Abstract
Objective
Adult polyglucosan body disease (APBD) is an autosomal recessive leukodystrophy characterized by neurogenic bladder, progressive spastic gait, and peripheral neuropathy. Polyglucosan bodies accumulate in the central and peripheral nervous systems and are often associated with glycogen branching enzyme (GBE) deficiency. To improve clinical diagnosis and enable future evaluation of therapeutic strategies, we conducted a multinational study of the natural history and imaging features of APBD.
Methods
We gathered clinical, biochemical, and molecular findings in 50 APBD patients with GBE deficiency from Israel, the United States, France, and the Netherlands. Brain and spine magnetic resonance images were reviewed in 44 patients.
Results
The most common clinical findings were neurogenic bladder (100%), spastic paraplegia with vibration loss (90%), and axonal neuropathy (90%). The median age was 51 years for the onset of neurogenic bladder symptoms, 63 years for wheelchair dependence, and 70 years for death. As the disease progressed, mild cognitive decline may have affected up to half of the patients. Neuroimaging showed hyperintense white matter abnormalities on T2 and fluid attenuated inversion recovery sequences predominantly in the periventricular regions, the posterior limb of the internal capsule, the external capsule, and the pyramidal tracts and medial lemniscus of the pons and medulla. Atrophy of the medulla and spine was universal. p.Y329S was the most common GBE1 mutation, present as a single heterozygous (28%) or homozygous (48%) mutation.
Interpretation
APBD with GBE deficiency, with occasional exceptions, is a clinically homogenous disorder that should be suspected in patients with adult onset leukodystrophy or spastic paraplegia with early onset of urinary symptoms and spinal atrophy.
Adult polyglucosan body disease (APBD) is a rare neurogenetic disorder that is clinically characterized by progressive pyramidal paraparesis, distal sensory deficit in the legs, and neurogenic bladder beginning in the 5th or 6th decade of life.1–3 The motor and sensory abnormalities are related to a combination of myelopathy with peripheral neuropathy.4 After about a decade of slow functional deterioration, most patients lose independent walking, and thereafter develop involvement of the arms.3,5 The disease often leads to premature death.6–9
The pathological hallmark of APBD is intracellular accumulation of polyglucosan bodies—containing amylopectinlike polysaccharide—in the central and peripheral nervous systems and in other tissues.1,9–13 Additional neuropathological findings include cerebral demyelination and gliosis, and loss of myelinated nerve fibers in peripheral nerves. These findings led to the discovery that most patients with APBD have an allelic form of glycogen storage disease type IV (GSD-IV) caused by glycogen branching enzyme (GBE) deficiency (Mendelian Inheritance in Man 232500).14–17 Although the presence of polyglucosan bodies in skin, muscle, or nerve tissues suggests the diagnosis, APBD is primarily confirmed by a significant reduction of GBE enzymatic activity in peripheral blood leukocytes or cultured skin fibroblasts.
However, the spectrum of GBE deficiency is broader than APBD, because GSD-IV patients can present either with a liver disease, usually in infancy, or a neuromuscular disease.16 The neuromuscular presentation of GSD-IV is heterogeneous, and 4 main variants have been described depending on the age at onset16,18,19 : (1) a perinatal form with multiple congenital contractures, hydrops fetalis, and perinatal death; (2) a congenital form with hypotonia, muscle wasting, neuronal involvement, inconsistent cardiomyopathy, and death in early infancy; (3) a childhood form dominated by myopathy or cardiopathy; and (4) an adult form presenting either as an isolated myopathy or as APBD.
The majority of APBD patients with GBE deficiency are of Ashkenazi Jewish ancestry and have a homozygous p.Y329S mutation in the GBE1 gene.3,15,17 Overall, the frequency of all glycogen storage diseases is 1:10,000, with GBE deficiency constituting about 3% of all glycogen storage diseases.16 Fewer than 50 patients with APBD have been described in the English medical literature. However, this disease is considered to be underdiagnosed.2,3,17
There is a critical lack of comprehensive overviews of the natural history and the neuroimaging features of APBD. Published clinical and imaging data are based on isolated case reports2,20,21 and small case series.1,14,15 Therefore, to improve the accuracy of clinical diagnosis and to enable future evaluation of therapeutic strategies, we conducted a multinational study of the clinical findings and neuroimaging features of APBD.
Patients and Methods
Patients
Clinical and laboratory data have been retrospectively collected from 50 APBD patients, that is, 47 families, confirmed by enzymatic and/or molecular testing from 4 reference centers in neurogenetics and neurometabolism in Israel (Hadassah-Hebrew University Medical Center, Jerusalem), the United States (Baylor Research Institute, Dallas, TX), France (Salpêtrière University Hospital, Paris), and the Netherlands (Center for White Matter Disorders, VU University Medical Center, Amsterdam). The study was approved by local ethics committees, and written informed consent was obtained for patients or their legal guardians as appropriate (NCT00947960). Neurological examination of all patients was conducted by experienced investigators from the study.
GBE enzymatic activity was measured in leukocytes—or fibroblasts—as described.14,22 The sequencing of the GBE1 gene was limited to exons and intron–exon junctions.15,23 Biochemical and molecular analyses were done in different laboratories.
