon rank-sum test or Kruskal-Wallis test was used for continuous variables, chi-square test was used for categorical variables. Survival curves were estimated by cumulative incidence treating death as the competing event and the k-sample test was used.22 To assess whether MTWA, BRS, or SDNN adds prognostic value in addition to LVEF, we fit four separate Cox proportional-hazards models (all adjusted for age and gender). The added prognostic value was assessed by likelihood ratio test. All tests were two-tailed with 5% significance level. All analyses were performed at the Vanderbilt University School of Medicine, Department of Biostatistics, Nashville, TN, U.S.A. using an open source software R version 3.0.2.
Results
A total of 60 patients with LVEF of 0·50 or less and 30 control patients with LVEF of more than 0·50 underwent serial testing. (Figure 1, upper part). Distribution of LVEF and clinical characteristics of the two patient groups are listed in Table 1. The median LVEF was 0·32 in DCM patients. During a median follow-up of 7·03 years, 23 deaths in the DCM group and one death in the control group were observed. Of these, 21 were categorized as cardiac deaths of where 10 were considered as arrhythmic. Seven patients had a RCA (2 VF and 5 fast VT). Four (Figure 1, marked*) out of these 7 patients died later an AD although an ICD was implanted. The remaining 3 patients together with those 10 patients who died an AD compromise 13 patients where life-threatening VTs were recorded. For further analysis the first event (either RCA or AD) was taken only. In 7 out of 10 ICD recipients (70%) ICD therapies (7 RCA events and 1 VT < 220/min events) were recorded. In 4 patients the ICD was unable to stop an electrical storm and the patient died an AD. Reasons for not implanting an ICD during later follow-up in a patient with LVEF of 0·30 or less were patient´s refusal or the treatment policy of the attending physician. For mortality rates see Table 2.
Figure 1.
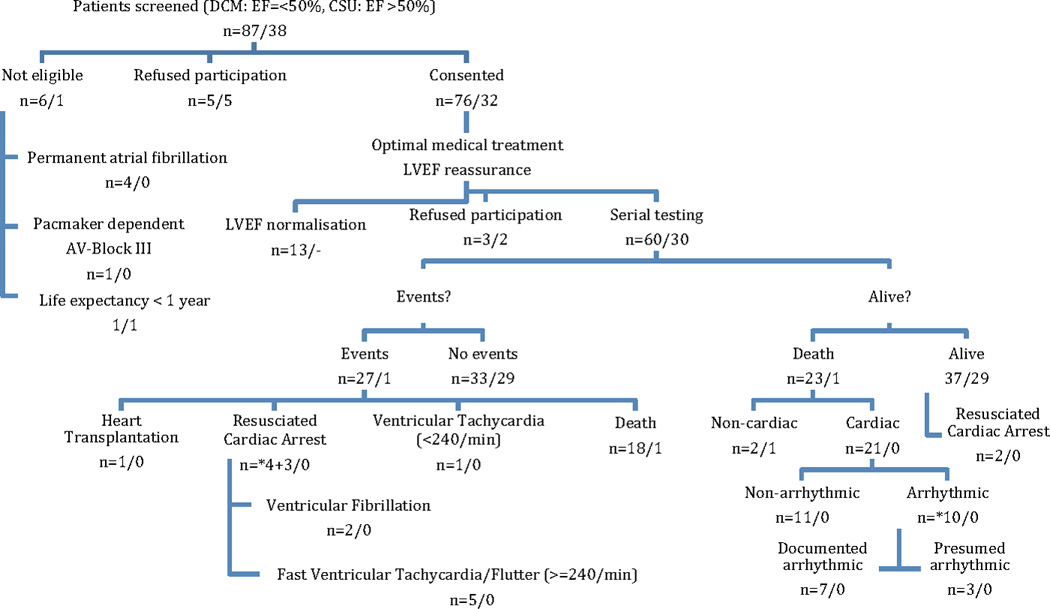
Patient Selection and Outcomes (2003–2012). Ineligibility was mainly due to permanent atrial fibrillation. Other patients refused participation or LVEF normalized. During a median follow-up of 7 years, 23+1 deaths were observed. Of these, 21 were categorized as cardiac and 10 as arrhythmic. Thirteen patients had life-threatening ventricular arrhythmias as their first event, namely resuscitated cardiac arrest in 7 patients and arrhythmic death in 6 patients. Out of 7 patients with resuscitated cardiac arrest *4 died later on from arrhythmic death, although an ICD was implanted, one from non-cardiac death and two are still alive.
