Abstract
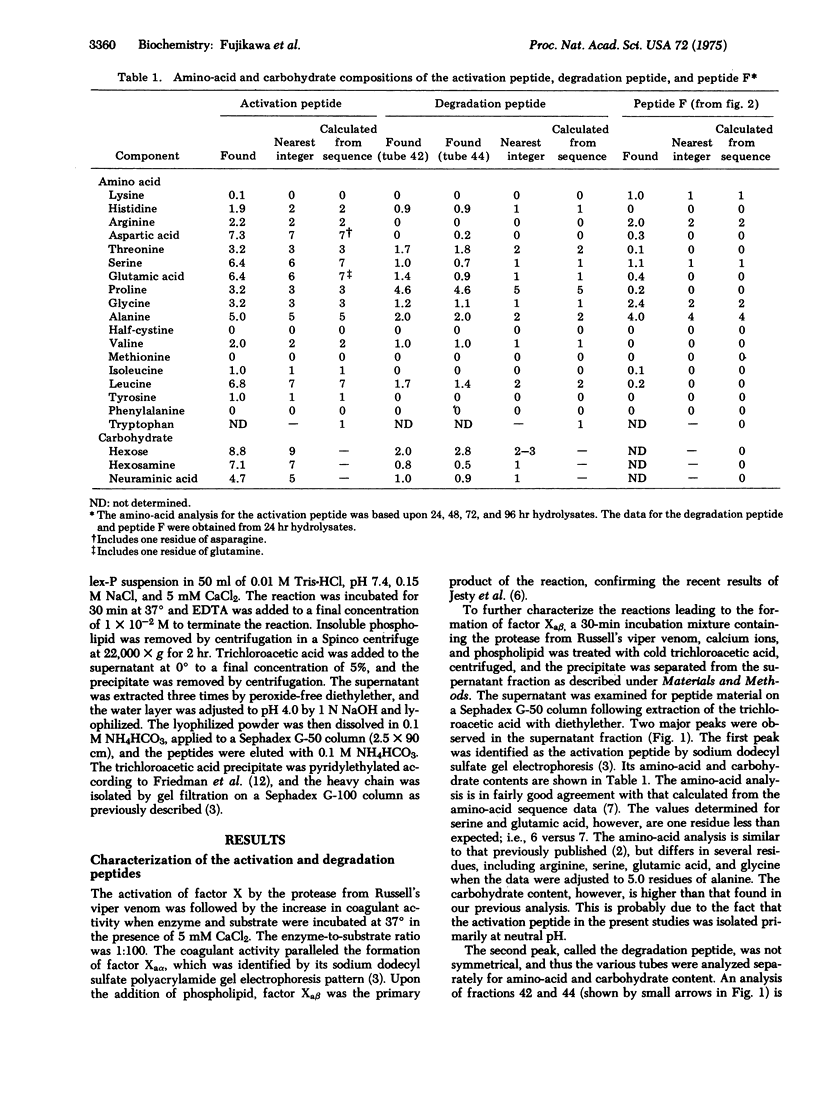
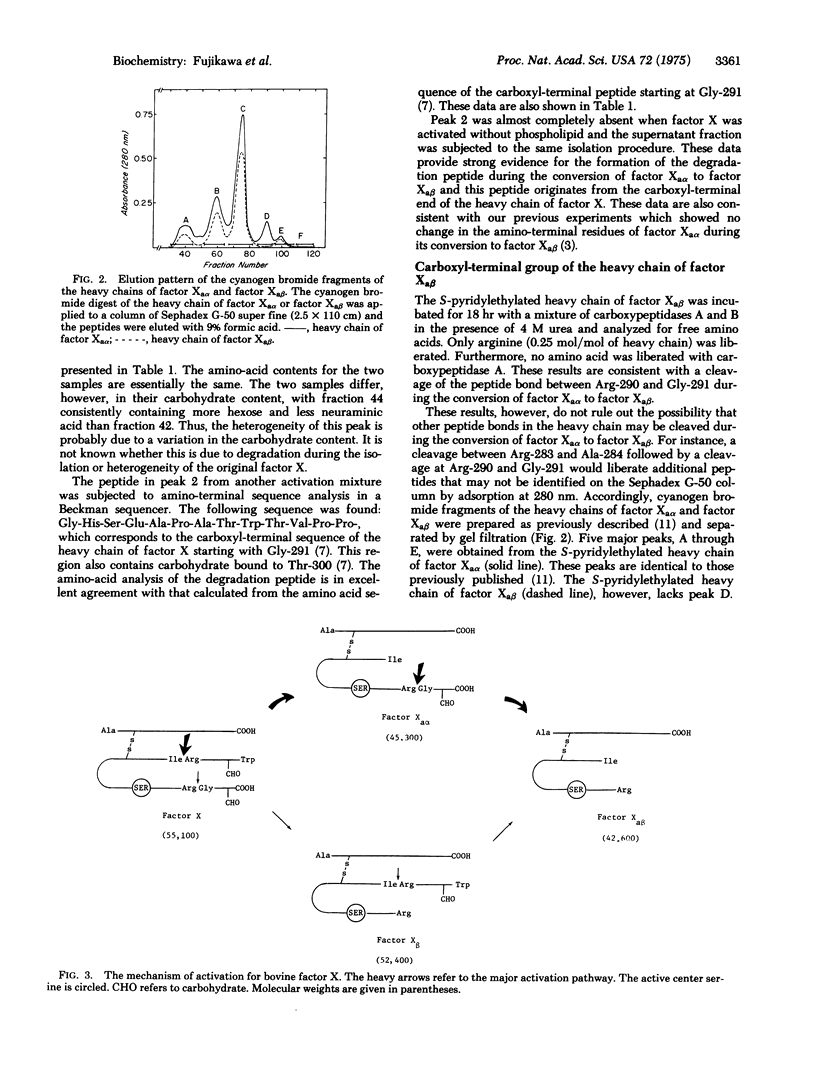
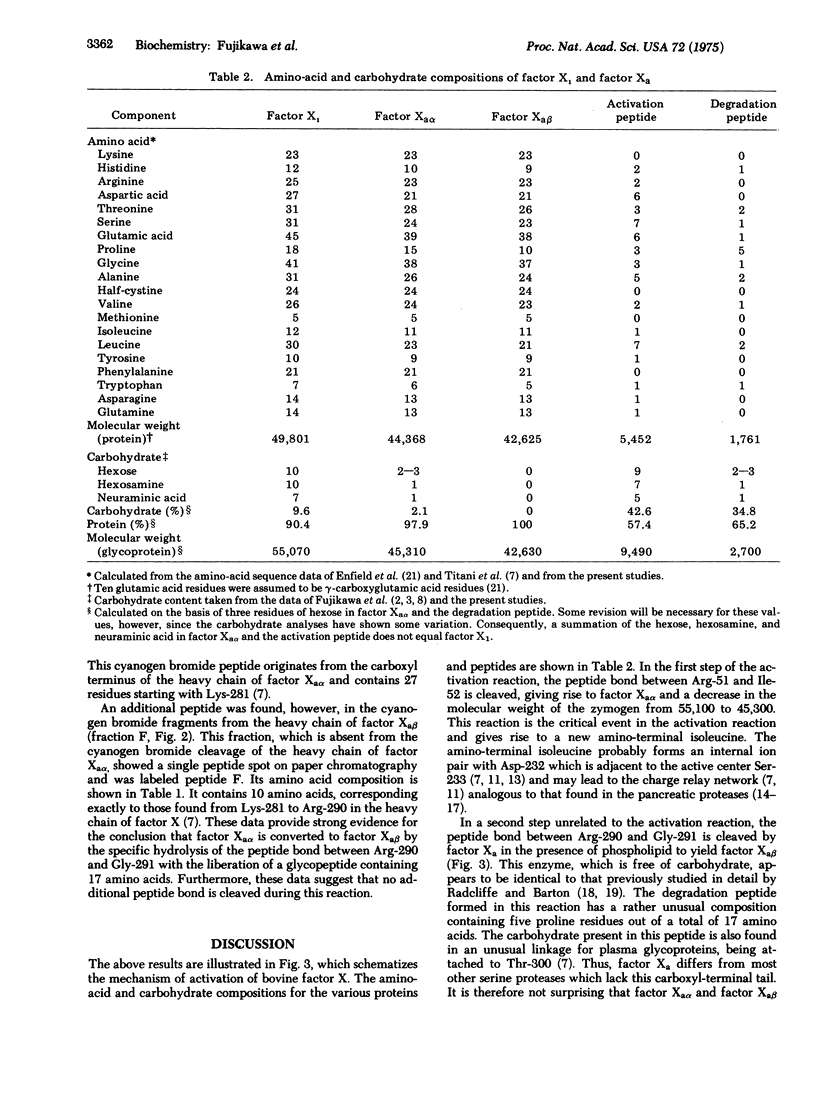
Bovine factor X (molecular weight 55,100) is a blood coagulation factor present in plasma in a precursor or zymogen form. It is a glycoprotein which has been isolated as a two-chain structure held together by one or more disulfide bonds. During the coagulation process, factor X is converted to a serine protease by the hydrolysis of a specific peptide bond in the amino-terminal region of the heavy chain. This cleavage occurs between Arg-51 and Ile-52, giving rise to factor Xaalpha (molecular weight 45,300) and an activation peptide (molecular weight 9500). Factor Xaalpha is then converted to factor Xabeta (molecular weight 42,600) by hydrolysis of a second specific peptide bond in the carboxyl-terminal region of the heavy chain. This cleavage occurs between Arg-290 and Gly-291, giving rise to a second glycopeptide (molecular weight 2700). Factor Xaalpha and factor Xabeta have equivalent coagulant activity, indicating that the cleavage of the second peptide bond is unrelated to the activation process.
Full text
PDF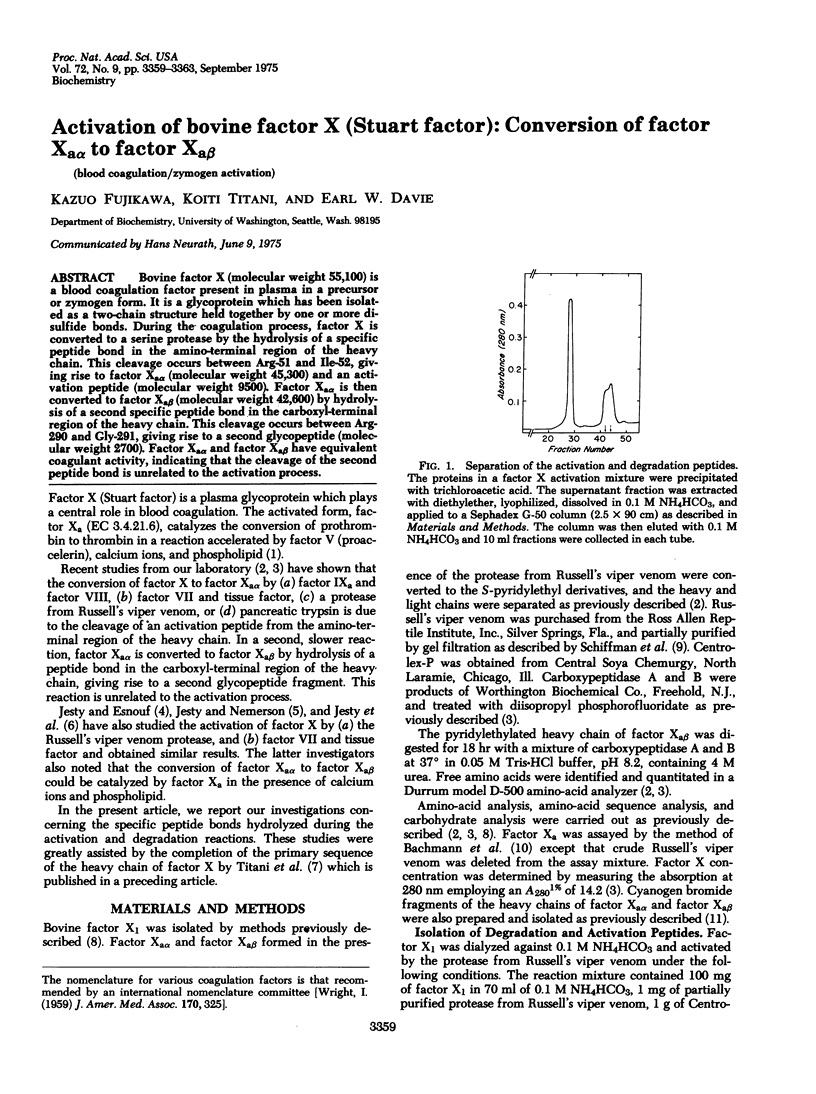

