Abstract
Objective
To describe the effect of lifting maneuver and quantity of weight lifted on the generation of intra-abdominal pressure.
Study Design
Forty-one women undergoing urodynamic evaluation performed four lifting maneuvers, each while lifting 0, 2.5, 5, 10, and 15 kg. The lifting maneuvers were routine activities including squatting with and without assistance, lifting from a counter and receiving weight. Pressure was recorded with a rectal microtip catheter. Each lift was performed twice and the average pressure change was analyzed.
Results
Controlling for potential confounding variables, repeated-measures ANOVA revealed a significant interaction between lift weight and lift maneuver (p= <0.001). Squatting was associated with generation of higher intra-abdominal pressure than lifting from a counter or receiving weights into outstretched arms (p= <0.001). Lifting ≥2.5 kg resulted in significant changes in intra-abdominal pressure regardless of lift maneuver (p= <0.001).
Conclusions
Both lifting maneuver and quantity of weight should be considered when counseling patients regarding postoperative lifting.
Keywords: Intra-abdominal pressure, weight lifting, postoperative instructions, pelvic floor surgery
Introduction
Each year, over 200,000 surgeries are performed in the United States for the treatment of pelvic organ prolapse (POP).1 The estimated lifetime risk of having surgery for either POP or urinary incontinence up to 80 years of age is 11.1%.2 Unfortunately, re-operation rates approach 29%.2, 3 With the aging American population, the demand for the care of pelvic floor disorders has been projected to increase by 45% over the next 30 years.4 Given the significant prevalence of POP and imperfect surgical outcomes, we seek ways to decrease incidence, as well as prevent recurrence of pelvic floor disorders. Increased intra-abdominal pressure (IAP) has been suggested as a potential contributor to pelvic floor dysfunction.
It has been established that women who lift heavy weights on a daily basis are at higher risk of developing a pelvic floor disorder.5 Woodman et al. demonstrated occupation type to be highly associated with the development of POP. Laborers and factory workers were at 7.7 times higher risk of developing POP when compared to all other professions.6 A study performed with a group of assistant nurses showed that these women have a 1.6 times higher risk of undergoing surgery for POP, as well as surgery for herniated lumbar discs.7 A study from Poland showed that the incidence of stress incontinence and POP increased with the weight of routinely lifted objects.8 It is postulated in these studies that chronic strenuous physical activity and heavy lifting leads to increased IAP. This pressure is transmitted to the pelvic floor muscles, nerves, and connective tissue and may lead to their injury, especially if performed chronically.
Unfortunately, unlike in orthopedics, pelvic floor muscles cannot be immobilized during the postoperative healing phase. The only way to protect the tissue from additional injury is to decrease the pressure in the abdominal cavity. This is why postoperative instructions after urogynecologic procedures are perceived to be important. Currently, urogynecologic postoperative instructions are empiric and inconsistent; it is unclear whether decreased lifting results in improved outcomes. Surgeons who perform reconstructive pelvic surgery typically develop their own instructions and recommended limitations.9 These instructions may be based on the surgeon’s educational background, experience, or “common sense,” however little scientific evidence is available to provide guidance. MacNeil interviewed four accomplished urogynecologic surgeons with varying approaches to postoperative activity instructions. These restrictions demonstrated a spectrum from imposing no limitations to strict weight based lifting restrictions. (MacNeil JS. Lifting and Exertion After Prolapse Surgery. OBGYN News 2007;42:23)
IAP changes associated with several common activities including lifting, jumping and coughing have been described.10,11,12 We sought to expand the current understanding of IAP generation by examining the relationship between weight lifting maneuver and quantity of weight lifted.
Materials and Methods
Between December 2005 and July 2006, forty-one women presenting for urodynamic studies as part of their evaluation of urinary incontinence or POP participated. The study was approved by the University’s Institutional Review Board for Human Use and all participants signed informed consent. All eligible patients seen during this time frame were offered participation in the study. To be eligible, patients aged 19 to 65 years had to be capable of lifting 15 kg from the floor, have no history of a musculoskeletal, spinal, or extremity condition that would limit their lifting ability (i.e. prior surgery, injury or arthritis) or be experiencing pain in the neck, back, shoulders or legs.
Subjects first completed a pelvic floor symptoms questionnaire (Pelvic Organ Prolapse Distress Inventory, POPDI)13 and demographic information was extracted from the chart. Prior to performing urodynamics, POP was quantified using the POP-Q quantification system14 and pelvic floor muscle strength was assessed with digital palpation.15 Intra-abdominal pressure was recorded using a transrectal microtip catheter (Millar® Houston, TX). Proper IAP transmission was tested with a cough test both prior to and after performing the lifting sequences.