Table 1.
Clinical characteristics
| Dilated cardiomyopathy n=60 |
Control subjects n=30 |
P-value | |
|---|---|---|---|
| Age, years, Med (range) | 57 (23–74) | 59 (28–73) | 0·59 |
| Male, (%) | 48 (80) | 17 (57) | 0·02 |
| BMI, kg/m2, Med | 27·7 (24·9–30·8) | 26·2 (24·1–29·1) | 0·21 |
| LVEF, %, Med (range) | 32 (13–50) | 67 (54–79) | <0·001 |
| LVEF = 40–50% | 11 (18) | 0 | |
| LVEF = 31–39% | 25 (42) | 0 | |
| LVEF = 25–30% | 16 (27) | 0 | |
| LVEF = < 25% | 8 (13) | 0 | |
| NYHA classification | 0·043 | ||
| NYHA I | 25 (42) | 15 (50) | |
| NYHA II | 24 (40) | 15 (50) | |
| NYHA III | 11 (18) | 0 | |
| Left bundle branch block (>120msec) | 27 (45) | 0 | <0·001 |
| Hypertension | 52 (87) | 23 (77) | 0·23 |
| Diabtes mellitus | 14 (23) | 2 (7) | 0·051 |
| Renal dysfunction | 17 (28) | 0 | 0·001 |
| Stroke | 6 (10) | 1 (3) | 0·27 |
| Syncope | 8 (13) | 1 (3) | 0·14 |
| Non-sustained VT | 12 (24) | 1 (3) | 0·84 |
| Medication | |||
| Beta-blocker | 54 (90) | 18 (60) | <0·001 |
| Sotalol | 1 (2) | 2 (7) | 0·21 |
| Amiodarone | 10 (17) | 0 | 0·018 |
| ACE inhibitors | 51 (85) | 11 (37) | <0·001 |
| Angiotensin receptor blockers | 22 (37) | 8 (27) | 0·34 |
| Diuretics | 38 (63) | 7 (23) | <0·001 |
| Follow-up, years, Med (mean±SD) | 1·8 6·6 6·8 (4·8±2·6) | 8·7 8·7 8·7 (8·5±1) | <0·001 |
| ICD implanted | 10 (17) | 0 | 0·018 |
| CRT-P / -D implanted | 7 (12) / 4 (7)* | 0 | <0·001 |
| ICD therapy | 7 (12) | 0 | 0·036 |
Data are n (%), unless otherwise indicated; Med=median (Q1–Q3); test used: Wilxocon test or chi-square test. Renal dysfunction= persistent serum creatinine >1.2mg/dl.
4 CRT-D upgrades during follow-up
Table 2.
Primary and secondary endpoints
| Dilated Cardiomyopathy, LVEF 0–30%, n=24 |
Dilated Cardiomyopathy, LVEF 31–50%, n=36 |
Per Person Year | Control Subjects, n=30 |
|
|---|---|---|---|---|
| All-cause mortality | n=13 (54%) | n=10 (28%) | 5.5% | n=1 (3%) |
| Cardiac death (CD) | n=14 (58%) | n=7 (19%) | 5% | 0 |
| Resusciated cardiac arrest (RCA) | n=3* (13%) | n=1*+3 (11%) | 1.7% | 0 |
| Arrhythmic death (AD) | n=4 (17%) | n=6 (17%) | 2.4% | 0 |
Out of 7 patients with resuscitated cardiac arrest
4 died later on from arrhythmic death, although an ICD was implanted.