Scoring of Brain and Spine Imaging
The imaging was reviewed by a neurologist expert in white matter diseases (R.S.) and a specialist in genetic metabolic disorders (F.M.) according to a standardized form.24 The main items that were scored comprised: the topography (lobe and region) and aspect of white matter abnormalities, the topography of brain or spinal atrophy, the occurrence of gray matter lesions, contrast enhancement, and restricted diffusion.
Statistics
For the Kaplan–Meier analyses, we assumed that patients are determined to have APBD at birth. Therefore, all time to event data (eg, time to bladder dysfunction, time to using a wheelchair) assumed that the initial time was the date of birth. The censoring time was the age at latest neurological exam. Finally, the symptoms were communicated via the caregiver or the patient directly. Differences between men and women were tested using log-rank test as well.
Results
Clinical Presentation
The clinical characteristics of our APBD cohort are described in Table 1. Although most of the patients were of Ashkenazi Jewish background, it appears that APBD is likely to be panethnic. We identified patients of Latin American, Pacific Islander, Caucasian, and Cambodian backgrounds. All but 3 APBD patients developed their symptoms after the age of 40 years; these subjects were of non-Jewish background. They include a male patient, previously published as having a mild hepatic form of GSD-IV, who in his early 30s developed typical APBD,25 and a 35-year-old female patient who presented with a subacute strokelike episode.
TABLE 1.
Clinical and Molecular Findings in a Cohort of 50 Adult Polyglucosan Body Disease Patients
| Characteristic | Value |
|---|---|
| Demographic, No. (%) | |
| Gender | |
| F | 23 (47) |
| M | 27 (53) |
| Ethnicity | |
| Ashkenazi | 37 (73) |
| Other Jews | 5 (12) |
| Other | 8 (15) |
| Age at onset, yr [range] | |
| Bladder dysfunction, n = 50 | 51 ±10 [20–71] |
| Difficulty walking, n = 44 | 53 ±5 [33–65] |
| Paresthesia, n = 24 | 53 ±6 [34–64] |
| Motor handicap, age at onset, yr [range] | |
| Walk with 1 cane, n = 29 | 58 ±4 [51–65] |
| Walk with 2 canes, n = 10 | 60 ±5 [53–68] |
| Walk with walker, n = 26 | 63 ±4 [55–70] |
| Wheelchair bound, n = 24 | 64 ±6 [51–73] |
| Age at examination, yr [range] | 59 ±7 [36–72] |
| Disease duration, yr [range] | 9 ±7 [2–40] |
| Neurological examination, No. (%) | |
| Romberg | |
| Positive | 14 (37) |
| Borderline | 9 (24) |
| Negative | 15 (39) |
| Tandem | |
| Unable | 28 (62) |
| Abnormal | 14 (31) |
| Normal | 3 (7) |
| LL spasticity | 45 (90) |
| UL spasticity | 6 (12) |
| LL reflexes, knee | |
| Increased | 23 (46) |
| Decreased/absent | 15 (30) |
| Normal | 12 (24) |
| LL reflexes (ankle) | |
| Increased | 6 (12) |
| Decreased/absent | 40 (80) |
| Normal | 4 (8) |
| UL reflexes | |
| Increased | 15 (30) |
| Decreased/absent | 9 (18) |
| Normal | 26 (52) |
| Plantar reflexes | |
| Extensor | 48 (96) |
| Flexor | 2 (4) |
| LL vibration sense | |
| Decreased | 46 (94) |
| Normal | 3 (6) |
| UL vibration sense | |
| Decreased | 14 (29) |
| Normal | 35 (71) |
| Attention deficit | 24 (48) |
| Memory deficit | 23 (46) |
| Eye movement abnormalities, No. (%) | |
| Pursuit | |
| Saccadic | 18 (39) |
| Normal | 28 (61) |
| Voluntary saccades | |
| Slow | 9 (20) |
| Normal | 37 (80) |
| Other features, No. (%) | |
| Orthostatic hypotension | 10 (31) |
| Cerebellar symptoms | 8 (16) |
| Bradykinesia | 7 (14) |
| Molecular findings, No. (% of 46 patients) | |
| Homozygous p.Y329S | 22 (48) |
| Heterozygous p.Y329S | 13 (28) |
| Other | |
| Heterozygous p.Y329S/p.L224P | 1 |
| Heterozygous p.Y329S/p.R565Q | 1 |
| Heterozygous p.Y329S/c.2003delA | 1 |
| Heterozygous p.Y329C/p.N556Y | 5 |
| Homozygous p.E242Q | 3 {related} |
All ages are indicated as mean ± standard deviation and range.
F= female; LL = lower limbs; M= male; UL = upper limbs.