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHMANN F., DUCKERT F., KOLLER F. The Stuart-Prower factor assay and its clinical significance. Thromb Diath Haemorrh. 1958 May 1;2(1-2):24–38. [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K. Basic mechanisms in blood coagulation. Annu Rev Biochem. 1975;44:799–829. doi: 10.1146/annurev.bi.44.070175.004055. [DOI] [PubMed] [Google Scholar]
- Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H., Titani K. Bovine factor X1 (Stuart factor). Primary structure of the light chain. Proc Natl Acad Sci U S A. 1975 Jan;72(1):16–19. doi: 10.1073/pnas.72.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- Fujikawa K., Coan M. H., Legaz M. E., Davie E. W. The mechanism of activation of bovine factor X (Stuart factor) by intrinsic and extrinsic pathways. Biochemistry. 1974 Dec 17;13(26):5290–5299. doi: 10.1021/bi00723a006. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factor X 1 (Stuart factor). Mechanism of activation by protein from Russell's viper venom. Biochemistry. 1972 Dec 19;11(26):4892–4899. doi: 10.1021/bi00776a003. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factors X 1 and X 2 (Stuart factor). Isolation and characterization. Biochemistry. 1972 Dec 19;11(26):4882–4891. doi: 10.1021/bi00776a002. [DOI] [PubMed] [Google Scholar]
