Abstract
The epidermis is maintained by epidermal stem cells (ESC) that reside in distinct niches and contribute to homeostasis and wound closure. Keratinocytes at the non-healing edges of venous ulcers (VUs) are healing-incompetent, hyper-proliferative and non-migratory suggesting deregulation of ESCs. To date genes which regulate ESC niches have been studied in mice only. Utilizing microarray analysis of VU non-healing edges, we identified changes in expression of genes harboring regulation of ESCs and their fate. In a prospective clinical study of ten VUs, we confirmed suppression of the bone morphogenetic protein receptor and GATA binding protein3 as well as inhibitors of DNA-binding proteins 2 and 4. We also found decreased levels of phosphorylated glycogen synthase kinase 3, nuclear presence of ß-catenin and overexpression of its transcriptional target, c-myc indicating activation of the Wnt pathway. Additionally, we found down-regulation of leucine-rich repeats and immunoglobulin-like domains protein 1, a gene important for maintaining ESCs in a quiescent state, and absence of keratin 15, a marker of the basal stem cell compartment suggesting local depletion of ESCs. Our study shows that loss of genes important for regulation of ESCs and their fate along with activation of ß-catenin and c-myc in the VU may contribute to ESC deprivation and a hyper-proliferative, non-migratory, healing incapable wound edge.
Keywords: non-healing edge, keratinocytes, quiescence, microarray, chronic wounds
INTRODUCTION
Skin renewal and homeostasis relies on resident populations of epidermal stem cells (ESCs) whose activity and fate are regulated by the distinct microenvironment, or niche, in which they reside (1-4). Under healthy conditions, a stem cell niche remains quiescent, and thus protected from inadvertent cell division and differentiation, which can result in depletion of the stem cell reservoir (3). To date, at least three distinct ESC niches have been identified: the bulge of the hair follicle (HF), the base of the sebaceous gland, and the basal layer of the interfollicular epidermis (IFE) (3, 5). It is still not clear whether the stem cells of the IFE produce a hierarchy of progenitors consisting of both identical daughter cells which replenish the stem cell pool, and transient amplifying cells which further divide and terminally differentiate (6) or, if they exist as only a single type of progenitor cell (7). In either case, they have ability to self–renew and differentiate, thus maintaining the integrity of the epidermis.
Under homeostatic conditions, ESCs behave unipotently, exclusively maintaining their respective tissues, however, in response to injury, stem cells within the IFE and HF niches give rise to daughter cells which migrate to re-epithelialize the epidermal defect (8-11). Previous study demonstrated the contribution of HF bulge stem cells to accelerate the early phase of re-epithelization in acute wounds (11). These cells, however, were not required to achieve complete closure as presumably this was accomplished by recruitment of epidermal cells from the IFE. More recent evidence supporting this notion and consistent with the presence of a hierarchy of progenitors in the IFE, indicates that it is the slow-cycling stem cells and not the transient amplifying cells that are involved as major contributors to long-term tissue repair (12). It seems likely that for healing to occur in the setting of larger wounds such as chronic ulcers, functional stem cells from both the IFE and HF are required.
Interestingly, HF stem cells have been shown to possess the capability to repair peripheral nerve and spinal cord injury, underscoring the potential of the skin to be an easily accessible source of autologous adult stem cells for regenerative medicine (13, 14). One area where regenerative therapy is in great need is the treatment of chronic wounds. Despite advances in growth factors and skin equivalents, up to 50% of chronic wounds present for more than one year remain recalcitrant to treatment (15). A therapeutic role for ESCs was recently demonstrated by a clinical pilot study where autologous scalp hair follicles transplanted into lower extremity ulcers enhanced epithelialization (16). Clearly, there is a great need to elucidate the role of stem cells in chronic wounds, as well as their regulatory mechanisms, for the purpose of developing novel therapeutic modalities to propel healing towards more favorable outcomes.
We have previously shown that keratinocytes at the edge of venous ulcers (VUs) are hyper-proliferative and healing-incompetent (17-19) suggesting deregulation of ESCs and their respective niches. Our additional finding that mRNA levels of c-myc are increased in the epidermis of chronic wounds (19) supports this notion as it has been shown that c-myc overexpression in mouse epidermis leads to excessive proliferation and differentiation, with subsequent loss of quiescence and depletion of the stem cell reservoir (20, 21).