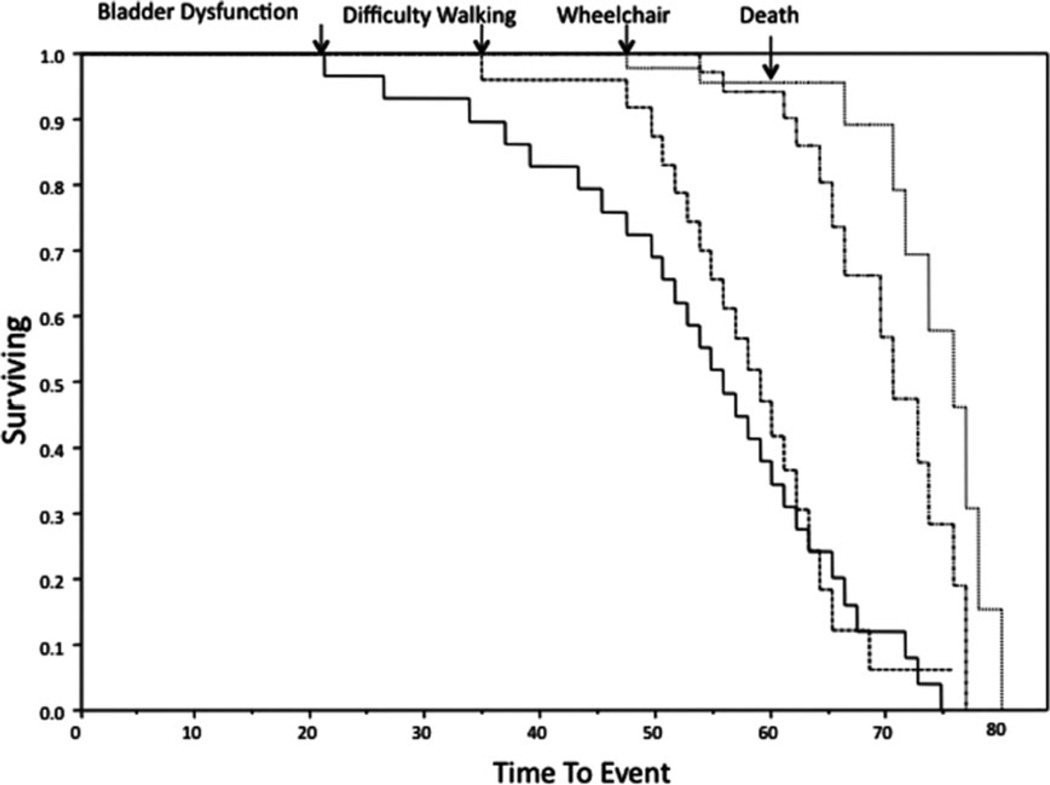
Bladder dysfunction was usually the first symptom that patients developed; 50% of patients were likely to develop it by the age of 51 years (Fig 1), a few years before developing gait difficulties at a median age of 53 years. Although in the present study we did not systemically ask men about sexual function, impotence was a common complaint. These 2 cardinal clinical signs, neurogenic bladder and gait disturbance, were followed in the majority of patients by paresthesias and hypoesthesia, most prominently in the legs, at a median age of 54 years. By age 62 years, 50% of patients were likely to need a walker, and by the median age of 63 years they needed a wheelchair for ambulation. In addition, almost half of APBD patients had mild attention and memory deficits as assessed by bedside neurological examination. Overall, patients with ABPD had a median survival probability of 70 years, which is about 8 years less than the general population (http://www.fis.org/public/survivalcurve-2010.html). There was no difference between men and women. In addition, affected siblings (ie, 8 patients from 3 families) followed the same disease course overall.
Figure 1.
Kaplan–Meier analyses indicate the natural history of 50 adult polyglucosan body disease patients for time to bladder dysfunction, difficulty walking, use of a wheelchair, and death.
On the more detailed neurological examination, some APBD patients showed a saccadic pursuit and occasional absence of convergence. Examination of the upper extremities tended to be normal, with only tendon reflex abnormality (see Table 1). Lower extremities usually showed weakness in an upper motor neuron distribution sometimes associated with distal weakness, deficit in vibration sense, and tendon reflex abnormalities reflecting the myelopathy and sensorimotor peripheral neuropathy. Gait was often spastic and unsteady. In some patients, gait became parkinsonianlike in advanced stages of the disease. When assessed by physicians, almost a third of the patients presented with orthostatic hypotension. In addition, 6 patients reported noteworthy diurnal fluctuations of motor symptoms (data not shown).
Electromyographic (EMG) and nerve conduction study results were available in 47 patients. Forty-two patients (89%) presented with a sensorimotor polyneuropathy characterized by markedly reduced compound motor action potentials and sensory nerve action potentials, often with moderately reduced conduction velocities. In most patients, prolonged F-wave latencies in nerves with relatively normal conduction as well as signs of denervation using proximal needle EMG studies indicated an additional proximal involvement. These findings were suggestive of a predominantly axonal polyradiculoneuropathy. When performed (n = 13), sural nerve biopsies were always diagnostic and showed abundant intraaxonal polyglucosan bodies. Muscle biopsies (n = 12) were normal or showed only signs of denervation, except in 2 patients in whom polyglucosan bodies were identified in myocytes.
Biochemical and Molecular Analyses
APBD diagnosis was confirmed based upon a reduction of GBE enzymatic activity in leukocytes or fibroblasts below 25% of controls (n = 43 patients) or the identification of GBE1 mutations (n = 46 patients) or both (n = 39 patients). For the 7 ABPD patients without enzymatic testing, GBE1 mutations were identified on both alleles. Among the 4 APBD patients for whom mutational analyses could not be performed or needed further molecular validation, all had a reduction of GBE activity in the range of 10 to 20% of normal in addition to intracellular accumulation of polyglucosan bodies in nerve or muscle tissues.