- Jesty J., Esnouf M. P. The preparation of activated factor X and its action on prothrombin. Biochem J. 1973 Apr;131(4):791–799. doi: 10.1042/bj1310791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesty J., Nemerson Y. Purification of Factor VII from bovine plasma. Reaction with tissue factor and activation of Factor X. J Biol Chem. 1974 Jan 25;249(2):509–515. [PubMed] [Google Scholar]
- Jesty J., Spencer A. K., Nemerson Y. The mechanism of activation of factor X. Kinetic control of alternative pathways leading to the formation of activated factor X. J Biol Chem. 1974 Sep 10;249(17):5614–5622. [PubMed] [Google Scholar]
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. Three-dimensional structure of tosyl-alpha-chymotrypsin. Nature. 1967 May 13;214(5089):652–656. doi: 10.1038/214652a0. [DOI] [PubMed] [Google Scholar]
- PAPAHADJOPOULOS D., HANAHAN D. J. OBSERVATIONS ON THE INTERACTION OF PHOSPHOLIPIDS AND CERTAIN CLOTTING FACTORS IN PROTHROMBIN ACTIVATOR FORMATION. Biochim Biophys Acta. 1964 Aug 19;90:436–439. doi: 10.1016/0304-4165(64)90220-x. [DOI] [PubMed] [Google Scholar]
