Abstract
CD36 is a scavenger receptor that functions in high affinity tissue uptake of long chain fatty acids (FA) and contributes under excessive fat supply to lipid accumulation and metabolic dysfunction. This review describes recent evidence regarding the CD36 FA binding site and a potential mechanism for FA transfer. It also presents the view that CD36 and FA signaling coordinate fat utilization based on newly identified CD36 actions that involve oral fat perception, intestinal fat absorption, secretion of the peptides cholecystokinin and secretin, regulation of hepatic lipoprotein output, activation of beta oxidation by muscle and regulation of the production of the FA derived bioactive eicosanoids. Thus abnormalities of fat metabolism and the associated pathology might involve dysfunction of CD36-mediated signal transduction in addition to the changes of FA uptake.
Keywords: fat taste, chylomicron, VLDL, CCK, secretin, calcium, FA binding, phospholipase, eicosanoid
INTRODUCTION
The recent emphasis on minimizing dietary fat to protect against obesity and associated coronary heart disease, combined with the increased availability of processed sugar, has led to increased consumption of low-fat-high-carbohydrate diets. In the United States, intake of dietary fat which in the 1960s approximated 42% of the total energy consumed has declined to 33% reflecting both an approximate 25% increase in total energy intake and increased consumption of carbohydrates (~50% of energy intake) and these trends have remained stable. The decline in percent fat consumed correlated with an increase rather than a decrease in the prevalence of obesity, the “American Paradox”. High intake of refined sugars and corn sweeteners, combined with increased dependence on processed food made much more palatable by added sugar and fat (57, 96) and a more sedentary lifestyle drive the obesity epidemic (97).
Dietary fat consumed in moderation is important for human health by supplying the body with essential fatty acids (FA) and fat soluble vitamins and by regulating satiety and energy homeostasis (2). Dietary fat consists mainly of triglycerides, TG (90-95%), but also of phospholipids, sterols, and fat soluble vitamins. TG absorption requires hydrolysis by lipases in the intestinal lumen to yield free FA and 2-monoacyl-glycerol (2-MG). The major FA of dietary TG are oleate, palmitate, stearate and linoleate. Polyunsaturated FA (linoleic and linolenic acids) are derived from phospholipids of vegetable origin and are considered essential because they cannot be synthesized de novo.
Lipid utilization is regulated across multiple tissues and the integrated cross talk between them determines lipid homeostasis. Lipid absorption in the gastrointestinal tract is coordinated by neural and humoral factors at several levels. Events that precede food intake such as seeing, smelling or thinking of food can induce modest salivary and gastric secretions via the autonomic nervous system and pancreatic and biliary secretions via the vagus nerve. These secretions are potentiated when fat intake occurs through various mechanisms involving the mouth, the proximal GI and to a lesser extent the distal GI (2, 46). The absorbed lipids are delivered to the circulation as components of the TG rich chylomicrons and very low density lipoproteins (VLDL). Fatty acids released from the TG contained in the lipoprotein particles by lipoprotein lipase in the vasculature, are taken up by various tissues. In adipose tissue, FA are primarily converted to TG for storage in cytosolic lipid droplets while in skeletal muscle they are used mainly for oxidation. Fatty acids taken up by the liver are reincorporated into TG and packaged into VLDL. During periods of energy demand such as with fasting or exercise, increased FA mobilization from adipocytes and VLDL secretion by the liver deliver FA to tissues, particularly skeletal muscle and heart.
This review will focus on our recent knowledge related to the role of membrane CD36 in fat metabolism. CD36 is a high affinity receptor for long chain FA that has been shown to facilitate net FA uptake into muscle and adipose tissues of rodents and humans (2, 28). CD36 has been recently implicated in several aspects related to FA metabolism including fat taste perception, fat intake, fat absorption, absorption-related peptide secretion and FA utilization by muscle and adipose tissues. The review will highlight new findings related to the above areas and present the hypothesis that a major role of CD36 is to coordinate cellular events involved in uptake and processing of the FA. The mechanisms underlying the various actions of CD36 are not completely clear but we will highlight the importance of CD36-mediated intracellular signaling induced by the FA-CD36 interaction.
As a result of its multiple ligands and signal transduction capabilities, CD36 has also a number of functions related to immune responses, inflammation, angiogenesis, atherogenesis and thrombosis. For these aspects of CD36 function and the link to metabolic pathologies the reader is referred to the following reviews (53, 62, 83).
STRUCTURE-FUNCTION OF CD36
The role of CD36 in cellular FA uptake identified in 1993 (1) is now supported by strong evidence generated in CD36 deficient rodents (15, 31) and humans (34, 94, 100). Polymorphisms in the CD36 gene have been linked to alterations in plasma lipid levels and to susceptibility to the metabolic syndrome (53).
Our knowledge related to the structure-function of CD36 remains incomplete but significant progress has been recently accomplished. CD36 is a heavily glycosylated 80kD integral membrane protein that is widely expressed. It is found on platelets, immune cells, adipocytes, myocytes, enterocytes, enteroendocrine cells, retinal and mammary epithelial cells and microvascular endothelial cells. Sometimes also referred to as GPIV, GPIIIb, PAS IV or FAT, CD36 is the founder member of an evolutionarily conserved protein family that includes the high density lipoprotein Scavenger Receptor B1 (SR-B1) and the lysosomal membrane protein LIMP2. These proteins share structural features that include a hairpin like membrane topology with two transmembrane domains and with both termini in the cytoplasm (Figure 1).
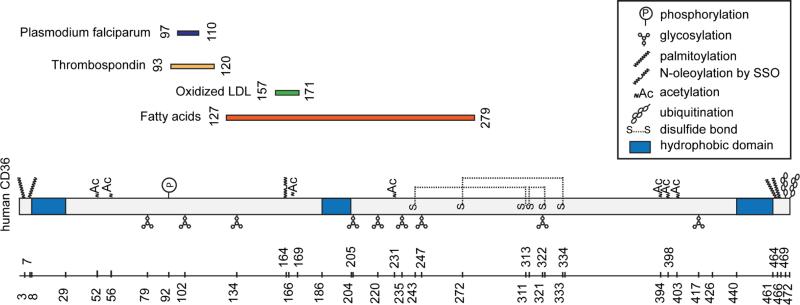
Figure 1. Schematic representation of CD36 binding domains and post-translational modifications.
CD36 has two short intracellular domains at both termini, two transmembrane segments and a large extracellular domain with a hydrophobic sequence where lipid ligands bind. The C-terminus can associate with Src tyrosine kinases, which initiate most of CD36 mediated signal transduction. The diagram highlights the post-translational modifications of the protein including its glycosylation, palmitoylation, ubiquitination, phosphorylation and acetylation. These modifications influence CD36 trafficking and function in FA uptake and signal transduction (see text for details). Both N- and C- termini contain two palmitoylation sites which localize CD36 to membrane lipid rafts. Binding sequences for CD36 ligands are aligned to the backbone of CD36 and the FA-binding site K164 is highlighted with a dotted line [for details see text and (44)].
The heavily glycosylated extracellular domain of CD36 includes three disulfide bridges in the carboxyl-terminal half that were shown important for CD36 membrane recruitment. The amino-terminal half contains binding domains for hexarelin, FA, oxidized LDL or phospholipids, thrombospondin, and P. falciparum-infected erythrocytes (9, 83). A stretch of hydrophobic amino acid residues (186–204) might loop into the outer leaflet of the membrane (14) and could be part of a binding pocket for lipid ligands of CD36, as discussed in the following section. The tails of the protein are cytoplasmic and short but the carboxyl tail is active in signal transduction through its ability to interact with a number of tyrosine kinases (9, 83). Several post-translational modifications, including phosphorylation, glycosylation, palmitoylation, or ubiquitination modulate levels or trafficking of CD36 and consequently its functions (Figure 1).
Identification of the FA binding pocket
CD36 function in FA uptake and FA signaling can be irreversibly inhibited by N-hydroxysuccinimidyl (NHS) esters of long-chain FA. These protein labeling reagents react rapidly to form stable bonds primarily with amino groups in lysine side chains. Sulfo-NHS esters contain a negatively charged sulfonate group that restricts membrane permeability. Esters of palmitate, myristate, and oleate were shown to effectively inhibit FA uptake by isolated adipocytes, a property used in early studies to identify CD36 as a membrane FA receptor (33). It is worth noting that the sulfo-NHS ester of oleate or SSO concentrations needed to inhibit FA binding to CD36 are 1-2 orders of magnitude lower than those initially used (200-1000 μM) with isolated adipocyte suspensions, where the added albumin scavenges much of the SSO necessitating higher concentrations. The SSO has been widely used and inhibits both CD36-mediated FA uptake (16, 89) and FA signaling (19, 43) in a variety of cell types. The molecular mechanism underlying SSO inhibition was recently explored in a study where Chinese hamster ovary (CHO) cells stably expressing human CD36 were SSO-treated before being subjected to proteomics. The target of SSO in human CD36 was identified as lysine 164 (Figure 1), a residue situated within a pocket previously suggested as a potential FA binding site based on simulations with the binding pocket of FA-binding proteins (5). Importance of Lys-164 for CD36-mediated FA uptake and FA-induced signaling was demonstrated using mutagenesis and expression in CHO cells. The CD36 lipid binding pocket has a preponderance of hydrophobic residues and our working model is that the alkyl chain of the FA would serve to direct and position the FA in the pocket. This interpretation is consistent with earlier data showing that a shorter chain NHS derivative such as sulfo-N-succinimidyl myristate bound to several proteins in adipocyte membranes, whereas SSO was recovered mainly on CD36. Once in the pocket the carboxyl group of the FA can form electrostatic interactions with Lys-164 and these would induce a conformational change in CD36 to promote FA uptake and/or FA-induced signaling. This interpretation is based on the observation that expression of a mutated CD36 where lysine 164 is substituted with alanine results in signaling that appears to mimic that induced by FA binding (Samovski and Abumrad, unpublished observations). In this context, FA interaction with lysines in the binding pocket of the intestinal FA-binding protein has been documented and shown to influence FA transfer from the protein to model membranes (88).