Keratin 15 (K15) (22) and leucine-rich repeats and immunoglobulin-like domains protein1 (LRIG1) (23) are characterized as putative ESC markers in the IFE. Furthermore, bone morphogenetic protein receptor1a (BMPR1a), GATA binding protein 3 (GATA3) and inhibitors of DNA-binding protein 2 and 4 (ID2, ID4) are known as genes which regulate ESCs and their fate in the HF bulge (24, 25). However, their expression in non-healing edges of chronic wounds has not been defined. Presence of hair follicles in the lower extremities is well documented in the literature (26), however, the clinical observation that hair is absent in the skin surrounding chronic wounds (27, 28) lends further support to our hypothesis that ESCs at the non-healing wound edge are depleted via loss of quiescence. To evaluate this, we assessed the expression of several ESC markers in the non-healing edges of VUs in a clinical study of ten patients.
MATERIALS AND METHODS
Skin Specimens Used in Study
Venous Ulcers
Institutional review board at Hospital for Special Surgery approval was obtained. Skin biopsies from the non-healing edges of two males and eight female patients, suffering from VUs were collected. Demographic characteristics of patient population are presented in Table 2. VU specimens were collected from discarded tissue after surgical debridement procedures as described previously (18). Before each biopsy, the ulcer was prepped in Betadine solution and the non-healing edge was identified by the surgeon. The biopsies where then processed in the following way: samples were either embedded in OCT compound (Tissue Tek, Fisher Scientific, Pittsburgh, PA) and frozen in liquid nitrogen for frozen sectioning, stored in formalin for paraffin embedding, snap frozen for protein isolation or stored in RNAlater (Ambion/Applied Biosystems, Austin, TX) for subsequent RNA isolation. Samples were standardized as previously described (18). Tissue morphology was evaluated initially using hematoxylin and eosin staining to assure that specimens contained full thickness epidermis and dermis. All chronic wound specimens showed hyperproliferative, hyper- and parakeratotic epidermis typical for non-healing edges of chronic ulcers (19).
Table 2.
Demographic characteristics of patient population.
| Patient sex | Male | 2 |
| Female | 8 | |
| Patient age | <40 years | 1 |
| 40-60 years | 6 | |
| >60 years | 3 | |
| Race | Caucasian | 3 |
| African - American | 1 | |
| Hispanic | 6 | |
| Number of previous debridements | 0 | 1 |
| 1-5 | 7 | |
| >5 | 2 |
Healthy human skin
Translational research on chronic wounds often faces the challenge obtaining site matched control skin. Faced with the difficulty in obtaining lower extremity skin from healthy individuals we utilized skin derived from reduction surgery. Four healthy skin specimens were obtained as discarded tissue from patients undergoing elective plastic surgery and utilized as healthy skin controls after Institutional review board approval from Hospital for Special Surgery was obtained. Adipose tissue was removed so that full thickness epidermis and dermis from each specimen were preserved for RNA isolation, snap frozen for protein isolation, or embedded in OCT or paraffin for immunohistochemical analyses.
RNA isolation and Quantitative Real-time PCR Analysis
RNA isolation and purification was performed using miRVana RNA isolation Kit (Ambion/Applied Biosystems, Austin, TX). For quantitative real-time PCR, 0.5 μg of total RNA from healthy skin and chronic wounds was reverse transcribed using Omniscript Reverse Transcription kit (Qiagen, Valencia, CA). Real-time PCR was performed in triplicate using the Opticon2 thermal cycler (Bio-Rad, Hercules, CA) and an iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Relative expression was normalized to levels of HPRT1. The primer sequences used were: HPRT1, forward (5'-AAAGGACCCCACGAAGTGTT-3') and reverse primer (5'-TCAAGGGCATATCCTACAACAA-3'); LRIG1 forward (5’-CTGGACGCGGAGCCTAAAC -3’) and reverse primer (5’- GCTGCGAATCTTGTTGTGCTG -3’); BMPR1a, forward (5’-TCAGACTCCGACCAGAAAAAGT-3’) and reverse primer (5’-TGGCAAAGCAATGTCCATTAGTT -3'); GATA3 forward (5’-GTGCTTTTTAACATCGACGGTC-3’) and reverse primer (5’-AGGGGCTGAGATTCCAGGG-3’), ID2 , forward (5'-GACCCGATGAGCCTGCTATAC- 3’) and reverse primer (5'-AATAGTGGGATGCGAGTCCAG 3’) ID4 , forward (5'-CGCTCACTGCGCTCAACAC- 3’) and reverse primer (5’-TCAGGCGGCCGCACACCT-3’) .Statistical comparisons of expression levels from chronic wound vs. healthy skin were performed using Student's t-test.