- Radcliffe R. D., Barton P. G. Comparisons of the molecular forms of activated bovine factor X. Products of activation with Russell's viper venom, insoluble trypsin, sodium citrate, tissue factor, and the intrinsic system. J Biol Chem. 1973 Oct 10;248(19):6788–6795. [PubMed] [Google Scholar]
- Radcliffe R. D., Barton P. G. The purification and properties of activated factor X. Bovine factor X activated with Russell's viper venom. J Biol Chem. 1972 Dec 10;247(23):7735–7742. [PubMed] [Google Scholar]
- Schiffman S., Theodor I., Rapaport S. I. Separation from Russell's viper venom of one fraction reacting with factor X and another reacting with factor V. Biochemistry. 1969 Apr;8(4):1397–1405. doi: 10.1021/bi00832a014. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Watson H. C. Three-dimensional structure of tosyl-elastase. Nature. 1970 Feb 28;225(5235):811–816. doi: 10.1038/225811a0. [DOI] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]
- Titani K., Hermodson M. A., Fujikawa K., Ericsson L. H., Walsh K. A., Neurath H., Davie E. W. Bovine factor X 1a (activated Stuart factor). Evidence of homology with mammalian serine proteases. Biochemistry. 1972 Dec 19;11(26):4899–4903. doi: 10.1021/bi00776a004. [DOI] [PubMed] [Google Scholar]