Interestingly, the binding pocket of CD36 contains another lysine, Lys-166, which does not bind SSO. This lysine was found to be acetylated (as well as Lys 52, 231, and 403) (Figure 1), which neutralizes its charge and increases hydrophobicity of the binding pocket. Potentially Lys-166 acetylation might regulate access to the binding pocket, or help position the FA in the site, which could regulate the FA-induced change in CD36 and subsequent signaling. For example, acetylation/de-acetylation regulates function of a number of proteins involved in glucose and FA metabolism (55).
Access of the FA binding pocket to a lipid transport tunnel within CD36
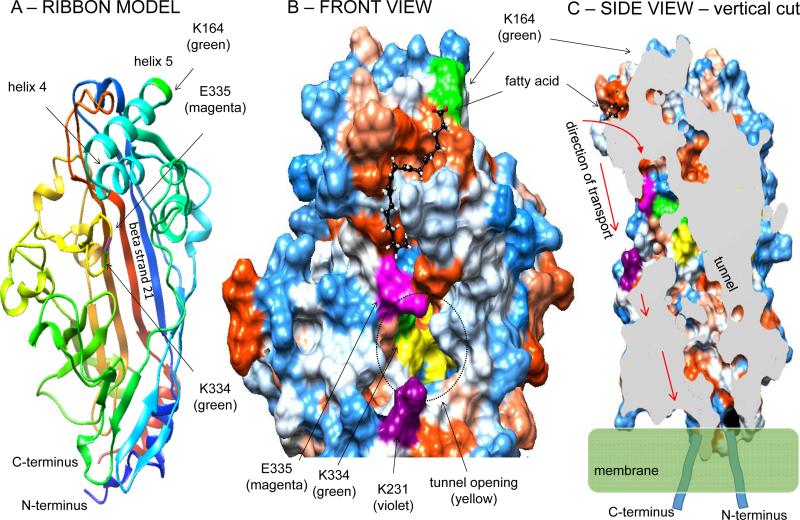
Insight into the potential mechanism of FA transport by CD36 can be derived from the recently reported crystal structure of the CD36 family member lysosomal LIMP-2 (68). The crystal structure identified the presence of a large cavity traversing the entire length of the molecule that was proposed to serve as a tunnel for lipid transfer to the membrane. We modeled CD36 structure and FA docking using respectively the Phyre2 (41) and SwissDock (30) servers as shown in (Figure 2A-C) and incorporated the tunnel feature identified in LIMP2. The model obtained predicts that the FA binding pocket forms a groove that slides towards the tunnel (Figure 2B). Interaction of the FA carboxyl group with Lys-164 (44) at the top of the pocket would position the FA so it can slide into the tunnel with its acyl chain leading the way. The interaction is also likely to induce a change in conformation, an interpretation consistent with the finding that substitution of Lys-164 by alanine results in signaling changes that mimic FA binding (Samovski D et al., unpublished observations). The change could move glutamate 335 situated at the tunnel top providing the FA with tunnel access. The FA would travel the tunnel to reach the plasma membrane where the tunnel outlet is likely to be buried. The tunnel has an opening on the CD36 surface, but our modeling and the SSO binding data (44) suggest that the FA would rarely access the tunnel though its surface opening. Lysine 231 present at the tunnel surface opening is acetylated (44) which could limit access of the FA to the tunnel surface entrance (Figure 2B). Whether two tunnel entry sites (via the FA binding pocket or the tunnel surface opening) are possible for the FA, whether this involves the FA type or different signaling functions would need to be tested experimentally.
Figure 2. The FA binding site on CD36 and its access to a transport tunnel within the molecule.
The structure of human CD36 exofacial domain (K36 – G436) was modeled using the Phyre2 prediction server (41) based on the recently reported crystal structure of the CD36 family member LIMP2 (68). 98% of residues modeled at >90% confidence. Fig 2-A: Ribbon model of CD36 structure highlighting the SSO-target residue K164. Also highlighted is K334 a secondary and rare SSO target (44). Figure 2-B: Detail of the CD36 structure showing the FA (oleic acid) docking site using the SwissDock server (30) (Full fitness -2409.97 kcal/mol; estimated ΔG -8.59kcal/mol). Figure 2-B: Surface hydrophobicity is indicated, with blue denoting hydrophilic residues and red hydrophobic residues. The FA is shown to dock within a hydrophobic pocket at the top of CD36 near helix 5 with the FA carboxylic tail in close proximity to lysine 164 (green residue), previously shown to bind the oleate derivative SSO (44). The FA pocket is shaped into a sliding groove that heads towards the tunnel present within the protein structure, modeled based on the one identified by the crystal structure of LIMP2 (68). The glutamic acid residue 335 (magenta color) is positioned at the site of FA entry into the tunnel. K164 might be important for correct positioning of the FA within the pocket possibly allowing it to slide into the tunnel, and for inducing a conformational change in the protein that initiates signaling. Fig. 2-C: A vertical slice through the CD36 modeled structure showing the rear side of the tunnel which is formed mainly by beta strand 21 and outlets close to the plasma membrane. The arrows show predicted direction of FA transport. Our working hypothesis is that the FA interacts with K164 within the hydrophobic groove which positions it to slide with the acyl chain leading the way into the tunnel. The outlet of the tunnel is likely buried in the charged layer of the membrane which would facilitate transport of the hydrophobic acyl chain through the bilayer.
The FA binding site overlaps with that of oxidized low density lipoproteins
An important feature of the FA-binding site identified in CD36 is that it would overlap with that of oxidized LDL (oxLDL) and oxidized phosphatidylcholine as these two ligands were proposed to interact with lysines 164 and 166 (38). In agreement with the possibility of a shared binding site, incubation with SSO (25 μM, 30 min) effectively inhibited oxLDL uptake by macrophages. The physiological implications of the commonality of binding site and lysine residues mediating interaction of FA and oxLDL with CD36 are unknown at the present time. However the findings might provide a framework for how oxLDL might induce dysfunction of CD36-regulated FA metabolism.
Functional implications of posttranslational changes in CD36
The varied post-translational modifications of CD36 that include ubiquitination, glycosylation, palmitoylation, acetylation, etc. are likely to exert regulatory effects on CD36 trafficking between plasma membranes and intracellular compartments and influence CD36-mediated ligand uptake or signaling functions. Ubiquitination of CD36 was documented on the two carboxy-terminal lysines 469 and 472 and is upregulated by long chain FA while inhibited by insulin (89). The two carboxyl-lysines might be important for CD36 induced protein associations since mutation of these two residues interferes with CD36's ability to induce Ca++ influx via the store-operated membrane channels, although release of calcium from intracellular stores appears normal (43). In contrast, mutation of Lys-164 the residue that interacts with the FA carboxyl group primarily abolishes CD36-mediated calcium release from intracellular stores in response to linoleic acid (44). These differences should help identify some of the molecular steps involved in CD36 regulation of Ca++ signaling. Changes in glycosylation can serve to acutely influence CD36 membrane recruitment as shown in the case of glucagon like peptide 2, which promoted chylomicron production by enhancing membrane CD36 content through inducing its glycosylation (36). Dephosphorylation of CD36 by intestinal alkaline phosphatase was reported to reduce FA uptake and fat absorption (56).
CD36 SIGNALING COORDINATES FAT METABOLISM
In addition to FA, CD36 recognizes a number of lipid ligands (anionic or oxidized phospholipids, diacylglycerol, cholesterol) and binds native (high, low and very low density; HDL, LDL and VLDL) and oxidized lipoproteins (oxLDL and oxHDL). Interaction of ligands with CD36 was shown in many cases to induce CD36-mediated intracellular signaling often initiated by Src tyrosine kinases and involving pathways linked to angiogenesis, inflammation or atherosclerosis (48, 83).
The relevance of CD36 mediated signaling to metabolic regulation and in particular to that involving cellular FA handling was underappreciated. CD36 binding of multiple ligands together with its association with other transmembrane proteins contributes to the diversity of its signal transduction. Our recent data indicate that ligands can differentially regulate CD36 signaling and downstream protein-protein interactions within metabolic protein clusters (Samovski et al., unpublished observations). A property of CD36 that has not been explored for its potential metabolic implications is CD36 interaction with extracellular matrix (ECM) proteins. CD36 binds thrombospondin 1 and collagen (type I and IV) in addition to its association with membrane integrins (β1, β2, β5) and tetraspanins (CD9, CD81) (9, 48, 83). CD36 is among the cellular adhesion receptors that were conserved during the evolution of metazoa (8) and so its interactions with the ECM might have modulatory influence on its functions including those involving nutrient signaling and uptake.