Protein Isolation and Western blot
Protein extracts for immunoblotting were prepared from healthy human skin explants and VU specimens, obtained in accordance with approved institutional protocol, using Tissue-PE LB Kit (Genotech) with addition of protease and phosphatase inhibitors (1mM phenylmethylsulfonyl fluoride, 20mM glycerophosphate, 8 mM sodium pyrophosphate, 1 ug/ml leupeptin, 1 ug/ml pepstatin A, and 1 ug/ml aprotinin (Roche, IN). The tissue and cell extracts were normalized for total protein concentration using the Bradford protein assay, and samples were stored at −80 C. Tissue and cell extracts were boiled for 5 min in 2x Lemlli sample buffer,separated by 10% SDS-PAGE and transferred to nitrocellulose membrane (VWR, Batavia, IL, USA). The membranes were blocked for 60 min in 5% bovine serum albumin in blocking solution (Tris-buffered saline (TBS), pH 7.4) at room temperature and then incubated with primary antibody at 4 °C overnight using 1:1000 dilution for anti-c-myc antibody (Abcam Inc., MA, USA). Jurkat Whole Cell Lysate (Santa Cruz, CA, USA) was used as a positive control. The membranes were washed three times for 5 min with TBS and 0.1% Triton X-100 and twice with TBS and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary anti-rabbit antibody using 1:2000 dilution (Cell Signaling, MA, USA). Blots were then washed three times for 5 min with TBS and 0.1% TritonX-100 and developed using Super Signal West Pico Chemiluminescent substrate (Pierce, IL, USA) and exposed on x-ray film (Eastman Kodak Co. Bio Max MR-Film). Antibody specific for GAPDH (Santa Cruz, CA, USA) was used for loading control.
Immunohistochemistry
Six μm thick tissue sections were serially cut on a microtome (HM 315, Carl Zeiss) and mounted on slides. Sections were de-waxed in xylene, re-hydrated and washed with 1XPBS. For antigen retrieval, paraffin sections were heated in 95°C water bath in Target Retrieval Solution (DAKO Corporation, Carpinteria, CA). Histological slides were treated with 0.1% H2O2 in Methanol for 30 minutes, rinsed with H2O, and blocked with 5% bovine serum albumin. Sections were then incubated with anti-ID2 and anti-ID4 antibodies (Santa-Cruz, Santa Cruz, CA). For GATA3, K15 and β-catenin staining we utilized frozen tissue specimens. Slides containing five micrometer thick skin sections were fixed in either 4% paraformaldehyde for 10mins (GATA3) or cold acetone for 1min (K15, β-catenin). All sections were blocked in 5% BSA. Mouse monoclonal antibodies detecting either GATA3 (Santa-Cruz, Santa Cruz, CA) or K15 (29) as well as rabbit polyclonal antibody detecting phosphorylated β-catenin (AbCam, Cambridge, MA) were diluted in 5% BSA in PBS and used for overnight incubation at +4C. Signal was visualized using Alexa-Fluor 488 or Alexa–Fluor 594 (Invitrogen, Carlsbad, CA) secondary antibodies and slides were mounted with mounting medium containing DAPI or propidium iodine to visualize nuclei (Vector Labs, Burlingame, CA). Nikon Eclipse E800 microscope was used for visualization and digital images were collected using SPOT Camera Advanced program.
RESULTS
Genes responsible for maintaining the hair follicle stem cell niche are found deregulated in non-healing edges of VUs
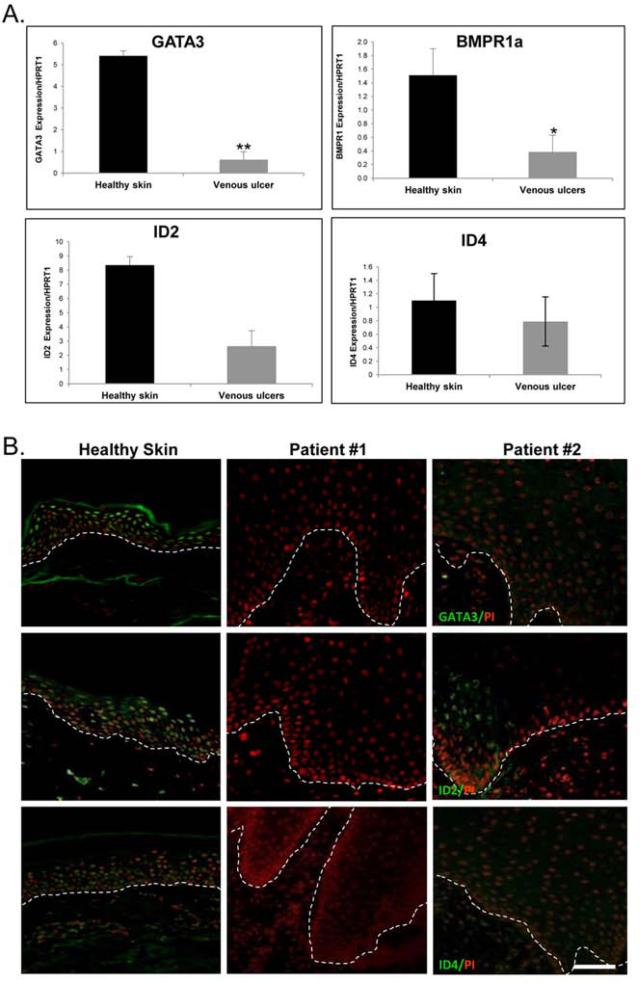
To assess regulation of genes presumed to be involved in the pathogenesis of VUs we utilized previous microarray profiles generated from the non-healing edges of VUs as described earlier (18). We found a deregulated set of genes known to be involved in maintaining ESC niches (Table 1). To confirm our microarray data, which demonstrated down-regulation of genes responsible for maintaining quiescence within the HF niche, we determined the expression of BMPR1a, GATA3, ID2, and ID4 in specimens collected from non-healing edges of VUs and compared it to that in healthy skin. qRT-PCR demonstrated marked suppression of all four genes in skin specimens derived from VUs as compared to healthy skin (Figure 1A.). Additionally, immunofluorescent staining revealed reduced nuclear presence of ID2 and ID4 and complete absence of GATA3 in all VUs tested (Figure 1B.), whereas their expression was detected throughout the epidermis of healthy skin further confirming marked suppression of the main regulators of HF stem cell niche quiescence in non–healing edges of VUs.