Test results related to primary and secondary endpoints
Table 3a demonstrates that LVEF, first BRS, HRV testing, creatinine and a history of syncope and both MTWA tests were associated with the endpoints of AD or RCA and other forms of death. Table 3b outlines all test results stratified according to the endpoints cardiac (n=21) and non-cardiac death (n=3). Besides LVEF, first BRS and first HRV assessment and both MTWA assessments were associated with cardiac and non-cardiac death. Figure 2 demonstrates that MTWA only was associated with AD or RCA (p<0·001). We fit a Cox proportional-hazards model for the association between EF and time to resuscitated cardiac arrest and arrhythmic death adjusted for age and gender. We then added MTWA, BRS, and SDNN to the EF model one at a time to assess the added prognostic value. The likelihood ratio test comparing the MTWA model, BRS model, and SDNN model to the EF only model revealed that only MTWA (p=0.018) but not BRS (p=0.349) or SDNN (p=0.192) added predictive information. (Table 4) Sensitivity, specificity, positive predictive value and negative predictive value regarding the primary endpoints AD or RCA for MTWA for all patients were 85%, 83%, 50% and 96% regarding 1st assessment (I1) and 100%, 71%, 25% and 100% regarding 2nd assessment (I2), respectively.
Table 3.
| a: Risk factors stratified by primary endpoints for first (I1) and second (I2) investigation. | |||||
|---|---|---|---|---|---|
| N | Alive n=64 |
Arrhythmic death/ resusciated cardiac arrest n=13 |
Death other n=13 |
P-value | |
| Autonomic tone | |||||
| BRS_I1, ms/mmHG, Med | 55 | 10·3 (6·3–18·9) | 6·3 (4·7–12·0) | 4·1 (1·6–8·1) | 0·012 |
| BRS_I2, ms/mmHG, Med | 65 | 10·2 (7·5–19·0) | 6·2 (5·0–10·9) | 14·2 (9·0–24·0) | 0·4 |
| LF/HF_I1, LF to HF ratio, Med | 49 | 2·4 (1·2–4·9) | 2·5 (1·8–3·7) | 1·3 (0·4–2·2) | 0·25 |
| LF/HF_I2, LF to HF ratio, Med | 67 | 3·4 (1·7–5·2) | 2·6 (1·9–3·7) | 5·5 (3·4–5·8) | 0·52 |
| HRV_I1, SDNN, ms, Med | 50 | 130 (100–158) | 102 (80–106) | 65 (56–91) | <0·001 |
| HRV_I2, SDNN, ms, Med | 53 | 121 (97–143) | 104 (94–123) | 99 (78–113) | 0·36 |
| Electrical substrate | |||||
| SAECG_I1 | 55 | 0·68 | |||
| Negative | 25 (69) | 6 (60) | 5 (56) | ||
| Non-negative | 11 (31) | 4 (40) | 4 (44) | ||
| SAECG_I2 | 57 | 0·58 | |||
| Negative | 30 (62) | 4 (67) | 1 (33) | ||
| Non-negative | 18 (38) | 2 (33) | 2 (67) | ||
| MTWA_I1 | 90 | <0·001 | |||
| Negative | 53 (83) | 2 (15) | 10 (77) | ||
| Non-negative | 11 (17) | 11 (85) | 3 (23) | ||