CD36 regulation of phospholipase activity and eicosanoid formation
A recent finding that needs to be integrated into the regulation of FA metabolism is the identification of CD36 influence on cytosolic calcium, the activation of phospholipases and eicosanoid production (19, 43), pathways that have pleiotropic cellular effects. CD36 regulation of cytosolic Ca++ can be dependent (19, 22, 90) or independent (43, 73) of direct FA interaction with CD36. Increases in free cytoplasmic Ca++ induced by the binding of an extracellular ligand to its surface receptor are a major cellular route for information relay and usually involve the release of stored calcium coupled with calcium influx across the plasma membrane. In non-excitable cells a predominant mechanism is the activation of phospholipase C to generate inositol 1,4,5-trisphosphate (IP3), which releases Ca++ from intracellular stores, primarily the endoplasmic reticulum (ER), into the cytosol. The reduced Ca++ level within the ER lumen then activates plasma membrane Ca++ influx, referred to as capacitative or store-operated calcium (SOC) entry. SOC function plays a major regulatory role influencing signal transduction pathways, cell functions as well as gene transcription (18, 42).
Among the metabolic pathways known to be regulated by capacitative calcium entry are those involving membrane phospholipid (PL) remodeling and the release of arachidonic acid (AA) from membrane PL by phospholipases A2 (PLA2) with subsequent AA conversion to bioactive eicosanoids (64). Eicosanoids have pleiotropic effects on cell function and influence metabolic homeostasis (37, 95). Eicosanoid production is tightly regulated with most AA being esterified in PL and the amount of free AA being controlled by the relative activities of PLA2s, AA-specific CoA synthetases and lysophospholipid acyl transferases (37, 49). Cytosolic Ca++-dependent phospholipase A2α (cPLA2α) plays a major role in AA release and is activated (together with other PLA2s) by a rise in intracellular Ca++, initially released from the ER and then sustained by influx via SOC. The activity of cPLA2α is regulated via phosphorylation and translocation to ER and nuclear membranes, where it releases AA from the sn-2 position of PL. Store-operated channels are regulated via phosphorylation by Src family kinases (4) which partner with CD36 and regulate mitogen activated protein kinases that include cPLA2s among their phosphorylation targets.
The role of CD36 in modulating SOC calcium flux, PL remodeling and AA release was documented in CHO cells stably expressing CD36. When these cells are treated with thapsigargin, an inhibitor of ER calcium uptake, they display SOC calcium influx, phosphorylation of cPLA2, translocation of cPLA2 to membranes, AA release and increased production of arachidonic acid-derived prostaglandin E2. These events are not observed in CHO cells lacking CD36 or in cells expressing a mutated form of the protein (43). Significantly altered eicosanoid profiles can be demonstrated in the tissues of the CD36 null mouse.
The importance of CD36's regulatory effects on calcium transients and phospholipid turnover is illustrated by several recent findings as described below. These include the role of CD36 in fat perception (22, 70), phospholipid remodeling and eicosanoid production (43), chylomicron production (93), release of intestinal peptides (90) during fat absorption, secretion of hepatic VLDL (66) and adaptation of myocardial rhythm to energy deprivation (73) as highlighted below.
Role of CD36 in perception of dietary fat by taste bud cells
The flavor of foods is perceived through a combination of all senses; taste, olfaction, somatosensation (e.g. texture, irritation, and temperature), vision and audition. The various inputs are integrated in the brain to converge in the perception we know as flavor (76). Considering the variety of sensory inputs and cognitive processes involved in flavor perception, it is not surprising that there is a wide variation among individuals in their sensory appreciation of foods (61). Perhaps this is best illustrated by the extreme differences between individuals in their ability to taste the bitter compounds 6-n-propylthiouracil (PROP), phenylthiocarbamide (PTC) and glucosinolates (naturally found in cruciferous vegetables) (92). While the majority of subjects (60 to 90%, depending on the population), perceive PROP/PTC as moderate to intensely bitter, 30% find it tasteless (98). The identification of taste receptors and of key elements involved in sensory transduction pathways has significantly enhanced our understanding of the molecular aspects underlying gustatory perception and its variation among individuals. For example, most of the variability in PROP taste sensitivity can be explained by polymorphisms in the bitter receptor gene TAS2R38 (7).
Existence of a taste component in perception of dietary fat
At present, the general consensus is that we perceive five taste qualities: sweet, sour, bitter, salty and umami (the savory, meaty taste of some amino acids). It is believed that these qualities evolved to help us find energy rich nutrients, maintain electrolyte balance and avoid potentially harmful substances. Although fat is still not included among the “primary” taste qualities, a growing body of evidence accumulated over the past decade from studies in humans and rodents now supports its inclusion as the sixth taste. In addition to smell and texture, fatty nutrients have a gustatory taste component which plays an important role in fat perception and might modify feeding behavior (25, 26, 61, 87). Two main classes of taste receptors for FA have been proposed based on molecular evidence and their study provided strong support for the existence of a fat taste component. They consist of the G protein-coupled receptors (GPR) (10) and CD36 (47). The GPRs that function in FA recognition are members of a large family usually with seven transmembrane segments and the family members that similar to CD36 recognize long chain FA are GPR120 and GPR40 (Table 1). Dietary fat is composed mainly of TG but the nutrient sensed is long chain FA which has signal transduction capabilities and is generated from TG digestion by lingual lipase. Rodents have strong lingual lipase activity and its inhibition reduces spontaneous preference for TG but not FA (39). Humans have low amounts of lingual lipase but it is secreted in the cleft of papillae and in proximity of taste buds and its activity is sufficient to generate FA from TG in the mouth. Indeed the concentration of long chain FA rises dramatically in the saliva of subjects asked to chew high fat-foods (26) and lipase inhibition by orlistat reduces sensitivity to the oral perception of triolein but not to that of oleic acid (71).
Table 1.
Secretions induced by fatty acids in taste bud cells and in enteroendocrine cells (EEC) and the membrane FA receptors involved. Circumvallate refers to the circumvallate papillae, Duod/Jejun refers to Duodenum/Jejunum.
| Peptide | Primary site | Fat ligand | FA Receptor | |
|---|---|---|---|---|
| Taste Cells | ||||
| GLP-1 | Circumvallate | LCFA | GPR120 | |
| Serotonin | Circumvallate | LCFA | CD36 | |
| EE Cells | ||||
| K cells | GIP | Duod/Jejun | LCFA | GPR40, 119, 120 |
| S cells | Secretin | Duod/Jejun | LCFA | CD36 |
| I cells | CCK | Duod/Jejun | LCFA | CD36, GPR40 |
| L cells | PYY | Ileum/colon | ShFA | GPR41, 43 |
| L cells | GLP-1 | Ileum/colon | LCFA, OEA | GPR40, 119, 120 |
The FA released by lingual lipase interact with the FA receptors CD36 and GPR120 on the surface of taste receptor cells. These cells which are present within taste buds localized in the gustatory papillae (fungiform, foliate and circumvallate) can establish synapses with afferent nerve fibers (VIIth and IXth) to transfer the gustatory signals to the brain (70). CD36 is expressed on taste bud cells of rodents, pigs and humans. Of the GPR that recognize long chain FA, GPR120 is detected in gustatory and non-gustatory epithelia while GPR40 is not expressed in gustatory tissues (25) suggesting that CD36 and GPR120 are likely to be the physiological receptors mediating fat taste perception.
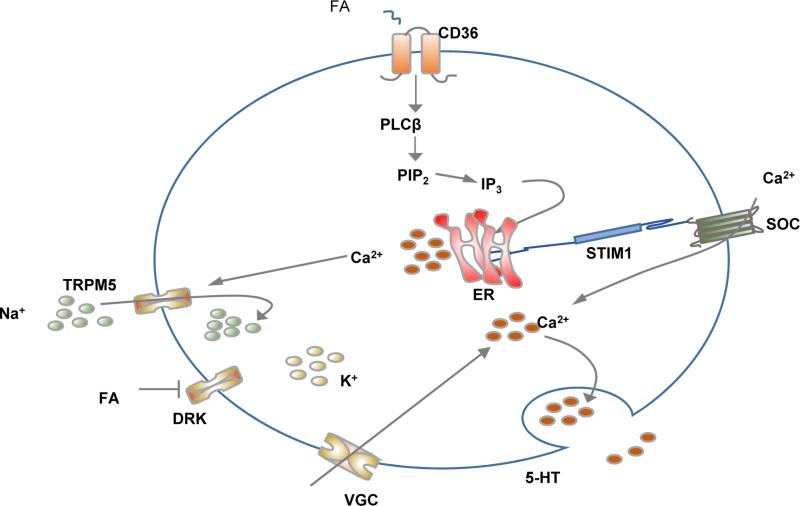
Interaction of the FA with taste bud cells expressing CD36 was shown by El-Yassimi et al., (22) to result in phospholipase C driven formation of IP3 which interacts with its receptor on the ER to release calcium (Fig. 3). Depletion of Ca++ in the ER lumen induces the Ca++ sensor STIM1 to activate membrane SOC and Ca++ flux from the extracellular milieu (19). There is also evidence that the initial rise in cytosolic Ca++ might induce depolarization of the cell membrane by activating Na+ influx trough the transient receptor potential M5 (TRPM5) channel, which was shown to mediate gustatory responses to sweet, savory, bitter and now fatty compounds (51, 80). TRPM5 activation coupled with FA inhibition of the delayed rectifying K+ (DRK) channel to block membrane repolarization would activate voltage gated calcium influx across the membrane (26). Regardless of the mechanisms mediating influx of Ca++ across the membrane, the ensuing sustained increase in cytosolic free Ca++ leads to the release of neurotransmitters notably serotonin (Figure 3) and the signal is transmitted to the gustatory nerves.