Table 1.
List of regulated genes known to be involved in maintaining of ESC niches when gene expression data from non-healing edges of VU's were compared to healthy skin. Fold changes, gene symbols and unigene comments are shown.
| Fold Change VU/ Healthy Skin | Gene Symbol | Unigene Comment | Function |
|---|---|---|---|
| −8.13 | GATA3 | GATA binding protein 3 | Transcription |
| −2.02 | TBX1 | T-box 1 | Transcription factor |
| −3.30 | BMPR1 | bone morphogenetic protein receptor, type IA | Receptor |
| 2.61 | MYCBP | c-myc binding protein | Regulator, Myc pathway |
| −7.52 | ID2 | inhibitor of DNA binding 2, helix-loop-helix protein | Transcription repressor |
| −70.42 | ID4 | inhibitor of DNA binding 4, helix-loop-helix protein | Transcription factor |
Figure 1. Downregulation of signals that control quiescence of ESCs in the epidermal edge of VUs.
A) Results of qRT-PCR demonstrating significant down- regulation of GATA3, BMPR1a, ID2 and ID4 in VUs as compared to healthy skin. All results are expressed as mean± SD from 10 VU and 4 healthy skin specimens. *p≤0.05; **p ≤0.01
B) Representative sections of immunofluorescent staining demonstrating diminished nuclear ID2 presence and, absence of ID4 and GATA3 in VU's when compared to healthy skin. Scale bar 50μm.
Morphology of specimens derived from the non-healing edges of VUs show a complete absence of hair follicles
To assess the presence of hair follicles in tissue specimens derived from non-healing edges of VUs, we stained specimens from ten patients using hematoxylin and eosin. Specimens were obtained using punch biopsies and therefore we were able to evaluate both epidermis as well as papillary and reticular dermis. Tissue morphology demonstrated the characteristic pattern of non-healing VU edges (19), marked by hyperproliferative epidermis and presence of dermal fibrosis (Supplemental Figure 1A). In 9 specimens, hair follicles were completely absent. Interestingly, only one examined non-healing edge VU specimen showed the presence of an arrector pili muscle, a muscle that is attached to hair follicles in mammals (Supplemental Figure 1B). However, a hair follicle was not apparent in near proximity of the arrector pili muscle, rather it was completely absent from the specimen. This finding complements the clinical observation of hair loss surrounding chronic wounds (27, 28), and demonstrates that not only is hair absent clinically, but that a complete absence of hair follicles is seen histologically as well thus further supporting the observed down-regulation of signals responsible for maintaince of HF stem cell niche at the non-healing edges of VUs.