Mutations of GBE1 were tested for in 46 patients (see Table 1). Mutations on both alleles were identified in 33 patients (72%), but in 13 patients (28%) only 1 mutation was found. By far the most common mutation was p.Y329S, which was present in 35 patients (76%) at a homozygous (n = 22) or heterozygous state (n = 13) and exclusively found in Ashkenazi Jewish patients. Patients who were only heterozygous for the p.Y329S mutation had the same disease characteristics, including GBE deficiency usually in the range of 10 to 20% of normal and clinical abnormalities, as patients who were homozygous for the same mutation. In addition, none of the patients’ parents available for examination had neurological symptoms suggestive of APBD.
In 2 Ashkenazi Jewish patients, the p.Y329S mutation was found in combination with mutations previously identified in GSD-IV patients (see Table 1) presenting with a hepatic form of the disease, p.L224P mutation26 and p.R565Q mutation (R. Froissart, unpublished data). One patient from the Pacific Islands also carried a p.Y329S mutation together with a frameshift c.2003delA mutation leading to a premature stop codon. Of note, the amino acid at position 329 is commonly involved in APBD. Indeed, besides the most common p.Y329S mutation, a p.Y329C mutation was found in 4 Ashkenazi Jewish patients and 1 Caucasian patient in combination with a p.N556Y mutation. Homozygous p.E242Q mutations were also found in 3 related Latin American patients. To our knowledge, these latest mutations— p.R565Q, c2003delA, p.Y329C, p.N556Y, and p.E242Q—have not been reported (http://www.hgmd.cf.ac.uk/ac/all.php). The 4 novel missense mutations are likely pathogenic; they affect conserved residues of the GBE protein and are predicted to alter protein function (http://genetics.bwh.harvard.edu/pph/), segregate with disease in families, are absent from control sequences (http://browser.1000genomes.org/index.html and http://www.ncbi.nlm.nih.gov/projects/SNP), and are associated with significantly reduced GBE enzymatic activity.
Three patients of non-Jewish background presented with atypical symptoms. One patient manifested Alzheimerlike dementia together with axonal neuropathy but normal gait and was a compound heterozygote for p.Y329C and p.N556Y mutations. The other 2 patients presented with subacute symptoms related to a strokelike episode for 1 and diaphragmatic failure for the other. The common p.Y329S mutation was not found in these 2 patients; further mutational analyses are in progress. In these 2 patients, the diagnosis was suspected on the basis of the magnetic resonance imaging (MRI) pattern and confirmed by the accumulation of polyglucosan bodies in peripheral tissues and decreased GBE enzymatic activity to 10 to 20% of normal in leucocytes.
