Abstract
This Research Update summarizes thirty years of studies on genetic influences on responses to the acute or chronic administration of nicotine. Early studies established that various inbred mice are differentially sensitive to the effects of the drug. Classical genetic analyses confirmed that nicotine effects on locomotion, body temperature and seizures are heritable. A significant inverse correlation between the locomotor and hypothermic effects and the density of nicotine binding sites suggested that differential expression α4β2-neuronal nicotinic acetylcholine receptor (nAChR) mediated some of this genetic variability. Subsequent studies with α4 and β2 nAChR null (decreased sensitivity) and gain of function mutants (increased sensitivity) supports the role of the α4β2*nAChR subtype. However, null mutant mice still respond to nicotine, indicating that other nAChR subtypes also mediate these responses. Mice differing in initial sensitivity to nicotine also differ in tolerance development following chronic treatment: Those mice that are initially more sensitive to nicotine develop tolerance at lower treatment doses than less sensitive mice, indicating that tolerance is an adaptive response to the effects of nicotine.. In contrast, the sensitivity of mice to pre-pulse inhibition of acoustic startle response is correlated with the expression of α7-nAChR. While genetic variability in nAChR expression and function is an important factor contributing to differences in response to nicotine, the observations that altered activity of opioid, glutamate, and cannabinoid receptors among others also change nicotine sensitivity reinforces the proposal that the genetics of nicotine response is more complex than differences in nAChRs.
1. Introduction
Evidence for the importance of genetic factors in mediating tobacco use in humans was first provided by the R.A. Fisher in 1958 [1]. Since then many different approaches, including twin studies and more recently genome wide association studies have firmly established that genetic factors are important components in tobacco use in humans (see reviews [2–6]).
Our research has used mouse models to investigate the role of genetics in mediating responses to nicotine. A useful initial step to assess the role of genetic factors on any response is the characterization of variability among defined genetic populations. The laboratory mouse is an excellent resource with which to begin the evaluation of genetic factors because of the availability of a large number of inbred strains. More recently, the mouse has been the species widely used to generate genetically modified lines, mostly gene knockout and knockin lines. Tests of the roles of specific genes on responses of interest are now possible.
2. Locomotor Activity and Body Temperature
2a. Inbred Mouse Strains and Classical Genetic Analysis
We initiated our studies on the role of genetic factors in mediating responses to nicotine using available inbred mouse strains. An early study examined the effect of an acute administration of nicotine by constructing full dose-response curves for several behavioral and physiological responses in four common inbred strains (BALB, C57BL/6, DBA/2 and C3H/IBG) [7]. Even with this fairly modest number of strains both quantitative differences (approximately a 4-fold difference in ED50 values for nicotine-induced hypothermia) and qualitative differences (locomotor depression in three of the inbred strains but locomotor activation in C3H mice in the open field arena) were observed. Certainly, genotype influenced response to nicotine in the mouse. However, with this limited number of mice a relationship between behavioral response and nicotinic receptor expression (measured with nicotine and α-bungarotoxin binding in tissue homogenates) could not be determined.
The observation of substantial strain differences in response to nicotine prompted two studies examining the heritability of these responses using a diallel cross. The parental strains for this analysis were the four strains screened initially (BALB, C57BL/6, DBA/2 and C3H/IBG) and A. All possible F1 hybrids were generated and tested for the effect of nicotine on hypothermia [8] and open-field activity [9]. Both analyses confirmed that strain differences exist and also demonstrated heritability of the nicotine-induced responses consistent with an additive/dominance model. A significant directional dominance toward increased sensitivity to nicotine that was particularly pronounced for the locomotor response was observed. That is, the hybrid mice were more sensitive to nicotine than predicted by the parental responses. This directional dominance was interpreted from an evolutionary point of view to be indicative of a selective advantage where increased sensitivity could protect against ingestion of toxic levels of nicotine.
The screen of inbred mice was subsequently expanded to include 19 strains [10]. A multi-component test battery was designed to allow the measurement of several different responses to nicotine in an individual mouse. The battery consisted of measurements of the effects of nicotine on respiratory rate, acoustic startle response, crosses and rears in the Y-maze, heart rate and body temperature. The efficiency of the test battery allowed construction of full nicotine dose-response curves for each strain. Substantial differences in ED50 values (4–5 fold for most tests) were observed among the strains, further establishing the importance of genetic factors in mediating nicotine-induced responses. Correlational analysis of the results revealed that the effects of nicotine on the activity measures and body temperature were very similar, a result confirmed by factor analysis. Overall these analyses indicated the existence of four groups of mice ranging from those that are very sensitive to nicotine (C57BL/10, C57BL/6 and A) to those that are very resistant (BUB and C58). Two additional subsets were also identified, one that is moderately sensitive (including DBA/1 and DBA/2) and a second that is moderately resistant (including C3H and CBA).