Components of the Wnt pathway are deregulated in non-healing edges of VUs
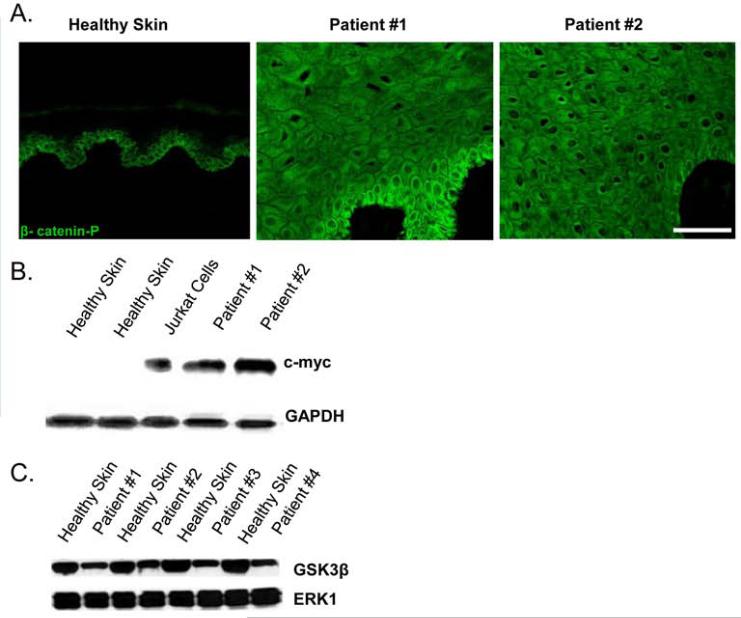
Using immunohistochemistry we have previously shown robust nuclear presence of β-catenin and c-myc at the edges of non-healing wounds (19). To further assess the status of the Wnt signaling pathway, which has been shown to regulate stem cell fate, we performed immunofluorescent staining using an antibody that recognizes the phosphorylated, activated form of β-catenin (tyrosine 412). We observed increased cytoplasmic presence and prominent nuclear localization of phosphorylated β-catenin in non-healing edges of VU specimens (Figure 2A). However, healthy skin specimens showed β-catenin present mainly at the membrane in the basal layer. Furthermore, western blot analysis showed that c-myc, a downstream target of β-catenin, was over-expressed in VUs as well as in Jurkat cells (positive control) while no c-myc protein was detected in healthy skin (Figure 2B). We utilized western blot analysis to assess GSK3β protein levels and demonstrated decrease of p-GSK3β in VUs as compared to healthy skin (Figure 2C.), indicating possible activation of the Wnt pathway.
Figure 2. Activation of the Wnt pathway in VUs is evident by decreased p-GSK3β, nuclearization of p-β-catenin, and induction of c-myc.
A) Immunofluorescence reveals activation of β-catenin in VU specimens as seen by the nuclear localization throughout the entire epidermis. Note that in healthy skin, localization of phosphorylated β-catenin is cytoplasmic and confined to the basal and first subrabasal layer. Scale bar 50μm. B) Western blot showing increased expression of c-myc in VUs and Jurkat cells (positive control) as compared to healthy skin controls. C) Western Blot demonstrating decreased levels of phosphorylated GSK3β in VUs as compared to healthy skin.
Stem cell markers of interfollicular epidermis are down- regulated at the non-healing edges of VUs
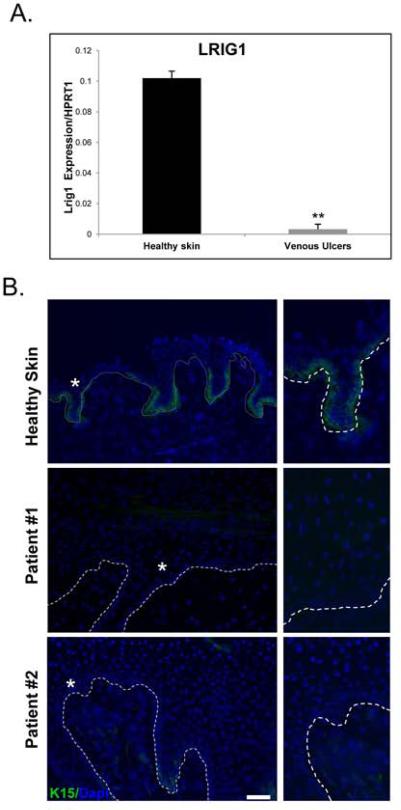
To evaluate the presence of ESCs and their regulatory mechanisms within the IFE, we determined the expression of LRIG1, which has been shown to both maintain quiescence and serve as an IFE stem cell marker (23). qRT-PCR demonstrated a striking down-regulation of LRIG1 in VUs as compared to healthy skin (Figure 3A). To further determine the presence of ESCs in the IFE, as well as those in the HF-bulge, we assessed K15 expression, which has been considered to be an ESC marker (30). Immunofluorescent staining revealed absence of K15 in tested non-healing VU edge specimens as compared to healthy skin where staining was evident in basal keratinocytes (Figure 3B). These data suggest down-regulation of stem cell markers implicated in maintenance of the IFE.
Figure 3. Loss of LRIG1 and K15 in VUs characterizes the hyperproliferative non-healing wound edge.