Cerebral and Spinal MR Characteristics
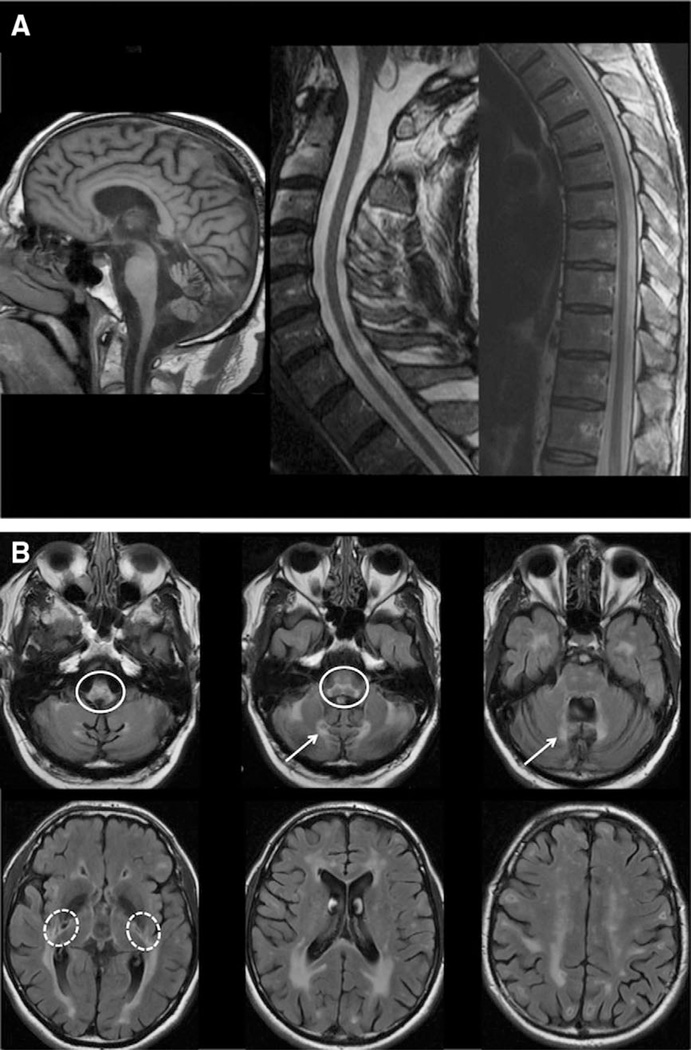
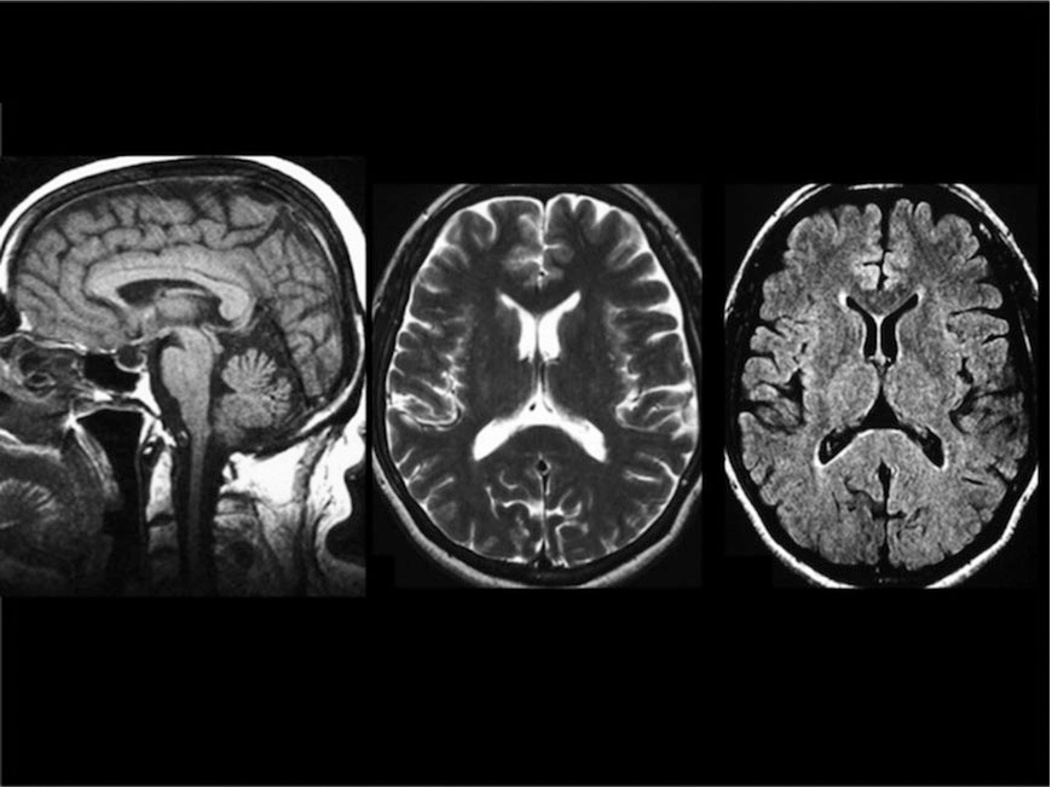
Brain and spine MRIs were available for review in 44 of 50 APBD patients. In general, patients with APBD tended to have a very similar MRI pattern (Table 2). The most consistent abnormalities were medullary and spinal atrophy, which were present in all the patients (Fig 2A). In 1 patient, this was initially the only finding despite significantly reduced GBE activity in leukocytes and homozygosity of the p.Y329S mutation (Fig 3). Cerebellar vermis and, less frequently, hemispheric atrophy were present to various degrees in most patients. A thin corpus callosum, usually mild, was a common feature as well. With the exception of the patient mentioned above, who only had medullary and spinal atrophy when she was diagnosed, hyperintense white matter abnormalities on T2 and fluid attenuated inversion recovery sequences were always present in the medulla and pons, usually in the pyramidal tracts and the medial lemniscus (see Fig 2B). In the cerebral hemispheres, white matter lesions tended to be symmetric, usually in the periventricular areas and mostly with occipital predominance. The temporal lobes were also frequently involved. Lesions were confluent or multifocal, but often both. The external capsule and the posterior limb of the internal capsule were often affected whereas the anterior limb was spared. The abnormal white matter areas had normal or hypointense signal on T1-weighted images (data not shown).
TABLE 2.
Main Brain and Spine Magnetic Resonance Imaging Features Analyzed in a Cohort of 44 Adult Polyglucosan Body Disease Patients
| Characteristic | Value |
|---|---|
| Age, yr (range) | 58 ±7 (39–73) |
| WM lesions | |
| Predominant affected regions | |
| Periventricular | 68% |
| Deep | 9% |
| Subcortical | 7% |
| None | 16% |
| Predominant affected lobes | |
| Occipital | 54% |
| Parietal | 7% |
| None | 39% |
| Capsules | |
| Internal posterior limb | 77% |
| Internal anterior limb | 7% |
| External | 67% |
| Cerebellum | |
| Hilus dentatus | 30% |
| Inferior peduncle | 21% |
| Mid peduncle | 23% |
| Superior peduncle | 14% |
| Midbrain | |
| Medial lemniscus | 89% |
| Pyramidal tract | 95% |
| Pons | |
| Medial lemniscus | 93% |
| Pyramidal tract | 91% |
| Medulla | |
| Decussatio, medial lemniscus | 95% |
| Pyramidal tract | 98% |
| Aspect of WM lesions | |
| Confluent | 84% |
| Multifocal | 70% |
| Symmetrical | 86% |
| Atrophy | |
| Ventricles, enlarged | 32%, 11% mild |
| Cerebral spaces, increased | 18%, 7% mild |
| Corpus callosum | 51%, 23% mild |
| Cerebellum hemispheres | 14% |
| Vermis | 70%, 20% mild |
| Medulla oblongata | 100% |
| Spine | 100% |
WM = white matter.