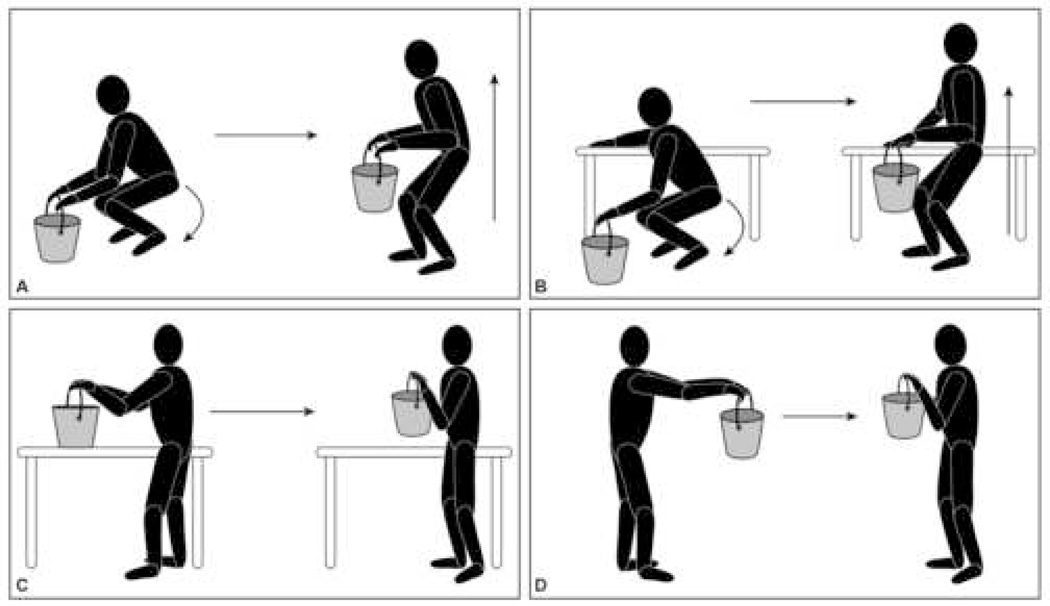
The subjects performed four separate lifting maneuvers. The weight lifting maneuvers and the specific weights to be lifted were established after a physical therapy consultation to ensure that they were safe and applicable to common daily human activities. Maneuver A was rising from a squatting position characterized by flexing at the knees with a straight back while bringing the weight up to the level of the xiphoid bone (Squat). Maneuver B was the same as Maneuver A, however, one hand was placed on a hip high counter to assist the subject during the lift (Squat Assist). Maneuver C was lifting the weight off a hip high countertop (Counter). Maneuver D was receiving the weight into slightly bent outstretched arms while standing (Receive). (Figure 1)
Figure 1.
Lifting Maneuvers: Squat (A), Squat Assist (B), Counter (C), Receive (D)
Each maneuver was performed while lifting 0, 2.5, 5, 10 and 15 kg. Each maneuver/weight combination lift was repeated twice (40 total lifts). Lifting within each maneuver began with 0 kg and was sequentially increased to 15 kg. The weights were contained in handled pails. The 0 kg lift was accomplished using an empty plastic milk jug. The average pressure increase in cm/H2O was used for data analysis.
Univariate analyses were conducted to identify variables, other than lift maneuver or lift weight, that could impact on IAP generation and create confounding. Natural-log transformation on the IAP outcome was performed to obtain a normal distribution. Normal probability plots of the residuals were examined to verify the effectiveness of the log transformation. Mixed-models repeated-measures ANOVA was performed across the different weights and lift maneuvers, while controlling for potential confounders. Interaction between lift maneuver and lift weight was examined. Tukey-Kramer Honestly Significant Difference identified differences in pressure generation between lift maneuver/lift weight groups, while adjusting for multiple comparisons.
A minimum sample size of 33 participants was calculated to identify an intra-abdominal pressure effect size of 5 cm/H20, a value chosen a priori as relevant, with a standard deviation of 10 cm/H20 (based on prior pilot data). Statistical analysis was performed using JMP IN 5.1®.
Results
A total of 41 women participated in the study. Patients had a mean age of 52.7 ± 9.6 years (range 25–65), body mass index (BMI) of 28.6 ± 4.1 Kg/m2, and all participants were Caucasian.