| MTWA_I2 | 73 | ||||
| Negative | 45 (71) | 0 | 3 (75) | 0·002 | |
| Non-negative | 18 (29) | 6 (100) | 1 (25) | ||
| Clinical variables | |||||
| History of syncope_I1 | 90 | 4 (6) | 4 (31) | 1 (8) | 0·026 |
| Non-sustained VT_I1 | 55 | 7 (20) | 5 (45) | 1 (11) | 0·14 |
| NYHA_I1 classification | 90 | <0·001 | |||
| NYHA I | 32 (50) | 6 (46) | 2 (15) | ||
| NYHA II | 30 (47) | 5 (38) | 4 (31) | ||
| NYHA III | 2 (3) | 2 (15) | 7 (54) | ||
| LVEF_I1, %, Med | 90 | 46 (32–67) | 33 (29–39) | 26 (22–35) | <0·001 |
| LVEF_I2, %, Med | 73 | 47 (36–67) | 31 (26–38) | 31 (24–41) | 0·006 |
| QTC_I1, ms, Med | 48 | 453 (426–466) | 464 (425–486) | 478 (466–494) | 0·18 |
| QTC_I2, ms, Med | 41 | 435 (403–465) | 459 (446–460) | 462 (452–471) | 0·41 |
| Crea_I1, mg/dl, Med | 38 | 1·0 (0·9–1·2) | 1·1 (1·1–1·3) | 1·2 (1·2–1·3) | 0·51 |
| Crea_I2, mg/dl, Med | 33 | 1·0 (0·9–1·2) | 1·1 (1·1–1·3) | 1·2 (1·2–1·3) | 0·44 |
| b: Risk factors stratified by secondary endpoints for first (I1) and second (I2) investigation | |||||
|---|---|---|---|---|---|
| N | Alive, n=66 |
Cardiac death, n=21 |
Non-cardiac death, n=3 |
P- value |
|
| Autonomic tone | |||||
| BRS_I1, ms/mmHG, Med | 55 | 10·3 (6·6–19·8) | 4·7 (1·7–8·3) | 8·2 (8·1–8·4) | 0·006 |
| BRS_I2, ms/mmHG, Med | 66 | 12·1 (7·0–18·8) | 13·8 (9·4–20·9) | 9·0 (6·4–11·6) | 0·7 |
| LF/HF_I1, LF to HF ratio, Med | 49 | 2·5 (1·3–5·1) | 2·0 (0·9–2·6) | 3·7 (2·0–5·4) | 0·3 |
| LF/HF_I2, LF to HF ratio, Med | 67 | 3·4 (1·7–5·1) | 2·3 (1·3–2·9) | 5·8 (5·6–5·9) | 0·14 |
| HRV_I1, SDNN, ms, Med | 50 | 130 (100–158) | 84 (63–102) | 96 (76–115) | 0·001 |
| HRV_I2, SDNN, ms, Med | 53 | 120 (96–142) | 125 (110–138) | 78 (68–89) | 0·25 |
| Electrical substrate | |||||
| MTWA_I1 | 90 | <0·001 | |||
| Negative | 54 (82) | 8 (38) | 3 (100) | ||
| Non-negative | 12 (18) | 13 (62) | 0 | ||
| MTWA_I2 | 73 | ||||
| Negative | 45 (69) | 1 (17) | 2 (100) | 0·02 | |
| Non-negative | 20 (31) | 5 (83) | 0 | ||
| Clinical variables | |||||
| NYHA_I1 classification | 90 | <0·001 | |||
| NYHA I | 33 (50) | 6 (29) | 1 (33) | ||
| NYHA II | 31 (47) | 6 (29) | 2 (67) | ||
| NYHA III | 2 (3) | 9 (43) | 0 | ||
| LVEF_I1, %, Med | 90 | 46 (32–67) | 29 (25–35) | 36 (36–49) | <0·001 |
| LVEF_I2, %, Med | 73 | 47 (35–67) | 26 (21–28) | 47 (41–53) | 0·003 |
Data are n (%), unless otherwise indicated; Med=median (Q1–Q3); I1, I2 Investigation 1 and 2, N is the number of non-missing values; tests used: Pearson Chi-square test, Kruskal-Wallis test.
Figure 2.