Figure 2.
Typical cerebral and spinal pattern is shown in adult polyglucosan body disease patients. (A) T1 sagittal scans show important medullary and spinal atrophy and mild vermian atrophy. (B) Fluid attenuated inversion recovery axial scans show hyperintense white matter abnormalities in the periventricular regions, with occipital predominance, the external capsule and the posterior limb of the internal capsule (dashed circles), the medial edges of the inferior and middle cerebellar peduncles (arrows), and the pyramidal tracts and medial lemniscus of the medulla and pons (plain circles).
Figure 3.
An adult polyglucosan body disease patient with medullary atrophy but normal white matter is shown in the early stages of her disease.
Discussion
We present the largest series of 50 APBD patients, which allowed us to delineate the natural history of the disease and depict the singular MRI pattern of APBD. Although most patients were of Ashkenazi Jewish origin, the diagnosis of APBD should also be considered in other ethnicities.27 Neurogenic bladder, spastic paraplegia, and axonal neuropathy are cardinal signs of the disease and present in 90% of the patients. Of note, bladder dysfunction is often the initial symptom, sometimes starting 1 or 2 decades before any difficulty walking or sensory deficit. This likely correlates with the degree of medullary and spinal atrophy that was observed in all APBD patients,2,28 even in the context of little or no cerebral white matter abnormalities. Therefore, APBD should be suspected in any adult patient with spastic paraplegia and significant neurogenic bladder symptoms associated with spinal atrophy. Besides the medullary and spinal atrophy, the demyelinating leukodystrophy in APBD is usually characterized by distinguishing features such as lesions of the posterior limb of the internal capsule, the external capsule, and the pyramidal tracts and medial lemniscus of the pons and medulla. Cognitive decline consisting of mild attention and memory deficit may also affect up to 50% of patients with APBD. Only 1 patient manifested Alzheimerlike dementia. However, standardized neuropsychological testing not routinely used in this study is needed to further evaluate cognitive functions. Moreover, occasional subacute presentations and significant fluctuations of symptoms present in some APBD patients suggest that, as in most other glycogen storage diseases, decreased glycogen degradation may lead to energy deficit.
The most common differential diagnoses of APBD are multiple sclerosis, hereditary spastic paraplegia, adrenomyeloneuropathy, and in men with early urinary symptoms, prostate hypertrophy. In older patients, differential diagnoses of APBD may include white matter leukoencephalopathies due to severe hypertension or NOTCH3 mutation.29 Due to the occasional late occurrence of bradykinesia, orthostatic hypotension, and cerebellar symptoms, some patients may be misdiagnosed with multiple system atrophy. Although there have been some reports of APBD resembling amyotrophic lateral sclerosis,30 we did not have any patient presenting with a motor neuron disease among our cohort. In addition, a few patients were diagnosed despite an atypical clinical presentation (ie, Alzheimerlike dementia, a strokelike episode, and a respiratory failure due to diaphragmatic dysfunction). Notably, these 3 patients were of non-Jewish descent, and their diagnosis was initially suspected solely based on imaging findings. Although Alexander disease, autosomal dominant leukodystrophy associated with LMNB1 duplication, and leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL; due to DARS2 mutations) can also present with medullary and spinal atrophy (but also signal abnormalities of the spinal cord in LBSL),31–33 we are not aware of other leukodystrophies presenting with a similar pattern of white matter lesions on MRI.
Although APBD patients showed a reduction to usually <25% of GBE activity (unlike the 50% activity measured in nonmanifesting carriers), in about a third of the patients, only 1 mutation (p.Y329S) was identified despite standard sequencing of the whole gene. However, large deletions, splice mutations, or mutations in regulatory regions of GBE1 were not searched in this study but are not excluded. For example, in 1 APBD patient heterozygous for the mutation p.Y329S at the genomic level, cDNA sequencing revealed only 1 mutated allele, indicating that the second allele is missing (Supplementary Fig). Transient expression experiments showed that the significant retention of GBE activity in the p.Y329S allele may be a reason for the mild disease compared to other GSD-IV phenotypes.26 Indeed, >80% of the patients in our series harbored the p.Y329S Jewish mutation.
The mechanism by which GBE deficiency causes a neurological disorder is not known.34 One hypothesis states that the polyglucosan inclusions mechanically disrupt normal cellular function such as intracellular transport. Because astrocytes are primarily involved in brain glycogen synthesis and utilization,35 another hypothesis is that decreased glycogen degradation leads to energy deficit in glial cells and therefore neurons. Here we report the first GSD-IV patient who initially presented with a mild GSD-IV hepatopathy and later developed typical symptoms of APBD.25 This emphasizes that there is a wide spectrum of clinical presentations that may be related to varying amounts of residual GBE enzymatic activities in target tissues and/or associated variants in other enzymes involved in glycogen synthesis or degradation.
In conclusion, this study provides the first quantitative natural history of APBD and opens ways to future therapeutic trials aimed at slowing or reversing disease progression. The delineation of the key MRI findings of ABPD should lead to a better and earlier recognition of the disease, even when the cardinal signs are missing.