Figure 3. Oral perception of fat.
The accumulating evidence supports gustatory cues in fat perception. Dietary fat is composed mainly of triglycerides (TG) but the nutrient sensed on the tongue is the FA released from TG digestion by lingual lipase. The FA interacts with CD36 on taste bud cells in the circumvalate and fungiform papillae (back and sides of the tongue, respectively) to induce intracellular calcium release from the ER. This in turn triggers calcium flux from membrane store operated calcium (SOC) channels leading to neurotransmitter release. The two FA receptors identified on taste bud cells in rodents and humans are CD36 and GPR120 (Table 1) and both were shown to impact sensitivity to oral perception of dietary fat. CD36 is the physiologically functional FA receptor with GPR120 acting to amplify signaling at high FA levels [see text and (69)]. TG: triglyceride, FA: fatty acid, PLC: phospholipase C, PIP2: phosphatidyl inositol-biphosphate, IP3: Inositol triphosphate or inositol 1,4,5-triphosphate, DG: diacylglycerol.
The increase in intracellular Ca++ that mediates neurotransmitter release and fat perception also induces the cephalic phase of digestion (47, 70) characterized by the release of small amounts of bile acids and by an increase in serum TG. Fat-induced cephalic phase responses also include transient secretions of intestinal cholecystokinin (CCK), pancreatic polypeptide, peptide tyrosine, tyrosine and of pancreatic insulin (46). These secretions prepare the organism for fat absorption.
CD36 and fat perception in animal models
Findings in rodents strongly support the physiological function of CD36 as a fat taste receptor. CD36 is expressed by taste receptor cells (47), where it often co-localizes with α-gustducin, a G protein involved in taste transduction that is frequently used as a taste bud marker. CD36 interaction with long chain FA induces calcium transients, neurotransmitter release and involves the neuronal gustatory pathway (22). Knockout of the CD36 gene (47) or reduction of CD36 expression in the tongue using RNA interference techniques (12) impair fat discrimination without affecting perception of other tastes such as sweetness. CD36 deletion also blunts the cephalic phase of pancreato-biliary secretions triggered by exposure of the tongue to fat (47). The physiologic relevance of CD36 in taste perception of dietary fat is reinforced by the finding that CD36 expression is sensitively modulated by dietary lipids. A recent study (58) that examined CD36 expression in mouse circumvallate papillae during the day-night cycle and with dietary manipulations showed CD36 downregulation in the dark period during food intake. This downregulation associated with a reduction in fat preference suggesting that CD36 sensing of dietary lipid might gradually decrease appetite for fat during a meal. In addition, exposure of the tongue to a drop of lipid for few minutes reduced CD36 protein expression in mouse gustatory papilla by ~twofold (58). This down-regulation of CD36 might involve its FA-induced ubiquitination, which has been demonstrated in cultured cells (89) and in vivo (93).
Both GPR40 and GPR120 were shown using the two bottle test to influence spontaneous preference for fat in rodents although the role of GPR40 might be indirect since it is not present in taste bud cells (10, 25). In contrast to CD36, GPR120 expression on taste buds appears unresponsive to ingested fat (58). Although the distinct physiological roles of CD36 versus GPR120 in fat taste perception remain incompletely defined, recent analysis of FA-induced calcium signaling in taste bud cells suggests that while both receptors are coupled to serotonin release, CD36 functions at low FA concentrations while GPR120 is only activated at high FA (69). A low concentration of linoleic acid fails to increase Ca++ in taste cells obtained from CD36−/− mice and a high concentration triggers a Ca++response that is much smaller than what is observed in WT mice. Thus GPR120 appears to be poorly responsive to long chain FA and might function in amplifying the response to high concentrations of dietary FA and other tastants consistent with its expression in a variety of taste cells responsive to various stimuli (10, 69).
CD36 and fat perception in humans
Compared with data from rodents, less is known about the role of CD36 as a lipid taste sensor in humans but recent findings are consistent with such a role. The first study to examine expression of CD36 in human lingual tissue demonstrated CD36 expression in the gustatory papillae (85) although no taste cell markers were used to confirm taste cell identity of the lingual cells expressing CD36. A more recent study with isolated human fungiform taste bud cells demonstrated co-expression of CD36 and GPR120 on taste cells. Selective knock-down of either CD36 or GPR120 in human fungiform taste cells showed that linoleic acid at low concentration induces Ca++ signaling via CD36 and not GPR120 (69). GPR120 displayed a poor response to linoleic acid while a GPR120 agonist induced strong calcium transients in these cells. These data were interpreted to suggest that while CD36 in taste cells would function in FA recognition and taste detection, GPR120 might be important in signal amplification for a more sustained taste experience at high concentrations of fatty food.
Two sensory studies (40, 71) that tested the effect of a common polymorphism in the CD36 gene (rs1761667 involving A/G substitution) provided support for the role of CD36 in the oral sensory perception of fat in humans. However, more work is required for full reconciliation of the two data sets obtained. In the first study, obese subjects carrying the A allele of rs1761667 that reduces CD36 expression in monocytes and platelets (54) had eight- fold higher oral detection thresholds for oleic acid and triolein indicating lower sensitivity for fat perception as compared with obese subjects who were non-carriers. The lipase inhibitor orlistat and solutions of oleic acid or triolein were used in this study to validate that the FA was the orally perceived tastant (71). The second study measured sensory fat perception by obese subjects using salad dressing samples containing fat concentrations well above detection thresholds. The findings showed that subjects homozygous for the A allele perceived more creaminess in salad dressing samples and reported liking more added fats than did those who were heterozygous or non-carriers (40). None of the genotype groups (AA, AG, or GG) discriminated creaminess or oiliness between different salad dressing samples with increasing (5-55% fat by weight) fat content and a nonfat control was not included. Thus definitive interpretation of the results must await further studies. In addition, it is worth noting that the relationship of taste detection thresholds as measured in the first study to fat perception at above-threshold levels in real-world settings as measured in the second study is often not a direct one (6, 72). For example, earlier findings suggested that different pathways might be potentially involved in perceiving threshold versus suprathreshold concentrations of tastants (6, 72). A simplistic and tentative interpretation of the data from the two studies would propose that subjects with low sensitivity to fat taste might display less taste “saturation” and more preference for food with high fat content.
Role of CD36-mediated FA uptake and signaling in absorption of dietary fat
In the small intestine of mice (67) and humans (52) CD36 is abundantly expressed in the proximal segment and localizes to the brush border membranes of duodenal and jejunal villi enterocytes. Epithelial cell immunochemical staining of CD36 is lower in the human and mouse ileum and colon (52, 90).
CD36 function in enterocyte FA uptake
In the intestines of the CD36 null mice, there is a defect in FA uptake by the proximal intestine, demonstrated in isolated enterocytes or in vivo from oleate enrichment of mucosal lipids after an oral triolein load (67). As a result more lipid (TG and cholesterol) reaches the distal small intestinal segments and the defect in proximal FA uptake is compensated for by uptake in the distal intestine (2). In addition to protein facilitated transfer, enterocytes might passively absorb long chain FA that are protonated (32) in the acidic microclimate next to the brush border. Passive and facilitated FA transfer would be coordinated with conversion to the acyl-CoA derivative, trapping the FA inside the cell (59).
Entry of long chain FA across the brush border membrane might also occur, as proposed recently through endocytosis within vesicles formed from lipid rafts containing caveolin-1 and CD36 (81). Lipid rafts are membrane domains enriched in cholesterol and sphingomyelin that contribute to the lateral compartmentalization of surface proteins and function as organizational centers in signal transduction and in the internalization of ligands and receptors. Caveolins 1-3 associate with lipid rafts to form smooth invaginations of the plasma membrane or caveolae. Caveolae endocytosis has been implicated in cholesterol transport and is proposed to traffic cholesterol between the plasma membrane and late endosomes and lysosomes (63). Caveolin-1 was also shown to influence FA uptake into cells (17). Mice deficient in caveolin-1 are lean and protected from high fat diet induced adiposity (75). Although caveolins might influence FA uptake by modulating CD36 localization to lipid rafts, CD36-independent effects of caveolin on FA uptake can be observed (17, 99). In the intestine caveolin-1 is expressed on enterocyte brush border membranes and the apically absorbed FA appears to be internalized by enterocytes in detergent resistant and caveolin-1 containing vesicles that also contain alkaline phosphatase and CD36. In cell lysates, alkaline phosphatase, CD36 and caveolin-1 co-immunoprecipitate consistent with functional interaction. In agreement with the in vitro data, caveolin-1 knockout mice displayed impaired FA absorption as more FA was recovered in the cecal contents of these mice (81). In addition these mice display metabolic inflexibility and reduced ability to perform the fuel switching necessary during fasting/feeding transitions (3) a phenotype shared by CD36 null mice (65).