In order to investigate whether the variation in acute response to nicotine is a consequence of variability in expression of nicotinic receptors, the binding of nicotine and α-bungarotoxin was measured in homogenates prepared from eight different brain regions. It is now well established that nicotine labels α4β2*-nAChR sites [11–12] (the * represents the potential for additional subunits[13]) and α-bungarotoxin labels α7-nAChR sites [14]. A significant overall negative correlation between the density of nicotine binding sites and ED50 values for nicotine effects on activity and body temperature was observed [15]. The correlation between α-bungarotoxin binding and these ED50 values was not statistically significant. This result indicated that the density of α4β2-nAChR was inversely correlated with sensitivity to locomotor and hypothermic effects of nicotine: the higher α4β2*-nAChR expression, the lower the dose of nicotine necessary to elicit a response. However, these results should be and have been regarded as merely suggestive.
2b. nAChR Null, Gain of Function Mutants and Natural Variants
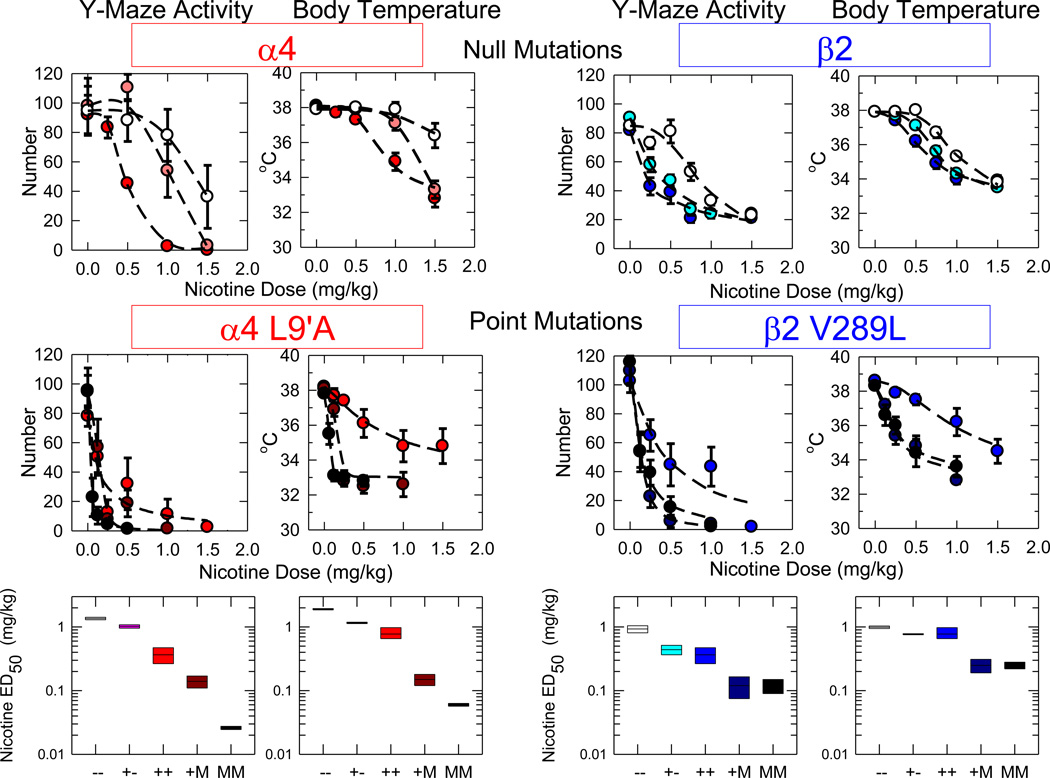
With the development of genetically modified mice the nAChR can either be deleted (null mutants) or mutated to enhance agonist sensitivity (gain of function) (see [16] for review). Both types of mutants have been generated for the Chrna4 and Chrnb2 genes, which encode the α4 and β2 nAChR subunits, respectively. The availability of these genetically modified mice allows the direct test of the effects of altered α4β2-nAChR expression on response to acute nicotine administration. The results presented in Figure 1 demonstrate the effect of deletion of either the α4 or β2 nAChR subunit gene or insertion of hyperactive α4 (L9’A) or β2 (V22’L) nAChR subunit gene on nicotine effects on Y-maze crosses or body temperature. Significant changes in sensitivity to acute nicotine injection were noted for mice from which wild-type versions of either the α4 or β2 nAChR subunits were deleted or replaced with a mutant hyperactive receptor subunit. Deletion of either α4 (new data) or β2 [17] resulted in a gene-dose dependent decrease in sensitivity to acute nicotine. In contrast, insertion of a hypersensitive version of either α4 (new data) or β2 [18] resulted in a gene-dose dependent increase in sensitivity to nicotine. It should be noted, that the null mutant mice still responded to acute nicotine administration illustrating that the α4β2-nAChR was not the only receptor subtype that regulates nicotine-induced hypomotility or hypothermia.
Figure 1.