Supplementary Material
Acknowledgments
This work was supported by Paris Hospitals Public Assistance (F.M.), the ZorgOnderzoek Nederland Medische Wetenschappen (ZonMw, TOP grant 9120.6002), the Optimix Foundation for Scientific Research (M.E.S., M.S.v.d.K.), the National Institute of Mental Health (B.L.F.), and the Adult Polyglucosan Body Disease Research Foundation (APBDRF) research grant (O.K., A.L.).
We thank the patients and their caregivers for their participation in this study; Drs S. Billot, D. Hervé, M. Sadeh, J. Chapman, A. Reches, O. Abramsky, Z. Argov, M. Rabey, M. Helman, I. Schlesinger, and D. Kidron for referral of patients; and M. Anand for statistical support.
We underscore the significant contribution of the late Dr G. Karpati at the initial stages of this work.
Potential Conflicts of Interest
F.M.: patents/patents pending, Assistance Publique des Hôpitaux de Paris. R.S.: consultancy, Shire HGT, Amicus Therapeutics, Genzyme Corporation; grants/grants pending, Shire HGT, Amicus Therapeutics, Genzyme Corporation; speaking fees, Shire HGT, Amicus Therapeutics, Genzyme Corporation; patents/patents pending, Baylor Research Institute; travel expenses, Shire HGT, Amicus Therapeutics, Genzyme Corporation.
Footnotes
Additional supporting information can be found in the online version of this article.
References
- 1.Robitaille Y, Carpenter S, Karpati G, DiMauro SD. A distinct form of adult polyglucosan body disease with massive involvement of central and peripheral neuronal processes and astrocytes: a report of four cases and a review of the occurrence of polyglucosan bodies in other conditions such as Lafora’s disease and normal ageing. Brain. 1980;103:315–336. doi: 10.1093/brain/103.2.315. [DOI] [PubMed] [Google Scholar]
- 2.Klein CJ, Boes CJ, Chapin JE, et al. Adult polyglucosan body disease: case description of an expanding genetic and clinical syndrome. Muscle Nerve. 2004;29:323–328. doi: 10.1002/mus.10520. [DOI] [PubMed] [Google Scholar]
- 3.Klein CJ. Adult polyglucosan body disease. Seattle, WA: University of Washington; 2009. [PubMed] [Google Scholar]
- 4.Cafferty MS, Lovelace RE, Hays AP, et al. Polyglucosan body disease. Muscle Nerve. 1991;14:102–107. doi: 10.1002/mus.880140203. [DOI] [PubMed] [Google Scholar]
- 5.Sindern E, Ziemssen F, Ziemssen T, et al. Adult polyglucosan body disease: a postmortem correlation study. Neurology. 2003;61:263–265. doi: 10.1212/01.wnl.0000073144.96680.cb. [DOI] [PubMed] [Google Scholar]
- 6.Negishi C, Sze G. Spinal cord MRI in adult polyglucosan body disease. J Comput Assist Tomogr. 1992;16:824–826. doi: 10.1097/00004728-199209000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Rifai Z, Klitzke M, Tawil R, et al. Dementia of adult polyglucosan body disease. Evidence of cortical and subcortical dysfunction. Arch Neurol. 1994;51:90–94. doi: 10.1001/archneur.1994.00540130124021. [DOI] [PubMed] [Google Scholar]
- 8.Berkhoff M, Weis J, Schroth G, Sturzenegger M. Extensive white-matter changes in case of adult polyglucosan body disease. Neuroradiology. 2001;43:234–236. doi: 10.1007/s002340000425. [DOI] [PubMed] [Google Scholar]
- 9.Bigio EH, Weiner MF, Bonte FJ, White CL. Familial dementia due to adult polyglucosan body disease. Clin Neuropathol. 1997;16:227–234. [PubMed] [Google Scholar]
- 10.Gray F, Gherardi R, Marshall A, et al. Adult polyglucosan body disease (APBD) J Neuropathol Exp Neurol. 1988;47:459–474. doi: 10.1097/00005072-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Busard HL, Gabreels-Festen AA, Renier WO, et al. Adult polyglucosan body disease: the diagnostic value of axilla skin biopsy. Ann Neurol. 1991;29:448–451. doi: 10.1002/ana.410290420. [DOI] [PubMed] [Google Scholar]
- 12.Ubogu EE, Hong ST, Akman HO, et al. Adult polyglucosan body disease: a case report of a manifesting heterozygote. Muscle Nerve. 2005;32:675–681. doi: 10.1002/mus.20384. [DOI] [PubMed] [Google Scholar]
- 13.Schroder JM, May R, Shin YS, et al. Juvenile hereditary polyglucosan body disease with complete branching enzyme deficiency (type IV glycogenosis) Acta Neuropathol. 1993;85:419–430. doi: 10.1007/BF00334454. [DOI] [PubMed] [Google Scholar]
- 14.Lossos A, Barash V, Soffer D, et al. Hereditary branching enzyme dysfunction in adult polyglucosan body disease: a possible metabolic cause in two patients. Ann Neurol. 1991;30:655–662. doi: 10.1002/ana.410300505. [DOI] [PubMed] [Google Scholar]