An initial analysis was performed to identify clinical variables that could impact on the generation of IAP (Table 1). Variables found to be significant included age, waist-to-hip ratio, pelvic floor muscle strength, POP-Q point Ba, POP-Q point C, and total vaginal length (p= <0.05). To determine possible associations between lift maneuver, lift weight and IAP while controlling for the potential confounding by the above identified variables, a mixed linear model was fit to the data. Such an approach is a generalization of repeated measure analysis of covariance. Due to non-normality, a natural log transformation was performed to normalize the data.
Table 1.
Subject Characteristics: relationship between parameters and intra-abdominal pressure generation
| Characteristic | Mean (SD) | p-value |
|---|---|---|
| Age | 52.73 (9.63) | .0017 |
| BMI (kg/m2) | 28.64 (4.05) | .1779 |
| Waist-to-Hip Ratio | .85 (.074) | <.0001 |
| POP-Q Point Ba (cm) | −.39 (1.88) | .0012 |
| POP-Q Point Bp (cm) | −1.63 (1.43) | .3642 |
| POP-Q Point C (cm) | −6.19 (3.32) | <.0001 |
| Total Vaginal Length (cm) | 9.62 (1.44) | .0004 |
| Pelvic Organ Prolapse Distress Inventory (POPDI, 0–300) | 111.55 (62.36) | .6641 |
| Pubococcygeal Muscle Strength (graded 1–4) | 2.32 (.69) | .0026 |
| POP-Q Stage: 0 1 2 3 4 |
4.9% 9.8% 70.7% 14.6% 0.0% |
* |
not analyzed
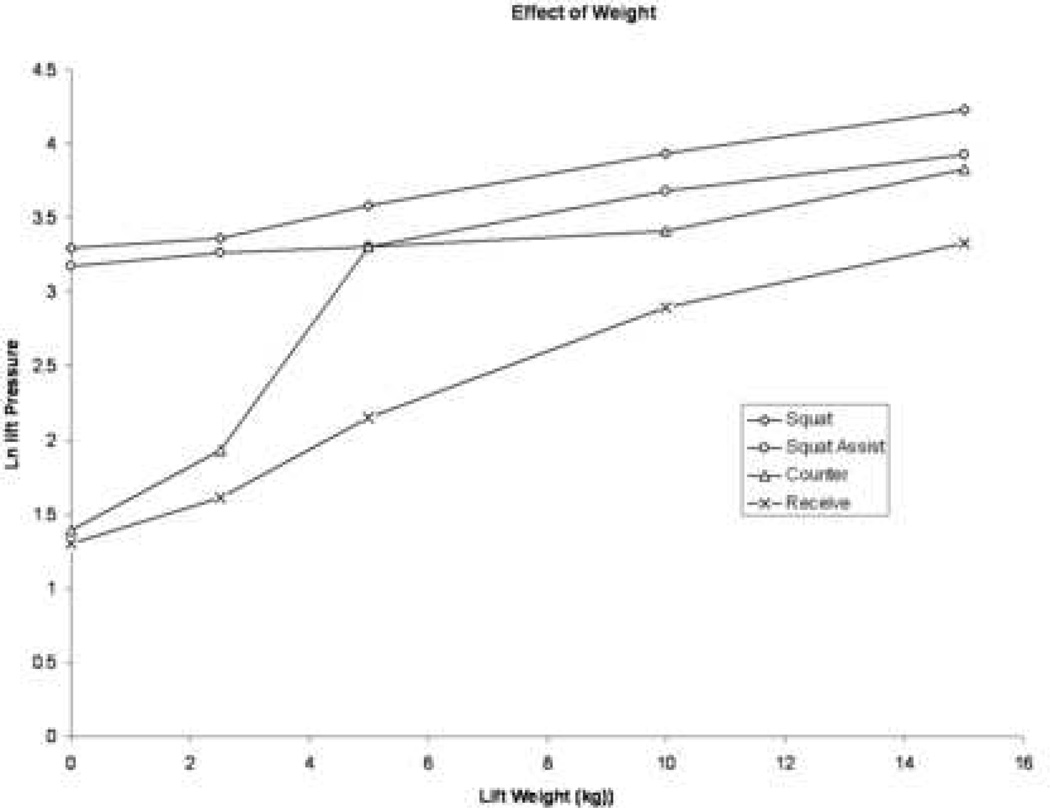
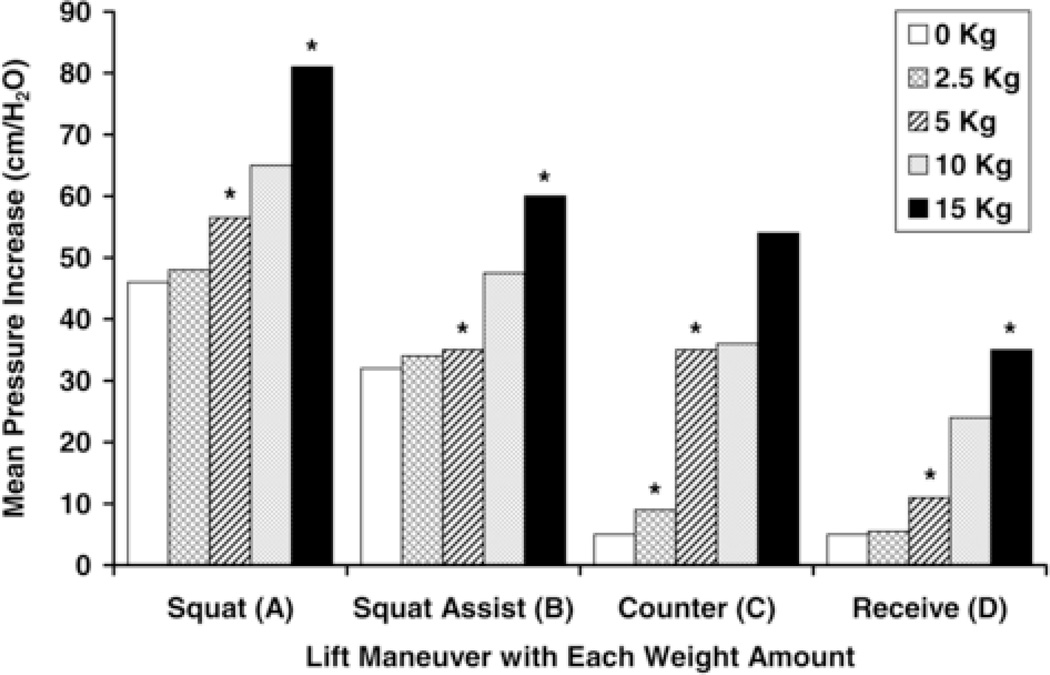
A significant interaction between weight quantity and lift maneuver (p= <0.001) was found, such that the relationship between weight lifted and IAP depended on the specific maneuver being performed (Figure 2). Therefore, the most appropriate interpretation of IAP generation is based on the combination of weight lifted and lift maneuver. Significant increases in IAP were generated between lifting 15 kg and 5 kg or less with Squat, between 15 kg and 5 kg or less with Squat Assist, between 5 kg or more and 2.5 kg or less when lifting from a Counter and between 15 kg and 5 kg or less when Receiving weights (all p< .05; Figure 3).