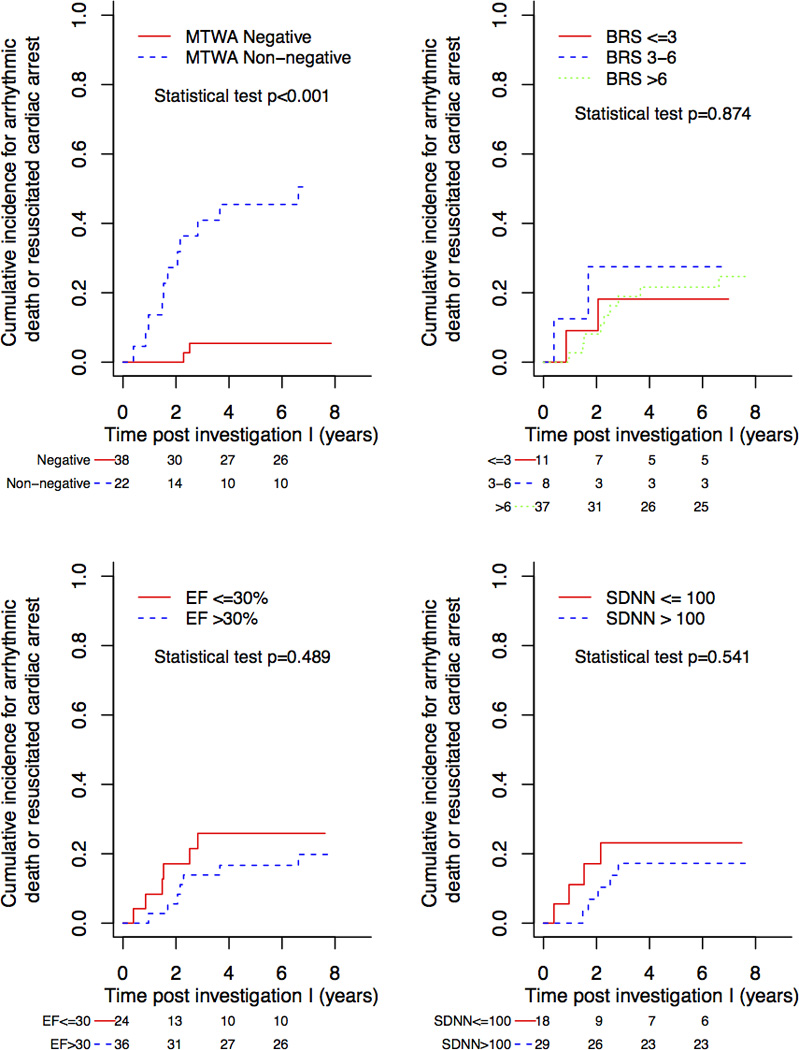
Kaplan-Meier curves showing the association between risk non-invasive tests and arrhythmic death or resuscitated cardiac arrest for dilated cardiomyopathy patients only.
Table 4.
Cox proportional-hazards model using baseline clinical variables for arrhythmic death / resuscitated death in dilated cardiomyopathy patients alone.
| Parameter | Hazard Ratio |
95%CI lower |
95% CI upper |
p-value |
|---|---|---|---|---|
| (LV)EF (10% increase) | 0.97 | 0.33 | 2.83 | 0.954 |
| MTWA (Non-negative:negative) | 5.37 | 1.61 | 46.71 | 0.018 |
| BRS (1 unit increase) | 1.04 | 0.95 | 1.14 | 0.349 |
| SDNN (10 units increase) | 0.86 | 0.69 | 1.08 | 0.192 |
Discussion
Our main findings are clinically important for primary prophylactic implantation of defibrillators: 1) during a median follow-up period of 7 years, the annual rate of fatal arrhythmic events is 1·7% and 2·4% for resuscitated cardiac arrest and arrhythmic death, respectively. 2) Among all noninvasive tests and in addition to LVEF an abnormal MTWA may complement risk stratification, although with false negative test results in 8% of patients.
Rationale for combined and multiple assessment
Our primary endpoint was AD or RCA. This is in contrast to other studies using sudden death as endpoint that is not synonymous with a fatal ventricular tachyarrhythmia. The rational for multiple testing and a projected follow-up of 10 years was the anticipated low risk of fatal arrhythmias in DCM patients.6 An annual total mortality rate of 5·5% compares favorably with data from previous studies.8, 23 We found that 44% of all deaths were arrhythmic. This is in agreement with Bardy et al. who demonstrated that sudden cardiac death accounts for up to 50% of all deaths in DCM patients.24 In DEFINITE the AD rate in ICD treated patients was 11%.3 This discrepancy can be explained by the different length of follow-up (mean 29 months versus median 7 years). We are in agreement with Ellenbogen et al.25 who observed that twice as many patients experienced appropriate ICD therapies compared with the patients dying a sudden, arrhythmic death: we observed that 7/10 patients had ICD intervention and 4/10 patients died an AD. Both CAT2 and AMIOVIRT8 have failed to demonstrate a survival benefit in patients treated with prophylactic ICDs. High numbers of ICD implants to save one life (14 ICDs within 5 years) have been already postulated in SCD-HeFT.4 In our study 13 out of 60 patients died an AD during follow-up.