Recent studies in HEK cells (99) showed that expression of CD36 enhanced FA uptake and promoted intracellular FA esterification (measured in minutes) without altering the rate of membrane FA translocation (measured in seconds). This contrasted with caveolin-1 which affects plasma membrane FA translocation but not FA esterification into TG. These observations would be consistent with our current working model of CD36 mediated FA uptake. Recent evidence documenting the tight coupling between CD36-mediated FA uptake and signaling and between FA signaling and cellular FA metabolism, suggests that the fraction of membrane FA uptake that is facilitated by CD36 serves to coordinate events related to the metabolic processing of the FA (2). Thus CD36-mediated FA uptake might function in a regulatory capacity rather than as a quantitative transport mechanism. This interpretation is also supported by our unpublished data indicating that FA binding to CD36 induces changes in downstream protein-protein interactions that are relevant to FA metabolism (Samovski D et al, unpublished).
FA uptake and signaling regulate chylomicron formation by the proximal intestine
A large part of the absorbed lipid in the CD36 deficient mouse bypasses the lymphatic system, which is normally the main route for delivery of intestinal lipid to the circulation. There is no evidence of lipid malabsorption based on blood appearance of intestinally derived TGafter an oral fat load. However the secreted TG are contained in lipoprotein particles that are smaller than the chylomicron particles released by the intestine of the WT mice (20). The smaller lipoproteins released by the intestines of CD36 null mice have a prolonged life in the circulation resulting in postprandial hypertriglyceridemia (20).
The mechanism underlying the effect of CD36 deficiency to impair chylomicron production is not totally clear but is likely to reflect the absence of CD36-mediated signaling (Figure 4). Although there is a demonstrated defect in FA uptake and incorporation into TG by the proximal intestine of CD36−/− mice, kinetic studies show that enterocyte CD36 functions in high affinity FA uptake similar to its role in other cell types (67). Based on the estimated micromolar concentration of free FA dissociated from micelles in the intestinal lumen, CD36 contribution to intestinal FA uptake would be limited to early stages of the digestive process when the FA concentrations reaching the proximal intestine are low. FA also downregulate levels of the CD36 protein in enterocytes which would further reduce its contribution to net FA absorption (2). As a result CD36-mediated FA uptake in the proximal intestine is likely to have a signaling function linked to chylomicron formation. One possibility is that it might contribute to the newly synthesized lipid pool accessible to the microsomal TG transfer protein for lipidation of apoprotein B protecting it from degradation and initiating formation of the primordial lipid particle.
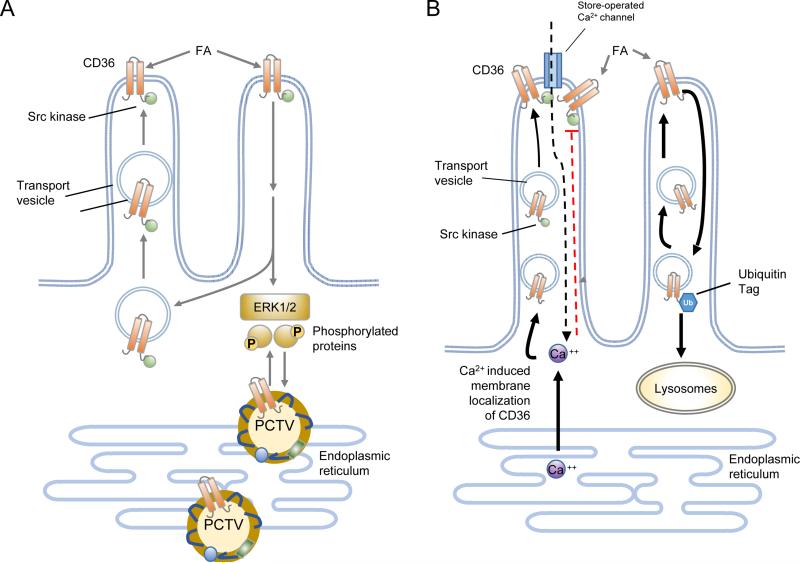
Figure 4. Working model of the molecular steps that may be involved in how intestinal CD36 facilitates FA uptake and chylomicron formation.
Panel A: CD36 expressed on the apical side of enterocytes of the proximal intestine interacts with fatty acids (FA) released from the digestion of dietary triglycerides. During the early phase of digestion when the FA concentrations are relatively low CD36 facilitates FA uptake probably by internalizing the FA within vesicles derived from membrane lipid rafts. The CD36-mediated FA uptake associates with intracellular signaling to promote events that facilitate chylomicron assembly. CD36 signaling is initiated in most cases via the Src kinases that associate with the C terminus of CD36 and downstream would involve the extracellular regulated kinase (ERK1/2). CD36 signaling may be important for phosphorylating proteins required to coordinate ER processing of prechylomicron vesicles (PCTV). CD36 has also been identified in the protein complex required for formation of the PCTV (82). Recent data indicate that CD36 signaling may be mediated by inositol triphosphate (IP3)-induced release of ER calcium (see Fig. 3). ER calcium release promotes membrane CD36 localization and also induces calcium influx via store operated calcium channels. The sustained increase in intracellular calcium could influence multiple events related to lipid processing or secretion. Panel B) CD36 is downregulated by FA via its ubiquitination on its carboxyl terminus which targets it to lysosomal degradation. This feedback loop may work to reduce CD36 function in the presence of excess FA supply. Inside the enterocytes the FA-induced decrease in CD36 associates with reduced activation of ERK1/2 which may serve to upregulate abundance of the microsomal triglyceride transfer protein (MTTP). Adapted from (2).
CD36 was identified together with liver FA binding protein (L-FABP) to be required for assembly of the multiprotein complex that generates the prechylomicron transport vesicle from the intestinal ER (82). CD36 signaling possibly to the extracellular regulated kinase 1/2 (ERK1/2) might influence phosphorylation of proteins important for ER lipid processing (Figure 4). It can be speculated that CD36's ability to influence store operated calcium flux and the secretion of bioactive compounds may contribute to its effects on chylomicron production. For example, in the liver CD36-mediated prostaglandin production was found to influence output of very low density lipoproteins (see section on hepatic CD36).
In addition to FA, CD36 may facilitate intestinal cholesterol absorption for optimal chylomicron production. Enterocytes isolated from CD36 null mice exhibit reduced cholesterol uptake (67) and in vivo cholesterol output into the lymph is reduced by 50% but like with FA the contribution of CD36-mediated uptake to net absorption is small as no changes in 24h fecal secretion are observed in CD36 null mice (2).
In summary the findings to date suggest that CD36 functions at different stages of fat digestion. CD36 mediates perception of FA in lingual taste bud cells and the initiation of the cephalic phase of digestion. In the small intestine CD36 transport provides the oleic acid needed for generation of oleoylethanolamide (OEA), which reduces food intake and the FA and cholesterol needed to initiate chylomicron production. Its contribution to the net absorption of either nutrient is small but CD36's signal transduction capabilities might play an important role in coordinating the incorporation of FA and cholesterol into esters for chylomicron production. CD36 appears required for assembly of the pre-chylomicron transport vesicle and its secretion from the ER.
In humans CD36 deficiency is rare in Caucasians but is relatively common (~6%) in persons of Asian and African descent and associates with altered levels of plasma cholesterol, TG and FA (53). Abnormal postprandial plasma lipids in humans with CD36 deficiency or with SNPs in the CD36 gene reflect in part abnormal peripheral clearance of plasma FA since impaired tissue FA uptake has been documented in humans with CD36 deficiency. However, contribution of defective lipid processing by the small intestine to the lipid abnormalities is suggested by findings of postprandial lipemia with high apoB48 levels and high concentration of chylomicron remnants in CD36 deficient subjects (45, 60).
Role of CD36 in enteroendocrine secretions
As the fatty food reaches the upper gut, a number of secretions by enteroendocrine cells (EEC) occur that influence the digestive process as well as satiety and overall energy homeostasis. Some of these secretions are known to be triggered by FA involving FA receptors such as GPR120, GPR40 and CD36 on EEC (2, 35, 90). Dysfunction of oral or gut fat sensing or a blunting of the pro-satiety effects of dietary fat might contribute to obesity (86). Among the satiety peptides, CCK and secretin are released in response to fat reaching the intestinal lumen, triggered by the nutrient interaction with receptors on I (CCK) and S (secretin) cells, mostly in the duodenum. Serotonin (5-hydroxytryptamine,) is released from EEC in the intestinal mucosa and from nerve terminals of the enteric nervous system and the intra-pancreatic nerves. Serotonin has a variety of effects acting through different receptor subtypes and it mediates some actions of secretin and CCK.
Enteroendocrine cells in the intestinal mucosa (Table 1), which constitute less than 1% of the epithelial cell population, are the FA sensors. These cells were shown to express the long chain FA receptors GPR40, GPR120 (21) and CD36 (90). EEC also express GPR41 and GPR43 which recognize short chain FA, GPR84, which is activated by medium chain FA and GPR119 that recognizes the oleic acid derivative OEA (35).