Figure 2.
Interaction effect of weight lifting maneuver and quantity of weight on intraabdominal pressure generation
Figure 3.
Mean Intra-abdominal Pressure Increase (cm/H2O) categorized by Lift Maneuver and Weight Quantity (kg). Within each lifting maneuver category, the weight quantities between which a significant IAP increase was observed are delineated with symbols.
Because the interaction displayed in Figure 2 indicates a quantitative interaction (i.e., the difference between IAP means generated by maneuvers differs based upon lift weight but the ranking of lift pressure means by maneuver remain constant across lift weight), we proceeded to examine univariate relationships. Initially, a univariate analysis was performed to evaluate differences in IAP generated by lift maneuver or lift weight separately. The amount of weight lifted produced different IAPs (p<0.0001), with lifting more than 2.5 kg resulting in significantly increased IAP. Similarly, IAPs generated by lift maneuver were also significantly different (p<0.0001). Specifically, using a squat maneuver with or without assistance (no significant difference between the two) was associated with the generation of the highest IAP, followed by lifting the weight from a counter. Receiving the weight generated the smallest increases in IAP.
Comment
Several studies have demonstrated the positive relationship between IAP and lifting weight/ load.10–12, 16 A positive relationship between IAP generation has been demonstrated by activation of the transverse abdominis muscle, as well as body lifting positions where the thighs meet the abdominal wall.11, 12 However, these studies were performed in men who may have different body and muscular compositions than women. Weir et al. studied IAP changes in healthy women while performing several activities; increases in IAP were also positively correlated with lifting weight.