MTWA
The role of MTWA in predicting arrhythmic death in DCM is controversial in the literature. In good accordance with our data most studies have shown that abnormal MTWA test results indicate an increased risk of death or fatal arrhythmias.11, 12, 14, 26 However, in most studies one of the reasons for the low PPV of MTWA testing in AD prediction is the reported high rates of abnormal results (37–51%) and the low event rate within a short follow-up period. In contrast, in our study the high event rate together with the overall high rate of negative MTWA test results contributed to the PPV of 50%. However, depending on the point in time of MTWA assessment the percentage of patients missed at risk is as high as 8%. In addition, in our population 5% of patients could not be included due to permanent atrial fibrillation.
SAECG and non-sustained VT
The role of SAECG is controversial in the literature. Some studies have indicated the usefulness of SAECG for risk stratification27, 28 whereas in others29 it was not a significant predictor for arrhythmias. Documentation of nsVT in Holter recordings has been shown to predict mortality in some studies6, 30 whereas others31 found no correlation. The role of PVS is unclear32, 33 and positive studies are rare.34 In the present study neither SAECG nor the presence of nsVT on Holter recordings were found to be useful in fatal arrhythmic risk prediction.
Markers of autonomic tone: HRV and BRS
It is common that HRV in heart failure patients is assessed by time domain analysis (SDNN) of Holter recordings and spectral analysis (LF/HF) of long-term Holter recordings. In our study, we performed additionally pharmacological BRS testing with phenylephrine bolus administration and together with short-term spectral analysis of prior data during resting supine baseline. Several studies indicated the usefulness of these markers in risk stratification3, 9, 12 whereas some studies also did not identify significant findings for prediction of arrhythmic mortality.5, 13 In our study only the first HRV and BRS were found to be predictive for cardiac death and for AD or RCA.
Role of LVEF
The reason to choose an upper LVEF limit of 0·50 was based on the ATRAMI study,18 where a relative risk of 2·5 for cardiac mortality in patients with an LVEF between 0·35 and 0·50 compared with patients with LVEF 0·50 or more was found. Primary preventive ICD trials used LVEF as main inclusion criterion with the hypothesis that fatal arrhythmic events play a major role. Of note, these studies were not designed to evaluate LVEF as risk stratification technique. One of our findings is that reduced LVEF is associated with increased cardiac mortality but not with increased AD or RCA. The majority of patients with sudden cardiac death have an LVEF of 0·30 or more and even above 0·40, respectively.35 In our study, 22% of patients with an LVEF of 0·30 or more had fatal ventricular arrhythmias as their first event.
Study limitations
This was a non-randomized, prospective, observational study performed to assess the risk of fatal arrhythmias during long-term follow-up. It is still possible that some of the events, defined as RCA, might not have led to death. Another limitation is the small sample size, which does not allow making firm conclusions. Therefore, any finding should be labeled as pilot study results.
Conclusions
One quarter of patients with non-ischemic, dilated heart disease and LVEF of 0·50 or less experience fatal arrhythmias. The occurrence of fatal arrhythmias is not dependent on the degree of LVEF reduction. In dilated cardiomyopathy none of the non-invasive risk stratification techniques appear useful in clinical decision-making. Therefore, the strategy to confine prophylactic ICD implantation to DCM patients with severely reduced LV function should be reconsidered.