Long chain FA stimulate CCK secretion by I cells in the mucosa of the duodenum, jejunum, and proximal ileum. CCK helps optimize fat digestion by regulating gastric emptying, gallbladder contraction, pancreatic secretion, and intestinal motility (11). Long chain FA induce S and K cells in the duodenum and jejunum to release, respectively, secretin and the glucose insulinotropic peptide (GIP). Secretin inhibits gastric emptying and synergizes with CCK to induce pancreatic secretions (13). GPR40 (50) and CD36 (90) have been implicated in mediating the effect of FA on CCK. Isolated I cells that express GPR40 respond to linoleic acid with increases in intracellular calcium and CCK release. CCK secretion induced by oleic acid is reduced in GPR40 null mice (50) (Table 4) The CD36 null mouse displays a 50% reduction in release of CCK and secretin in response to gastric administration of oil. Diminished release in response to FA is also observed with CD36 deficient intestinal segments in vitro. In EEC expressing CD36 release of CCK and secretin involves FA-induced increases in calcium and the second messenger cAMP (90). GPR40 is present on EEC expressing the insulinotropic peptides (incretins, GIP and glucagon-like peptide 1, GLP-1) and its disruption reduces secretion of these peptides (21). Release of GLP-1 is stimulated in vivo by FA reaching the distal intestine and involves GPR120 (35). CD36 expression on EEC does not alter release of the incretins (Sundaresan S., unpublished observations) but in vivo incretin release in response to intragastric lipid administration is increased in CD36−/− mice probably reflecting the higher concentration of lipid reaching the distant small intestine.
Fatty acid binding to CD36 on enteroendocrine cells associates with increases in intracellular calcium and cAMP (69, 90). Ligand binding to the GPRs activates intracellular heterotrimeric G proteins and distinct signal transduction pathways depending on the type of G-protein coupling (35).
CD36 function in the intestine might impact fat-induced satiety through various mechanisms. CD36-mediated CCK secretion has pro-satiety effects mediated by receptors on vagal afferents in the duodenum, which signal to the brain (11). The small intestine generates OEA, a pro-satiety lipid from absorbed oleic acid that acts centrally to prolong inter-meal intervals and reduce feeding frequency (74). Generation of OEA is CD36-dependent and disruption of CD36 or peroxisome proliferator activated receptor alpha the OEA target, impairs its prosatiety effects (Table 1). The satiety inducing property of fat can be blunted by factors such as the simultaneous ingestion of carbohydrates and the release of endocannabinoids by the palatable fat-sugar mix (e.g. in processed food) (84).
Hepatic CD36 regulates VLDL output. High CD36 in some forms of NAFLD may be an adaptation to lipid accumulation
In the mouse liver CD36 is expressed on endothelial, parenchymal, and Kupffer cells. Expression level is low but it increases during fasting possibly to enhance hepatic uptake of FA mobilized from adipose tissue. Activation of the transcription factors liver X, pregnane X and aryl hydrocarbon receptors by xenobiotics, bacteria, or cytokines induces CD36 expression and lipid deposition in the liver. Consumption of a high-fat diet increases liver CD36, hepatic FA uptake and TG accumulation, and these are prevented by CD36 deletion. However, TG accumulation in response to consumption of a high-fructose diet, which enhances hepatic de novo lipogenesis (DNL) is exacerbated by CD36 deletion.
Humans who have nonalcoholic fatty liver disease (NAFLD) have high hepatic CD36 levels (29). However several findings suggest that the role of CD36 in hepatic FA metabolism is not limited to enhancing FA flux and TG accumulation. In NAFLD increased output of TG in VLDL is usually not paralleled with increases in VLDL-apoB output (23). Recent genetic studies associated CD36 level with serum apoproteinB and with VLDL particle number in addition to VLDL-TG (54). These associations suggested influence of CD36 on VLDL secretion in humans. In agreement with this, a recent study (66) documented that CD36 deletion results in 60% suppression of VLDL output in vivo, and from incubated liver slices in vitro. The effect of CD36 deletion was mediated, at least in part, by enhancing formation of hepatic prostaglandins D2, F2, and E2. Treatment of CD36-deficient slices with inhibitors of cyclooxygenases reversed the reduction in TG secretion. Interestingly, CD36 deletion in ob/ob mice exacerbated the obesity-associated spontaneous steatosis in this model. Livers of ob/ob mice had 5-fold more CD36 primarily on Kupffer cells, but also on hepatocytes, and exhibited steatosis that was driven by increased DNL. CD36 deletion exacerbated the steatosis, by impairing hepatic TG and apoB secretion through increasing prostaglandin levels (66). These findings suggested an unappreciated role of CD36 in regulating VLDL secretion, which might have relevance to some forms of fatty liver. They also provide insight into the association reported in humans between CD36 protein expression and serum levels of apoB and VLDL particle number (54).
CD36 influences heart Ca++ dynamics and functional adaptation to nutrient deprivation
Historically, based on studies in rodents and humans, CD36 in heart and skeletal muscle has been viewed as a FA transporter delivering a major fraction of FA uptake (28, 91). During acute metabolic challenges such as feeding, fasting, or exercise, muscle can adjust FA utilization rates to FA availability. This metabolic flexibility is required for maintenance of cellular homeostasis, since the muscle has limited capacity for FA storage. FA uptake rates that exceed the oxidative capacity of muscle result in ectopic FA accumulation and subsequently lead to deleterious metabolic consequences that compromise function (78). CD36 contributes to the metabolic adjustments of muscle FA uptake and its deletion abolishes the muscle's metabolic flexibility and ability to adapt to fasting (65). This role of CD36 is the result of the exquisite regulation of its recruitment to the sarcolemma by energy status or exercise involving the activation of PI3 Kinase (PI3K)–Akt and AMP kinase (AMPK) signaling (28, 77). The two signaling pathways converge downstream to induce phosphorylation and activation of the AS160 Rab GAP (GTPase Activating Protein) a master regulator of vesicular trafficking (77). In further support of vesicular trafficking of CD36, its sarcolemmal recruitment was shown to involve vesicle-associated membrane proteins (VAMPs) (79). In insulin resistant human muscle, CD36 trafficking is disrupted with chronic relocation of CD36 to the sarcolemma (24, 27) which associates with FA accumulation.
The metabolic implications of CD36 mediated signal transduction in heart and skeletal muscle have been addressed only recently. CD36 was found to modulate myocardial Ca++ metabolism and FA cycling into phospholipids (73). CD36 deletion in mice altered myocardial proteins functioning in ER Ca++ handling, increased heart lysophospholipid content and altered FA composition of phospholipids. Eicosanoid production by the heart was dramatically altered (Gross RW, unpublished observations). These alterations impaired the functional adaptation of the heart to nutrient deprivation, as evidenced by electrical anomalies observed after an 18h fast. These included slow Ca++ transients, delayed signal propagation and atrioventricular block in CD36 null mice with incidence of sudden death (73). Our recent findings (Samovski et al, Diabetes 2015 Epub) document the direct role of CD36-mediated signal transduction in FA activation of AMPK and subsequently of FA β-oxidation. Together the above data suggest an important regulatory role of CD36 mediated signal transduction in muscle. The implications with regard to the maintenance of metabolic flexibility and insulin sensitivity will need to be explored further as they are relevant to obesity associated insulin resistance and heart disease.
CONCLUSIONS
The role of CD36 as a high affinity pathway for cellular FA uptake and utilization and its influence on lipid metabolism have been well documented in mice and relevance of the data to humans has also been demonstrated [reviewed in (2)]. CD36 transduces intracellular signals initiated by Src kinase partners. Although CD36 signaling effects on pathways related to inflammation and atherosclerosis have been well studied [reviewed in (83)], the influence of CD36 signaling on cellular fat metabolism has not been examined. Recent findings uncovered the role of CD36 signaling in regulating Ca++ activation of phospholipases that release AA from membrane PL, remodel membrane FA composition and result in the formation of the pleiotropically bioactive eicosanoids. Emerging evidence described in this review supports importance of CD36-mediated signaling in fat taste perception, chylomicron formation, FA-induction of gut peptide secretion, hepatic VLDL output, Ca++ dynamics and the activation of mitochondrial FA oxidation by muscle cells. Together the findings support our hypothesis that CD36-dependent FA signaling is integral to FA utilization and could play a crucial role in dysfunction of FA metabolism and disease risk. The evidence for a key metabolic role of CD36 in humans is strong [reviewed in (53)], and understanding the mechanisms that mediate and regulate CD36 function in fat utilization is of significant interest in light of the high morbidity and economic burden consequent to obesity-related diseases worldwide.
ACKNOWLEDGMENTS
The authors’ research was supported in part by grants from the National Institute of Health (DK-033301, DK-60022 to NAA), by Nutrition and Obesity Research Center grant P30 DK-056341, by grant GACR 13-04449 from the Czech Science Foundation to OK. We thank Terri Pietka for assistance with figure design and text editing.