A) Decreased expression of LRIG1 in VUs (n=10) as compared to healthy skin (n=4) demonstrated by qRT-PCR; **p ≤0.01. B) Immunofluorescent staining with K15 specific antibody reveals complete absence of K15 in VU specimens in contrast to healthy skin where K15 is expressed throughout the basal layer of the epidermis. Asterisks demarcate enlarged areas presented on the right. Scale bar 50μm.
DISCUSSION
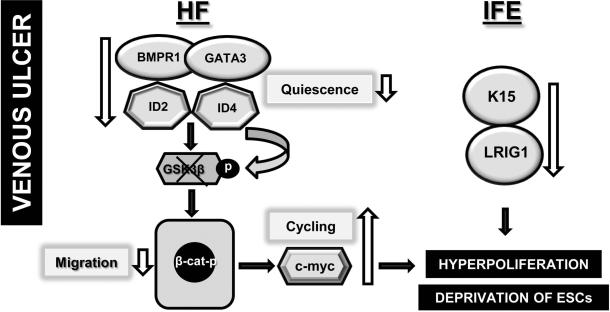
In this study we analyzed tissue samples obtained from ten patients suffering from non-healing VUs to evaluate genes and markers associated with the fate of ESCs. We found that signals which regulate quiescence of ESCs, namely BMPR1, GATA3, ID2 and ID4, are downregulated in VUs, pointing to loss of ESC control. Furthermore, we observed a decrease in GSK3ß and, as a consequence, an increase in phosphorylated nuclear ß-catenin and c-myc, suggesting activation of the Wnt pathway. In addition, we found down-regulation of LRIG1 and K15 in the IFE of the non-healing edge of VUs. Taken together, these findings suggest that loss of quiescence and de-regulation of the ESC niche may affect frequent cycling and subsequent ESC depletion possibly resulting in the hyperpoliferative epidermis characteristic of a non-healing VU edge (Figure 4).
Figure 4.
Diagram shows how fate of ESCs may contribute to inhibition of healing in VUs.
The non-healing edges of chronic wounds are characterized by hyperproliferative keratinocytes marked by activated β-catenin and c-myc (19). β-catenin signaling has multiple roles in stem cell lineage determination within epithelial tissues such as the HF, where nuclear stabilization of β-catenin stimulates resting bulge stem cells to proliferate (31). β-catenin has also been linked to BMP signaling as conditional ablation of the BMPR1a gene enhanced β-catenin stabilization and activated quiescent SCs to enter the proliferative phase (24). In addition, GATA3, which appears to suppress terminal differentiation of ESCs within their niche by inhibiting the expression of keratin 10 (K10) was found to be strongly reduced (32). Previously, we have shown suppression of the early differentiation marker K10 in non-healing edges of VUs (18). Our finding that BMPR1a is suppressed in VUs correlates with the observed increase in β-catenin and down-regulation of GATA3, which together may lead to loss of quiescence and a hyperproliferative, non-healing epidermis.
It is apparent that the Wnt pathway, known to regulate stem cell fate, (33) is deregulated in VUs and this is further confirmed by the observed decrease in GSK3ß phosphorylation, indicating Wnt pathway activation. C-myc, a transcriptional target of β-catenin, also plays an important role in controlling ESC behavior as it has been implicated in the proposed conversion of ESCs to transit amplifying cells. Its over-expression can lead to excessive proliferation and differentiation (34). Induction of c-myc in the non-healing edges of VUs suggests a loss of quiescence with depletion of the SC niche and is consistent with previous findings showing impaired wound healing and a reduction of label-retaining bulge cells upon c-myc over-expression in transgenic mice (20, 21).
“Inhibitor of DNA binding” genes, ID2 and ID4, are presumed to maintain quiescence in the HF bulge SC niche as they are preferentially expressed in the undifferentiated, slow cycling cells as compared to their progeny (25, 35). The family of ID helix-loop-helix (HLH) proteins, which are universally expressed, negatively regulate basic HLH (bHLH) transcriptional regulators that promote cell lineage determination (36). Therefore, the observed down-regulation of ID2 and ID4 in VUs may contribute to both loss of quiescence and the previously described deregulation of keratinocyte differentiation markers (18).
LRIG1 has been identified as a marker of human IFE stem cells, although LRIG1 positive cells have also been found in the junctional zone of the murine hair follicle (23). LRIG1 has been shown to regulate SC quiescence in the IFE niche and an inverse relationship between the levels of LRIG1 and c-myc were previously demonstrated in the basal layer of human IFE (37). Here we show a striking suppression of LRIG1 in non-healing VUs along with a concomitant increase in c-myc, indicating depletion of the IFE SC niche. Decreased levels of LRIG1 are associated with psoriasis-like lesions in mice and poorly differentiated squamous cell carcinomas suggesting a role for LRIG1 and loss of SC quiescence in skin disorders characterized by hyperproliferation (38).