- 15.Lossos A, Meiner Z, Barash V, et al. Adult polyglucosan body disease in Ashkenazi Jewish patients carrying the Tyr329Ser mutation in the glycogen-branching enzyme gene. Ann Neurol. 1998;44:867–872. doi: 10.1002/ana.410440604. [DOI] [PubMed] [Google Scholar]
- 16.Kishnani PS, Koeberl D, Chen Y-T. In: Glycogen storage diseases. Scriver CR, Beaudet AL, Sly WS, et al., editors. New York, NY: McGraw-Hill; 2009. MMBID Online, http://genetics.accessmedicine.com. [Google Scholar]
- 17.Ziemssen F, Sindern E, Schroder JM, et al. Novel missense mutations in the glycogen-branching enzyme gene in adult polyglucosan body disease. Ann Neurol. 2000;47:536–540. [PubMed] [Google Scholar]
- 18.Maruyama K, Suzuki T, Koizumi T, et al. Congenital form of glycogen storage disease type IV: a case report and a review of the literature. Pediatr Int. 2004;46:474–477. doi: 10.1111/j.1442-200x.2004.01916.x. [DOI] [PubMed] [Google Scholar]
- 19.Moses SW, Parvari R. The variable presentations of glycogen storage disease type IV: a review of clinical, enzymatic and molecular studies. Curr Mol Med. 2002;2:177–188. doi: 10.2174/1566524024605815. [DOI] [PubMed] [Google Scholar]
- 20.Milde P, Guccion JG, Kelly J, et al. Adult polyglucosan body disease. Arch Pathol Lab Med. 2001;125:519–522. doi: 10.5858/2001-125-0519-APBD. [DOI] [PubMed] [Google Scholar]
- 21.Vucic S, Pamphlett R, Wills EJ, Yiannikas C. Polyglucosan body disease myopathy: an unusual presentation. Muscle Nerve. 2007;35:536–539. doi: 10.1002/mus.20720. [DOI] [PubMed] [Google Scholar]
- 22.Akman HO, Sheiko T, Tay SK, et al. Generation of a novel mouse model that recapitulates early and adult onset glycogenosis type IV. Hum Mol Genet. 2011;20:4430–4439. doi: 10.1093/hmg/ddr371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tay SK, Akman HO, Chung WK, et al. Fatal infantile neuromuscular presentation of glycogen storage disease type IV. Neuromuscul Disord. 2004;14:253–260. doi: 10.1016/j.nmd.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 24.van der Knaap MS, Breiter SN, Naidu S, et al. Defining and categorizing leukoencephalopathies of unknown origin: MR imaging approach. Radiology. 1999;213:121–133. doi: 10.1148/radiology.213.1.r99se01121. [DOI] [PubMed] [Google Scholar]
- 25.McConkie-Rosell A, Wilson C, Piccoli DA, et al. Clinical and laboratory findings in four patients with the non-progressive hepatic form of type IV glycogen storage disease. J Inherit Metab Dis. 1996;19:51–58. doi: 10.1007/BF01799348. [DOI] [PubMed] [Google Scholar]
- 26.Bao Y, Kishnani P, Wu JY, Chen YT. Hepatic and neuromuscular forms of glycogen storage disease type IV caused by mutations in the same glycogen-branching enzyme gene. J Clin Invest. 1996;97:941–948. doi: 10.1172/JCI118517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruno C, Servidei S, Shanske S, et al. Glycogen branching enzyme deficiency in adult polyglucosan body disease. Ann Neurol. 1993;33:88–93. doi: 10.1002/ana.410330114. [DOI] [PubMed] [Google Scholar]
- 28.Massa R, Bruno C, Martorana A, et al. Adult polyglucosan body disease: proton magnetic resonance spectroscopy of the brain and novel mutation in the GBE1 gene. Muscle Nerve. 2008;37:530–536. doi: 10.1002/mus.20916. [DOI] [PubMed] [Google Scholar]
- 29.Labauge P. Magnetic resonance findings in leucodystrophies and MS. Int MS J. 2009;16:47–56. [PubMed] [Google Scholar]
- 30.Segers K, Kadhim H, Colson C, et al. Adult polyglucosan body disease masquerading as “ALS with dementia of the Alzheimer type”: an exceptional phenotype in a rare pathology. Alzheimer Dis Assoc Disord. 2012;26:96–99. doi: 10.1097/WAD.0b013e31821cc65d. [DOI] [PubMed] [Google Scholar]
- 31.Farina L, Pareyson D, Minati L, et al. Can MR imaging diagnose adult-onset Alexander disease? AJNR Am J Neuroradiol. 2008;29:1190–1196. doi: 10.3174/ajnr.A1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuster J, Sundblom J, Thuresson AC, et al. Genomic duplications mediate overexpression of lamin B1 in adult-onset autosomal dominant leukodystrophy (ADLD) with autonomic symptoms. Neurogenetics. 2011;12:65–72. doi: 10.1007/s10048-010-0269-y. [DOI] [PubMed] [Google Scholar]
- 33.Scheper GC, van der Klok T, van Andel RJ, et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat Genet. 2007;39:534–539. doi: 10.1038/ng2013. [DOI] [PubMed] [Google Scholar]
- 34.Akman HO, Raghavan A, Craigen WJ. Animal models of glycogen storage disorders. Prog Mol Biol Transl Sci. 2011;100:369–388. doi: 10.1016/B978-0-12-384878-9.00009-1. [DOI] [PubMed] [Google Scholar]
- 35.Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.