Abbreviations
- FA
fatty acids
- SSO
sulfo-N-succinimidyl-oleate
- VLDL
very low density lipoproteins
- oxLDL
oxidized low density lipoproteins
- SOC
store operated calcium channel
- ER
endoplasmic reticulum
- CCK
cholecystokinin
- GPR
G-protein coupled receptor
- OEA
oleoylethanolamide
Footnotes
DISCLOSURE STATEMENT
The authors have no conflicts to disclose
LITTERATURE CITED
- 1.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–8. [PubMed] [Google Scholar]
- 2.Abumrad NA, Davidson NO. Role of the Gut in Lipid Homeostasis. Physiological Reviews. 2012;92:1061–85. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asterholm IW, Mundy DI, Weng J, Anderson RG, Scherer PE. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 2012;15:171–85. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babnigg G, Bowersox SR, Villereal ML. The role of pp60c-src in the regulation of calcium entry via store-operated calcium channels. J Biol Chem. 1997;272:29434–7. doi: 10.1074/jbc.272.47.29434. [DOI] [PubMed] [Google Scholar]
- 5.Baillie AGS, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol. 1996;153:75–81. doi: 10.1007/s002329900111. [DOI] [PubMed] [Google Scholar]
- 6.Bartoshuk LM. The psychophysics of taste. Am J Clin Nutr. 1978;31:1068–77. doi: 10.1093/ajcn/31.6.1068. [DOI] [PubMed] [Google Scholar]
- 7.Behrens M, Meyerhof W. Bitter taste receptor research comes of age: from characterization to modulation of TAS2Rs. Semin Cell Dev Biol. 2013;24:215–21. doi: 10.1016/j.semcdb.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Bentley AA, Adams JC. The evolution of thrombospondins and their ligand-binding activities. Mol Biol Evol. 2010;27:2187–97. doi: 10.1093/molbev/msq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–34. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 10.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–82. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2007;14:63–7. doi: 10.1097/MED.0b013e3280122850. [DOI] [PubMed] [Google Scholar]
- 12.Chen CS, Bench EM, Allerton TD, Schreiber AL, Arceneaux KP, 3rd, Primeaux SD. Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1346–55. doi: 10.1152/ajpregu.00582.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chey WY, Chang TM. Secretin, 100 years later. J Gastroenterol. 2003;38:1025–35. doi: 10.1007/s00535-003-1235-3. [DOI] [PubMed] [Google Scholar]
- 14.Coburn C, Abumrad NA, editors. Structure function of CD36 and evidence for it's role in facilitating fatty acid transport. Wiley-VCH Publishers GMBH & Co; 2003. [Google Scholar]
- 15.Coburn CT, Knapp FF, Jr., Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice [In Process Citation]. J Biol Chem. 2000;275:32523–9. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 16.Coort SL, Willems J, Coumans WA, van der Vusse GJ, Bonen A, Glatz JF, Luiken JJ. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol Cell Biochem. 2002;239:213–9. [PubMed] [Google Scholar]
- 17.Covey SD, Brunet RH, Gandhi SG, McFarlane N, Boreham DR, Gerber GE, Trigatti BL. Cholesterol depletion inhibits fatty acid uptake without affecting CD36 or caveolin-1 distribution in adipocytes. Biochem Biophys Res Commun. 2007;355:67–71. doi: 10.1016/j.bbrc.2007.01.135. [DOI] [PubMed] [Google Scholar]
- 18.Decrock E, De Bock M, Wang N, Gadicherla AK, Bol M, Delvaeye T, Vandenabeele P, Vinken M, Bultynck G, Krysko DV, Leybaert L. IP3, a small molecule with a powerful message. Biochim Biophys Acta. 2013;1833:1772–86. doi: 10.1016/j.bbamcr.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Dramane G, Abdoul-Azize S, Hichami A, Vogtle T, Akpona S, Chouabe C, Sadou H, Nieswandt B, Besnard P, Khan NA. STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. J Clin Invest. 2012 doi: 10.1172/JCI59953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–7. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–7. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem. 2008;283:12949–59. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 23.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–31. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galindo MM, Voigt N, Stein J, van Lengerich J, Raguse JD, Hofmann T, Meyerhof W, Behrens M. G protein-coupled receptors in human fat taste perception. Chem Senses. 2012;37:123–39. doi: 10.1093/chemse/bjr069. [DOI] [PubMed] [Google Scholar]
- 26.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: Advances and challenges. Prog Lipid Res. 2013;53C:82–92. doi: 10.1016/j.plipres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Glatz JF, Angin Y, Steinbusch LK, Schwenk RW, Luiken JJ. CD36 as a target to prevent cardiac lipotoxicity and insulin resistance. Prostaglandins Leukot Essent Fatty Acids. 2013;88:71–7. doi: 10.1016/j.plefa.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 29.Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T, Laitinen S, Kolak M, Fisher RM, Hamsten A, Auvinen P, Yki-Jarvinen H. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–7. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 30.Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:W270–7. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002;109:1381–9. doi: 10.1172/JCI14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton JA, Guo W, Kamp F. Mechanism of cellular uptake of long-chain fatty acids: Do we need cellular proteins? Mol Cell Biochem. 2002;239:17–23. [PubMed] [Google Scholar]
- 33.Harmon CM, Abumrad NA. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J Membr Biol. 1993;133:43–9. doi: 10.1007/BF00231876. [DOI] [PubMed] [Google Scholar]
- 34.Hirano K, Kuwasako T, Nakagawa-Toyama Y, Janabi M, Yamashita S, Matsuzawa Y. Pathophysiology of human genetic CD36 deficiency. Trends Cardiovasc Med. 2003;13:136–41. doi: 10.1016/s1050-1738(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 35.Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol Pharm Bull. 2008;31:1847–51. doi: 10.1248/bpb.31.1847. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh J, Longuet C, Maida A, Bahrami J, Xu E, Baker CL, Brubaker PL, Drucker DJ, Adeli K. Glucagon-Like Peptide-2 Increases Intestinal Lipid Absorption and Chylomicron Production via CD36. Gastroenterology. 2009;137:997–1005. e4. doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins CM, Cedars A, Gross RW. Eicosanoid signalling pathways in the heart. Cardiovasc Res. 2009;82:240–9. doi: 10.1093/cvr/cvn346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kar NS, Ashraf MZ, Valiyaveettil M, Podrez EA. Mapping and characterization of the binding site for specific oxidized phospholipids and oxidized low density lipoprotein of scavenger receptor CD36. J Biol Chem. 2008;283:8765–71. doi: 10.1074/jbc.M709195200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol. 2003;285:17. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]
- 40.Keller KL. Genetic influences on oral fat perception and preference: Presented at the symposium “The Taste for Fat: New Discoveries on the Role of Fat in Sensory Perception, Metabolism, Sensory Pleasure and Beyond” held at the Institute of Food Technologists 2011 Annual Meeting, New Orleans, LA, June 12, 2011. J Food Sci. 2012;77:1750–3841. doi: 10.1111/j.1750-3841.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 41.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–71. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 42.Kiviluoto S, Vervliet T, Ivanova H, Decuypere JP, De Smedt H, Missiaen L, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate receptors during endoplasmic reticulum stress. Biochim Biophys Acta. 2013;1833:1612–24. doi: 10.1016/j.bbamcr.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Kuda O, Jenkins CM, Skinner JR, Moon SH, Su X, Gross RW, Abumrad NA. CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J Biol Chem. 2011;286:17785–95. doi: 10.1074/jbc.M111.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuda O, Pietka TA, Demianova Z, Kudova E, Cvacka J, Kopecky J, Abumrad NA. Sulfo-N-succinimidyl Oleate (SSO) inhibits fatty acid uptake and signaling for intracellular calcium via binding CD36 lysine 164. SSO also Inhibits oxLDL uptake by macrophages. J Biol Chem. 2013 doi: 10.1074/jbc.M113.473298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuwasako T, Hirano K, Sakai N, Ishigami M, Hiraoka H, Yakub MJ, Yamauchi-Takihara K, Yamashita S, Matsuzawa Y. Lipoprotein abnormalities in human genetic CD36 deficiency associated with insulin resistance and abnormal fatty acid metabolism. Diabetes Care. 2003;26:1647–8. doi: 10.2337/diacare.26.5.1647-a. [DOI] [PubMed] [Google Scholar]
- 46.Lambert JE, Parks EJ. Postprandial metabolism of meal triglyceride in humans. Biochim Biophys Acta. 2012;1821:721–6. doi: 10.1016/j.bbalip.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–84. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leslie CC. Regulation of arachidonic acid availability for eicosanoid production. Biochem Cell Biol. 2004;82:1–17. doi: 10.1139/o03-080. [DOI] [PubMed] [Google Scholar]
- 50.Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140:903–12. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu P, Shah BP, Croasdell S, Gilbertson TA. Transient receptor potential channel type M5 is essential for fat taste. J Neurosci. 2011;31:8634–42. doi: 10.1523/JNEUROSCI.6273-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lobo MV, Huerta L, Ruiz-Velasco N, Teixeiro E, de la Cueva P, Celdran A, Martin-Hidalgo A, Vega MA, Bragado R. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J Histochem Cytochem. 2001;49:1253–60. doi: 10.1177/002215540104901007. [DOI] [PubMed] [Google Scholar]