Our objective was to elucidate the role of both quantity of weight lifted and lifting maneuver on the generation of IAP. Our data demonstrates that IAP generation is intrinsically linked to both, as verified by a formal test of interaction. In fact, lifting 0 kg using the squat maneuver generated more intra-abdominal pressure than lifting 10 kg off a counter or receiving 15 kg into outstretched arms.
Gynecologic postoperative activity restrictions are inconsistent among surgeons and no literature exists to guide counseling on activity and weight lifting limits. Fitzgerald reported physician recommended activity restrictions ranging from 1 to 104 weeks duration and between 1 and 50 pounds in a survey of 287 gynecologic surgeons.9 Overly restrictive weight-lifting instructions are at risk of being ignored or impossible to follow, and delayed return to activities and work may cause unnecessary financial/social hardship. Furthermore, many unavoidable physiologic functions (breathing, coughing, walking, valsalva) are associated with increases in IAP.10
In light of the significant differences in IAP generation based on the combination of lifting maneuver and quantity of weight, we suggest that rather than assigning one specific weight lifting limit, we should counsel our patients to limit activities that have the greatest increases in IAP (ie. squat lifting), especially in combination with additional weight.
We hypothesized that using a hand on the counter to assist with squat lifting would lessen pressure transmission to the pelvic floor. However, no difference in IAP generation between lifting from a squat position with or without hand assistance was demonstrated. Thus, we no longer counsel women to preferentially use a squat assist maneuver. Based on the finding that lower IAP was generated using the Receive maneuver compared to the Squat maneuver, we counsel women to lift their children/grandchildren by having them handed over to them rather than lifting them from the ground. Hence, a much heavier child could be handled with the Receive maneuver, while still minimizing IAP generation in comparison to using the Squat maneuver.
Obesity has been demonstrated as a risk factor for the development of pelvic floor disorders,17 as well as being a condition associated with chronically increased IAP.18 It has been reported that the mean IAP in non-obese patients is 6.7 cm/H20, compared to 12.1 cm/H20 in morbidly obese patients.19 Obesity may impact on the pelvic floor by the chronic state of increased pressure, and may explain the increased prevalence of stress urinary incontinence in these patients.20 In our analysis, BMI, height and weight were not significant determinants of IAP generation. However, waist-to-hip ratio was found to be a significant variable. Larger ratios were associated with higher pressure generation. Central adiposity may be a more sensitive measure of the effect obesity has on the generation of IAP than BMI itself.17
Strengths of this study include the controlled manner in which the weights were lifted, allowing for meaningful comparisons of IAP generation across the different lift maneuvers and weight amounts. The maneuvers were designed with attention to the functional applicability to routine human activities. Furthermore, an appropriate statistical correction method (Tukey’s HSD) was employed to account for the multiple comparisons performed across weight amounts, lift maneuvers, and predictive factors.
One weakness of this study is that the order of lift maneuvers and weights lifted was not varied between or within subjects. To control for the effects of fatigue, it would have been ideal to vary these parameters randomly or systematically. However, the difficulties involved in guiding the participants through the various combinations of the 40 weight lifting repetitions made it logistically difficult to accomplish.
A potential weakness of the study is that subjects were presenting for care of pelvic floor disorders, which theoretically, could have affected the trans-rectal measurement of IAP. However, the majority of our subjects had Stage II POP or less (85.4%), which is consistent with published population POP prevalence studies.21 Analysis did demonstrate an effect of anterior vaginal wall position and vaginal apex in the generation of IAP, however there was no clear trend that advancing prolapse was associated with higher or lower pressure values. Furthermore, these factors were controlled for in the statistical model. Posterior wall prolapse was not a significant factor in IAP generation. The advantage of studying women with pelvic floor disorders is that they constitute the population of women to which the results should be generalizable. It is hoped that this information will be useful in determining the role of IAP generation in regards to activity restrictions and surgical durability.
Although we have demonstrated the role of lifting using several different maneuvers and weight quantities on the generation of IAP, the study design did not allow for comment on the physiologic effects of increased IAP on the pelvic floor musculature, nerves, or connective tissue. We are also not able to extrapolate this information with respect to impact on healing after surgery or the risk of POP recurrence after reconstructive pelvic surgery.
In conclusion, both lifting maneuver and quantity of weight should be considered when counseling patients regarding postoperative activity restrictions, however, it is unclear at this time whether this may impact on surgical outcomes. Studies are needed to more fully elucidate the mechanism between IAP and pelvic floor disorders.