K15 was one of the first markers to be associated with HF bulge stem cells (22). Although its precise role in keratinocyte biology has yet to be determined, K15 expression may be associated not only with stem cell subpopulations but also with basal-like cells which respond to disruption of healthy epidermal proliferation and differentiation (30). In a murine full-thickness wound model, progeny of K15 bulge cells were shown to migrate to the epidermis to aid in repair, although K15 expression was found only temporarily in the IFE post-wounding (10). In a more recent chemical injury model, K15 was initially up-regulated and found in the basal and suprabasal layers of the IFE, then transiently down-regulated before being re-established in the basal layer of the repaired epidermis (30). In healthy skin specimens, K15 was found throughout the basal layer, yet at the non-healing edge of VUs, K15 was absent, suggesting loss of a subpopulation of cells capable of epidermal repair and homeostasis. Nevertheless, during acute wound healing, stem cells respond rapidly to the injury by increasing proliferation and reducing differentiation until tissue is restored (39). One can speculate that the observed loss of signals that control the stem cell niche in non-healing edges of VUs are due to changes in the chronic wound microenvironment to which stem cells are exposed, since it is well established that the chronic wound cytokine milieu significantly differs from that present during acute wound healing (40).
Our data suggest that in VUs, deregulation of signals which control SC quiescence in both the HF and IFE niches can be correlated to a frequent cycling, presented as a hyperproliferative epidermis of VU's wound edges, and possibly, depletion of the SC pool. This is further supported by the absence of HF and IFE stem cell markers in healing-incompetent epidermal tissue at the VU wound edge. Taken together, our findings begin to shed light on the possibility that ESC function is impaired in chronic wounds and furthermore, may provide a rationale for implementing stem cell therapy and regenerative medicine strategies for non-healing ulcers of various etiologies.
To the best of our knowledge this is the first report correlating the loss of SC niche signaling and deprivation of ESCs to the hyperproliferative non-healing edge of chronic wounds. We show that BMPR1, GATA3, ID2 and ID4 are down-regulated at the non-healing edge of VUs. Additionally, we report decreased levels of phosphorylated GSK3ß, indicating activation of the Wnt pathway, which was confirmed by strong nuclear presence of ß-catenin and overexpression of c-myc. Down-regulation of LRIG1, a gene important for maintaining ESCs in a quiescent, non-dividing state was accompanied by an absence of K15, a marker of the basal stem cell compartment, suggesting local deprivation of ESCs, leading to hyper-proliferative epidermis found at the edge of VUs. Although current observations necessitate mechanistic studies that may prove to be challenging approach in human subjects, they provide a basis for further clinical studies evaluating new therapeutic strategies to reverse de-regulation of ESC niches, and restore a fully functional, healing-competent epidermis in patients with VU's.
Supplementary Material
Figure S1. Loss of hair follicles and hyperpoliferative epidermis is characteristic of non-healing wound edge in VUs. A.) Typical histology is shown. B.) In 1/10 specimens an arrector pili muscle (arrow) was found but no evidence of hair follicles.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants NR008029 and NR013881 (MTC), University of Miami SAC Award SAC 2013-19 and The Skin Disease Research Center, Department of Dermatology and Cutaneous Surgery at the University of Miami Miller School of Medicine.
LIST OF ABBREVIATIONS
- BMPR1
bone morphogenetic protein receptor
- ESCs
epidermal stem cells
- GATA3
GATA binding protein 3
- HF
hair follicle
- HLH
helix-loop-helix
- ID
DNA-binding protein inhibitor
- IFE
interfollicular epidermis
- K10
keratin 10
- K15
keratin 15
- LRIG1
leucine-rich repeats and immunoglobulin-like domains protein 1
- VUs
venous ulcers
Footnotes
The authors state no conflict of interests.