Acknowledgments
Funding Sources: Research reported in this publication was partly supported by the National Center for Advancing Translational Science of the National Institute of Health (NIH) under Award Number UL1TR000445 and NIH 5P01 HL56693. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564–1575. doi: 10.1056/NEJM199412083312307. [DOI] [PubMed] [Google Scholar]
- 2.Bansch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, Block M, Gietzen F, Berger J, Kuck KH. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT) Circulation. 2002;105:1453–1458. doi: 10.1161/01.cir.0000012350.99718.ad. [DOI] [PubMed] [Google Scholar]
- 3.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation I. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 4.Olshansky B, Wood F, Hellkamp AS, Poole JE, Anderson J, Johnson GW, Boineau R, Domanski MJ, Mark DB, Lee KL, Bardy GH. Investigators SC-H. Where patients with mild to moderate heart failure die: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Am Heart J. 2007;153:1089–1094. doi: 10.1016/j.ahj.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Grimm W, Christ M, Bach J, Muller HH, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883–2891. doi: 10.1161/01.CIR.0000100721.52503.85. [DOI] [PubMed] [Google Scholar]
- 6.Meinertz T, Hofmann T, Kasper W, Treese N, Bechtold H, Stienen U, Pop T, Leitner ER, Andresen D, Meyer J. Significance of ventricular arrhythmias in idiopathic dilated cardiomyopathy. Am J Cardiol. 1984;53:902–907. doi: 10.1016/0002-9149(84)90522-8. [DOI] [PubMed] [Google Scholar]
- 7.Pezawas T, Grimm M, Ristl R, Kivaranovic D, Moser FT, Laufer G, Schmidinger H. Primary preventive cardioverter-defibrillator implantation (Pro-ICD) in patients awaiting heart transplantation. A prospective, randomized, controlled 12-years follow-up study. Transpl Int. 2014 Aug 31; doi: 10.1111/tri.12436. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Strickberger SA, Hummel JD, Bartlett TG, Frumin HI, Schuger CD, Beau SL, Bitar C, Morady F. Investigators A. Amiodarone versus implantable cardioverter-defibrillator:randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia--AMIOVIRT. J Am Coll Cardiol. 2003;41:1707–1712. doi: 10.1016/s0735-1097(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 9.Fauchier L, Babuty D, Cosnay P, Fauchier JP. Prognostic value of heart rate variability for sudden death and major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1999;33:1203–1207. doi: 10.1016/s0735-1097(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann J, Grimm W, Menz V, Muller HH, Maisch B. Heart rate variability and baroreflex sensitivity in idiopathic dilated cardiomyopathy. Heart. 2000;83:531–538. doi: 10.1136/heart.83.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klingenheben T, Zabel M, D'Agostino RB, Cohen RJ, Hohnloser SH. Predictive value of T-wave alternans for arrhythmic events in patients with congestive heart failure. Lancet. 2000;356:651–652. doi: 10.1016/s0140-6736(00)02609-x. [DOI] [PubMed] [Google Scholar]
- 12.Hohnloser SH, Klingenheben T, Bloomfield D, Dabbous O, Cohen RJ. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: results from a prospective observational study. J Am Coll Cardiol. 2003;41:2220–2224. doi: 10.1016/s0735-1097(03)00467-4. [DOI] [PubMed] [Google Scholar]
- 13.Grimm W, Christ M, Sharkova J, Maisch B. Arrhythmia risk prediction in idiopathic dilated cardiomyopathy based on heart rate variability and baroreflex sensitivity. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S202–S206. doi: 10.1111/j.1540-8159.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- 14.Salerno-Uriarte JA, De Ferrari GM, Klersy C, Pedretti RF, Tritto M, Sallusti L, Libero L, Pettinati G, Molon G, Curnis A, Occhetta E, Morandi F, Ferrero P, Accardi F. Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: results of the ALPHA Study. J Am Coll Cardiol. 2007;50:1896–1904. doi: 10.1016/j.jacc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Breithardt G, Cain ME, el-Sherif N, Flowers NC, Hombach V, Janse M, Simson MB, Steinbeck G. Standards for analysis of ventricular late potentials using high-resolution or signal-averaged electrocardiography. A statement by a Task Force Committee of the European Society of Cardiology, the American Heart Association, and the American College of Cardiology. Circulation. 1991;83:1481–1488. doi: 10.1161/01.cir.83.4.1481. [DOI] [PubMed] [Google Scholar]
- 16.Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol. 2002;13:502–512. doi: 10.1046/j.1540-8167.2002.00502.x. [DOI] [PubMed] [Google Scholar]