Acknowledgements
Cynthia J. Brown, MD, MSPH: physical therapy consultation
Josephine H. Taylor: graphics and artwork
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as an Oral Poster at the 2007Annual Meeting of the Society of Gynecologic Surgeons, Orlando, FL, April 14, 2007.
References
- 1.Hamilton Boyles S, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188:108–115. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 2.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Clark AL, Gregory T, Smith VJ, Edwards R. Epidemiologic evaluation of reoperation for surgically treated pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2003;189:1261–1267. doi: 10.1067/s0002-9378(03)00829-9. [DOI] [PubMed] [Google Scholar]
- 4.Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184:1496–1503. doi: 10.1067/mob.2001.114868. [DOI] [PubMed] [Google Scholar]
- 5.Sustersic O, Kralj B. The influence of obesity, constitution and physical work on the phenomenon of urinary incontinence in women. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:140–144. doi: 10.1007/BF02001082. [DOI] [PubMed] [Google Scholar]
- 6.Woodman PJ, Swift SE, O’Boyle AL, Valley MT, Bland DR, Kahn MA, et al. Prevalence of severe pelvic organ prolapse in relation to job description and socioeconomic status: a multicenter cross-sectional study. Int Urogynecol J. 2006;17:340–345. doi: 10.1007/s00192-005-0009-2. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen S, Hein HO, Gyntelberg F. Heavy lifting at work and risk of genital prolapse and herniated lumbar disc in assistant nurses. Occup Med (Lond) 1994;44:47–49. doi: 10.1093/occmed/44.1.47. [DOI] [PubMed] [Google Scholar]
- 8.Gedymin K, Starczewski A, Torbe A. [The influence of individual and total lifted objects on the equilibrium of the uterus] Med Pr. 1986:373–336. [PubMed] [Google Scholar]
- 9.Fitzgerald MP, Shisler S, Shott S, Brubaker L. Physical limitations after gynecologic surgery. J Pelvic Surg. 2001;7:136–139. [Google Scholar]
- 10.Weir LF, Nygaard IE, Wilken J, Brandt D, Janz KF. Postoperative activity restrictions. Any Evidence? Obstet Gynecol. 2006;107:305–309. doi: 10.1097/01.AOG.0000197069.57873.d6. [DOI] [PubMed] [Google Scholar]
- 11.Cresswell AG, Grundstrom H, Thorstensson A. Observations on intra-abdominal pressure and patterns of abdominal intra-muscular activity in man. Acta Physiol Scand. 1992;144:409–418. doi: 10.1111/j.1748-1716.1992.tb09314.x. [DOI] [PubMed] [Google Scholar]
- 12.Harman EA, Frykman PN, Clagett ER, Kraemer WJ. Intra-abdominal and intra-thoracic pressures during lifting and jumping. Med Sci Sports Exerc. 1988;20:195–201. doi: 10.1249/00005768-198820020-00015. [DOI] [PubMed] [Google Scholar]
- 13.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185:1388–1395. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 14.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarshov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 15.Brink CA, Wells TJ, Sampselle CM, Taillie ER, Mayer R. A digital test for pelvic muscle strength in older women with urinary incontinence. Nurs Res. 1989;38:196–199. [PubMed] [Google Scholar]
- 16.Mairiaux Ph, Malchaire D, Vandiepenbeeck, Bellelahom L. Reproducibility of intra-abdominal pressure when lifting. Scand J Rehab Med. 1988;20:83–88. [PubMed] [Google Scholar]
- 17.Brown J, Grady D, Ouslander J, Herzog A, Varner R, Posner S. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999;94:66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 18.Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obes Surg. 2005;15:1225–1232. doi: 10.1381/096089205774512546. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez NC, Tenofsky PL, Dort JM, Shen LY, Helmer SD, Smith RS. What is normal intra-abdominal pressure? Am Surg. 2001;67:243–248. [PubMed] [Google Scholar]
- 20.Noblett KL, Jensen JK, Ostergard DR. The relationship of body mass index to intraabdominal pressure as measured by multichannel cystometry. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:323–326. doi: 10.1007/BF02765589. [DOI] [PubMed] [Google Scholar]
- 21.Nygaard I, Bradley C, Brandt D. Pelvic Organ Prolapse in Older Women: Prevalence and Risk Factors. Obstetrics & Gynecology. 2004;104:489–497. doi: 10.1097/01.AOG.0000136100.10818.d8. [DOI] [PubMed] [Google Scholar]