- 53.Love-Gregory L, Abumrad NA. CD36 genetics and the metabolic complications of obesity. Curr Opin Clin Nutr Metab Care. 2011;14:527–34. doi: 10.1097/MCO.0b013e32834bbac9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Love-Gregory L, Sherva R, Schappe T, Qi JS, McCrea J, Klein S, Connelly MA, Abumrad NA. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum Mol Genet. 2011;20:193–201. doi: 10.1093/hmg/ddq449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, Kelstrup CD, Dmytriyev A, Choudhary C, Lundby C, Olsen JV. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2:419–31. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lynes M, Narisawa S, Millan JL, Widmaier EP. Interactions between CD36 and global intestinal alkaline phosphatase in mouse small intestine and effects of high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1738–47. doi: 10.1152/ajpregu.00235.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–64. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One. 2011;6:25. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mashek DG, Coleman RA. Cellular fatty acid uptake: the contribution of metabolism. Curr Opin Lipidol. 2006;17:274–8. doi: 10.1097/01.mol.0000226119.20307.2b. [DOI] [PubMed] [Google Scholar]
- 60.Masuda D, Hirano K, Oku H, Sandoval JC, Kawase R, Yuasa-Kawase M, Yamashita Y, Takada M, Tsubakio-Yamamoto K, Tochino Y, Koseki M, Matsuura F, Nishida M, Kawamoto T, Ishigami M, Hori M, Shimomura I, Yamashita S. Chylomicron remnants are increased in the postprandial state in CD36 deficiency. J Lipid Res. 2009;50:999–1011. doi: 10.1194/jlr.P700032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mattes RD. Accumulating evidence supports a taste component for free fatty acids in humans. Physiology & Behavior. 2011;104:624–31. doi: 10.1016/j.physbeh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–48. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mundy DI, Li WP, Luby-Phelps K, Anderson RG. Caveolin targeting to late endosome/lysosomal membranes is induced by perturbations of lysosomal pH and cholesterol content. Mol Biol Cell. 2012;23:864–80. doi: 10.1091/mbc.E11-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 65.Nahle Z, Hsieh M, Pietka T, Coburn CT, Grimaldi PA, Zhang MQ, Das D, Abumrad NA. CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress. J Biol Chem. 2008;283:14317–26. doi: 10.1074/jbc.M706478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nassir F, Adewole OL, Brunt EM, Abumrad NA. CD36 deletion reduces VLDL secretion, modulates liver prostaglandins, and exacerbates hepatic steatosis in ob/ob mice. J Lipid Res. 2013;54:2988–97. doi: 10.1194/jlr.M037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;282:19493–501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 68.Neculai D, Schwake M, Ravichandran M, Zunke F, Collins RF, Peters J, Neculai M, Plumb J, Loppnau P, Pizarro JC, Seitova A, Trimble WS, Saftig P, Grinstein S, Dhe-Paganon S. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature. 2013;504:172–6. doi: 10.1038/nature12684. [DOI] [PubMed] [Google Scholar]
- 69.Ozdener MH, Subramanian S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan N. CD36 and GPR120 mediated Ca2+ signaling in human taste bud cells: differential responses to fatty acids and obesity. Gastrenterology. 2014 doi: 10.1053/j.gastro.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Passilly-Degrace P, Chevrot M, Bernard A, Ancel D, Martin C, Besnard P. Is the taste of fat regulated? Biochimie. 2014;96:3–7. doi: 10.1016/j.biochi.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 71.Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 2012;53:561–6. doi: 10.1194/jlr.M021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity (Silver Spring) 2010;18:959–65. doi: 10.1038/oby.2009.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pietka TA, Sulkin MS, Kuda O, Wang W, Zhou D, Yamada KA, Yang K, Su X, Gross RW, Nerbonne JM, Efimov IR, Abumrad NA. CD36 Protein Influences Myocardial Ca2+ Homeostasis and Phospholipid Metabolism: CONDUCTION ANOMALIES IN CD36-DEFICIENT MICE DURING FASTING. J Biol Chem. 2012;287:38901–12. doi: 10.1074/jbc.M112.413609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piomelli D. A fatty gut feeling. Trends Endocrinol Metab. 2013;24:332–41. doi: 10.1016/j.tem.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Razani B, Park DS, Miyanaga Y, Ghatpande A, Cohen J, Wang XB, Scherer PE, Evans T, Lisanti MP. Molecular cloning and developmental expression of the caveolin gene family in the amphibian Xenopus laevis. Biochemistry. 2002;41:7914–24. doi: 10.1021/bi020043n. [DOI] [PubMed] [Google Scholar]
- 76.Rolls ET. Taste, olfactory and food texture reward processing in the brain and the control of appetite. Proc Nutr Soc. 2013;71:488–501. doi: 10.1017/S0029665112000821. [DOI] [PubMed] [Google Scholar]
- 77.Samovski D, Su X, Xu Y, Abumrad NA, Stahl PD. Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase. J Lipid Res. 2012;53:709–17. doi: 10.1194/jlr.M023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwenk RW, Dirkx E, Coumans WA, Bonen A, Klip A, Glatz JF, Luiken JJ. Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes. Diabetologia. 2010;53:2209–19. doi: 10.1007/s00125-010-1832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1504–13. doi: 10.1152/ajpregu.00364.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siddiqi S, Sheth A, Patel F, Barnes M, Mansbach CM. Intestinal Caveolin-1 is important for dietary fatty acid uptake. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2013 doi: 10.1016/j.bbalip.2013.05.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siddiqi S, Saleem U, Abumrad NA, Davidson NO, Storch J, Siddiqi SA, Mansbach CM., 2nd A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J Lipid Res. 2010;51:1918–28. doi: 10.1194/jlr.M005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silverstein RL, Li W, Park YM, Rahaman SO. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc. 2010;121:206–20. [PMC free article] [PubMed] [Google Scholar]
- 84.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–90. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Simons PJ, Kummer JA, Luiken JJ, Boon L. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 2011;113:839–43. doi: 10.1016/j.acthis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 86.Stewart JE, Newman LP, Keast RS. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin Nutr. 2011;30:838–44. doi: 10.1016/j.clnu.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 87.Stewart JE, Keast RS. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int J Obes (Lond) 2012;36:834–42. doi: 10.1038/ijo.2011.155. [DOI] [PubMed] [Google Scholar]
- 88.Storch J, McDermott L. Structural and functional analysis of fatty acid-binding proteins. J Lipid Res. 2009;50(Suppl):S126–31. doi: 10.1194/jlr.R800084-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009 doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sundaresan S, Shahid R, Riehl TE, Chandra R, Nassir F, Stenson WF, Liddle RA, Abumrad NA. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. Faseb J. 2013;27:1191–202. doi: 10.1096/fj.12-217703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanaka T, Nakata T, Oka T, Ogawa T, Okamoto F, Kusaka Y, Sohmiya K, Shimamoto K, Itakura K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res. 2001;42:751–9. [PubMed] [Google Scholar]
- 92.Tepper BJ, Williams TZ, Burgess JR, Antalis CJ, Mattes RD. Genetic variation in bitter taste and plasma markers of anti-oxidant status in college women. Int J Food Sci Nutr 60 Suppl. 2009;2:35–45. doi: 10.1080/09637480802304499. [DOI] [PubMed] [Google Scholar]
- 93.Tran TT, Poirier H, Clement L, Nassir F, Pelsers MM, Petit V, Degrace P, Monnot MC, Glatz JF, Abumrad NA, Besnard P, Niot I. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem. 2011;286:25201–10. doi: 10.1074/jbc.M111.233551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watanabe K, Ohta Y, Toba K, Ogawa Y, Hanawa H, Hirokawa Y, Kodama M, Tanabe N, Hirono S, Ohkura Y, Nakamura Y, Kato K, Aizawa Y, Fuse I, Miyajima S, Kusano Y, Nagamoto T, Hasegawa G, Naito M. Myocardial CD36 expression and fatty acid accumulation in patients with type I and II CD36 deficiency. Ann Nucl Med. 1998;12:261–6. doi: 10.1007/BF03164911. [DOI] [PubMed] [Google Scholar]
- 95.Watkins SM, Hotamisligil GS. Promoting atherosclerosis in type 1 diabetes through the selective activation of arachidonic acid and PGE(2) production. Circ Res. 2012;111:394–6. doi: 10.1161/CIRCRESAHA.112.273508. [DOI] [PubMed] [Google Scholar]
- 96.Welsh JA, Sharma A, Cunningham SA, Vos MB. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation. 2011;123:249–57. doi: 10.1161/CIRCULATIONAHA.110.972166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Willett WC, Stampfer MJ. Current evidence on healthy eating. Annu Rev Public Health. 2013;34:77–95. doi: 10.1146/annurev-publhealth-031811-124646. [DOI] [PubMed] [Google Scholar]
- 98.Wooding S, Bufe B, Grassi C, Howard MT, Stone AC, Vazquez M, Dunn DM, Meyerhof W, Weiss RB, Bamshad MJ. Independent evolution of bitter-taste sensitivity in humans and chimpanzees. Nature. 2006;440:930–4. doi: 10.1038/nature04655. [DOI] [PubMed] [Google Scholar]
- 99.Xu S, Jay A, Brunaldi K, Huang N, Hamilton JA. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 2013;52:7254–61. doi: 10.1021/bi400914c. [DOI] [PubMed] [Google Scholar]
- 100.Yamashita S, Hirano K, Kuwasako T, Janabi M, Toyama Y, Ishigami M, Sakai N. Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mol Cell Biochem. 2007;299:19–22. doi: 10.1007/s11010-005-9031-4. [DOI] [PubMed] [Google Scholar]