- 17.Gratze G, Fortin J, Holler A, Grasenick K, Pfurtscheller G, Wach P, Schonegger J, Kotanko P, Skrabal F. A software package for non-invasive, real-time beat-to-beat monitoring of stroke volume, blood pressure, total peripheral resistance and for assessment of autonomic function. Comput Biol Med. 1998;28:121–142. doi: 10.1016/s0010-4825(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 18.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 19.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 20.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 21.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death. Structure, function, and time-dependence of risk. Circulation. 1992;85:I2–I10. [PubMed] [Google Scholar]
- 22.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 23.Schaer B, Theuns DA, Sticherling C, Szili-Torok T, Osswald S, Jordaens L. Effect of implantable cardioverter-defibrillator on left ventricular ejection fraction in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2010;106:1640–1645. doi: 10.1016/j.amjcard.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Sudden Cardiac Death in Heart Failure Trial I. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 25.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation I. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776–782. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- 26.Molon G, Cohen RJ, de Santo T, Costa A, Barbieri E. Clinical use of microvolt T-wave alternans in patients with depressed left ventricular function eligible for ICD implantation: mortality outcomes after long term follow-up. Int J Cardiol. 2013;168:3038–3040. doi: 10.1016/j.ijcard.2013.04.133. [DOI] [PubMed] [Google Scholar]
- 27.Mancini DM, Wong KL, Simson MB. Prognostic value of an abnormal signal-averaged electrocardiogram in patients with nonischemic congestive cardiomyopathy. Circulation. 1993;87:1083–1092. doi: 10.1161/01.cir.87.4.1083. [DOI] [PubMed] [Google Scholar]
- 28.Fauchier L, Babuty D, Cosnay P, Poret P, Rouesnel P, Fauchier JP. Long-term prognostic value of time domain analysis of signal-averaged electrocardiography in idiopathic dilated cardiomyopathy. Am J Cardiol. 2000;85:618–623. doi: 10.1016/s0002-9149(99)00821-8. [DOI] [PubMed] [Google Scholar]
- 29.Galinier M, Albenque JP, Afchar N, Fourcade J, Massabuau P, Doazan JP, Legoanvic C, Fauvel JM, Bounhoure JP. Prognostic value of late potentials in patients with congestive heart failure. Eur Heart J. 1996;17:264–271. doi: 10.1093/oxfordjournals.eurheartj.a014844. [DOI] [PubMed] [Google Scholar]
- 30.Becker R, Haass M, Ick D, Krueger C, Bauer A, Senges-Becker JC, Voss F, Hilbel T, Niroomand F, Katus HA, Schoels W. Role of nonsustained ventricular tachycardia and programmed ventricular stimulation for risk stratification in patients with idiopathic dilated cardiomyopathy. Basic Res Cardiol. 2003;98:259–266. doi: 10.1007/s00395-003-0398-7. [DOI] [PubMed] [Google Scholar]
- 31.Singh SN, Fisher SG, Carson PE, Fletcher RD. Prevalence and significance of nonsustained ventricular tachycardia in patients with premature ventricular contractions and heart failure treated with vasodilator therapy. Department of Veterans Affairs CHF STAT Investigators. J Am Coll Cardiol. 1998;32:942–947. doi: 10.1016/s0735-1097(98)00338-6. [DOI] [PubMed] [Google Scholar]
- 32.Grimm W, Hoffmann J, Menz V, Luck K, Maisch B. Programmed ventricular stimulation for arrhythmia risk prediction in patients with idiopathic dilated cardiomyopathy and nonsustained ventricular tachycardia. J Am Coll Cardiol. 1998;32:739–745. doi: 10.1016/s0735-1097(98)00306-4. [DOI] [PubMed] [Google Scholar]
- 33.Pezawas T, Stix G, Kastner J, Wolzt M, Mayer C, Moertl D, Schmidinger H. Unexplained syncope in patients with structural heart disease and no documented ventricular arrhythmias: value of electrophysiologically guided implantable cardioverter defibrillator therapy. Europace. 2003;5:305–312. doi: 10.1016/s1099-5129(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 34.Gatzoulis KA, Vouliotis AI, Tsiachris D, Salourou M, Archontakis S, Dilaveris P, Gialernios T, Arsenos P, Karystinos G, Sideris S, Kallikazaros I, Stefanadis C. Primary prevention of sudden cardiac death in a nonischemic dilated cardiomyopathy population: reappraisal of the role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2013;6:504–512. doi: 10.1161/CIRCEP.113.000216. [DOI] [PubMed] [Google Scholar]
- 35.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–1209. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]