REFERENCES
- 1.Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol. 2009;217(2):169–80. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Fu X, Sheng Z. Cutaneous stem cells: something new and something borrowed. Wound Repair Regen. 2007;15(6):775–85. doi: 10.1111/j.1524-475X.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 3.Braun KM, Prowse DM. Distinct epidermal stem cell compartments are maintained by independent niche microenvironments. Stem Cell Rev. 2006;2(3):221–31. doi: 10.1007/s12015-006-0050-7. [DOI] [PubMed] [Google Scholar]
- 4.Boehnke K, Falkowska-Hansen B, Stark HJ, Boukamp P. Stem cells of the human epidermis and their niche: composition and function in epidermal regeneration and carcinogenesis. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs136. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180(2):273–84. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghadially R. 25 years of epidermal stem cell research. J Invest Dermatol. 2012;132(3 Pt 2):797–810. doi: 10.1038/jid.2011.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doupe DP, Jones PH. Interfollicular epidermal homeostasis: dicing with differentiation. Exp Dermatol. 2012;21(4):249–53. doi: 10.1111/j.1600-0625.2012.01447.x. [DOI] [PubMed] [Google Scholar]
- 8.Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. 2009;18(11):921–33. doi: 10.1111/j.1600-0625.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Cotsarelis G. Is the hair follicle necessary for normal wound healing? J Invest Dermatol. 2008;128(5):1059–61. doi: 10.1038/jid.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351–4. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 11.Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2008;128(5):1311–8. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- 12.Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489(7415):257–62. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 13.Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle. 2008;7(12):1865–9. doi: 10.4161/cc.7.12.6056. [DOI] [PubMed] [Google Scholar]
- 14.Bickenbach JR, Stern MM, Grinnell KL, Manuel A, Chinnathambi S. Epidermal stem cells have the potential to assist in healing damaged tissues. J Investig Dermatol Symp Proc. 2006;11(1):118–23. doi: 10.1038/sj.jidsymp.5650009. [DOI] [PubMed] [Google Scholar]
- 15.Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25(1):73–8. doi: 10.1016/j.clindermatol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez F, Garde C, Poblet E, Jimeno B, Ortiz J, Martinez ML, et al. A pilot clinical study of hair grafting in chronic leg ulcers. Wound Repair Regen. 2012;20(6):806–14. doi: 10.1111/j.1524-475X.2012.00846.x. [DOI] [PubMed] [Google Scholar]
- 17.Pastar I, Stojadinovic O, Tomic-Canic M. Role of keratinocytes in healing of chronic wounds. Surgical technology international. 2008;17:105–12. [PubMed] [Google Scholar]
- 18.Stojadinovic O, Pastar I, Vukelic S, Mahoney MG, Brennan D, Krzyzanowska A, et al. Deregulation of keratinocyte differentiation and activation: A hallmark of venous ulcers. J Cell Mol Med. 2008;12(6B):2675–90. doi: 10.1111/j.1582-4934.2008.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, et al. Molecular Pathogenesis of Chronic Wounds: The Role of beta-Catenin and c-myc in the Inhibition of Epithelialization and Wound Healing. Am J Pathol. 2005;167(1):59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11(8):558–68. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 21.Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28(2):165–8. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- 22.Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G. Human hair follicle bulge cells are biochemically distinct and possess an epithelial stem cell phenotype. J Investig Dermatol Symp Proc. 1999;4(3):296–301. doi: 10.1038/sj.jidsp.5640233. [DOI] [PubMed] [Google Scholar]
- 23.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4(5):427–39. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104(24):10063–8. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22(4):411–7. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 26.Kuck M, Schanzer S, Ulrich M, Garcia Bartels N, Meinke MC, Fluhr J, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Optics. 2012;17(10):101504. doi: 10.1117/1.JBO.17.10.101504. [DOI] [PubMed] [Google Scholar]
- 27.Grey JE, Harding KG, Enoch S. Venous and arterial leg ulcers. BMJ. 2006;332(7537):347–50. doi: 10.1136/bmj.332.7537.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26(4):286–95. doi: 10.1055/s-0029-1242204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waseem A, Dogan B, Tidman N, Alam Y, Purkis P, Jackson S, et al. Keratin 15 expression in stratified epithelia: downregulation in activated keratinocytes. J Invest Dermatol. 1999;112(3):362–9. doi: 10.1046/j.1523-1747.1999.00535.x. [DOI] [PubMed] [Google Scholar]
- 30.Troy TC, Arabzadeh A, Turksen K. Re-assessing K15 as an epidermal stem cell marker. Stem Cell Rev. 2011;7(4):927–34. doi: 10.1007/s12015-011-9243-9. [DOI] [PubMed] [Google Scholar]
- 31.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–73. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163(3):609–23. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2005;97(3):173–83. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130(21):5241–55. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 35.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113(Pt 22):3897–905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 37.Jensen KB, Watt FM. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc Natl Acad Sci U S A. 2006;103(32):11958–63. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanemura A, Nagasawa T, Inui S, Itami S. LRIG-1 provides a novel prognostic predictor in squamous cell carcinoma of the skin: immunohistochemical analysis for 38 cases. Dermatol Surg. 2005;31(4):423–30. doi: 10.1111/j.1524-4725.2005.31108. [DOI] [PubMed] [Google Scholar]
- 39.Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nature reviews Cancer. 2012;12(3):170–80. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- 40.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Loss of hair follicles and hyperpoliferative epidermis is characteristic of non-healing wound edge in VUs. A.) Typical histology is shown. B.) In 1/10 specimens an arrector pili muscle (arrow) was found but no evidence of hair follicles.