Effects of α4 or β2 gene deletion or gain of function mutations on acute responses to nicotine. Mice differing in either α4 genotype (α4 wild-type, heterozygotes or null mutants or wild-type, α4 L9’A homozygotes or hemizygotes) or β2 genotype (β2 wild-type, heterozygotes or null mutants or wild-type, \β2 V289L homozygotes or hemizygotes) were injected intraperitoneally with the indicated doses of nicotine (free base). Activity in the Y-maze was measured for 3 min beginning 3 min after injection and rectal body temperature was measured 15 min after injection. Each point on the dose-response curves represents data from 5–16 mice at each dose. The different α4 genotypes are represented by red symbols (++, wild-type, red; +−, heterozygote, pink; −−, null mutant, white; +M, α4L9’A hemizygote, dark red; MM, α4 9’AA homozygote, darkest red). The different β2 genotypes are represented by blue symbols (++, wild-type, blue; +−, heterozygote, light blue; −−, null mutant, white; +M, β2VL hemizygote, darker blue and MM β2 LL homozygote, darkest blue). The lines for each genotype represent the least squares curved fit of the data to modified Hill equations. The effect of variation of genotype on the ED50 ±SEM values (mg/kg) calculated from the Hill fits are shown in the lower panels. Note that ED50 values increase with gene deletion and decrease with introduction of a gain of function mutation.
The studies described above concentrated on the relationship between the density of α4β2*-nAChR expression and response to nicotine. However, a polymorphism representing a single point mutation in the Chrna4 gene changed in the primary sequence of the subunit (ala/thr difference at position 529) and an alteration of receptor function [19]. This mutation was originally identified in the long sleep (LS) and short sleep (SS) mice that were selected for their differential sensitivity to ethanol and also differ in response to nicotine [20]. Recombinant inbred (RI) strains generated by inbreeding mice isolated from a F2 cross of these mice were tested for their responses to acute nicotine administration. RI mice differing in the Chrna4 polymorphism were also differentially sensitive to nicotine-induced hypomotility and hypothermia [21]. In heterologous expression systems, α4β2-nAChR can assemble with two alternative stoichiometries [(α4)2(β2)3 with high agonist sensitivity to agonists (HS form) and (α4)3(β2)2 with lower agonist sensitivity (LS)] [22–25]. These alternate stoichiometries are also found in mouse brain [26–27]. The observation that the A529T polymorphism affects the relative expression of the two alternative stoichiometric forms of the α4β2-nAChR receptor with intrinsic differences in sensitivity to activation by nicotinic agonists [28] suggests that this and perhaps other point mutations in a receptor subunit can alter nicotine responses by changing the ratio of HS to LS α4β2-nAChR. In addition, the relative expression of HS and LS forms of α4β2-nAChR can also be influenced by the 3’ untranslated region of mRNA encoding the α4 nAChR subunit protein [29]. A mechanism such as this could contribute to the differential expression of HS and LS forms of the receptor throughout the brain and to alter relative sensitivity to of α4β2-nAChR to agonists, including nicotine, and change response to the drug. However, it is unknown whether alterations in ratio of HS and LS forms contribute to genetically determined differences in response to nicotine.
3. Antinociception
Investigating the differential responses of inbred strains has also been extended to an examination of the role of nicotine as an anti-nociceptive [30]. Significant differences in the potency of nicotine as an antinociceptive were noted among the seven inbred strains using two tests for thermal pain. The ED50 values for the tail-flick and hot plate tests were significantly positively correlated (r = 0.89). In addition, the ED50 values for these responses showed a significant negative correlation to the density of α4β2*-nAChR binding sites in the hindbrain. These inverse correlations are reminiscent of those observed for the analysis of nicotine effects on locomotor depression and hypothermia [15] and indicate the importance of α4β2*-nAChR in mediating these anti-nociceptive effects of nicotine. This assignment is consistent with results obtained with α4 and β2 null mutant mice each of which required higher nicotine doses to block the thermal pain than those required for wild-type mice [12] However, testing of two F1 hybrids (C57BL/6 × CBA and C57BL/6 × DBA) indicated that the genetic architecture of the antinociceptive action of nicotine may not be simple: Over-dominance toward increased sensitivity of the C57BL/6 × DBA F1 hybrid was similar to the result from the diallel crosses for locomotor activity [9] and hypothermia [8]. However, over-dominance toward decreased sensitivity was observed for the C57BL/6 × CBA F1 hybrid. It should also be noted that deletion of the α4β2*-nAChR subtype had a greater effect on the ED50 or maximal response for the hot-plate test than for the tail flick test. The α4 L9’S heterozygotes, which express a hypersensitive α4 subunit, showed enhanced sensitivity on the hot plate test with less effect for tail flick [30], further indicating a more important role for the α4β2*-nAChR subtype in mediating the response to nicotine for the hot plate.
The α5 nAChR subunit is an auxiliary subunit that does not function as a classical α nAChR subunit, but can co-assemble with αβ nAChR subunit pairs to occupy the fifth position in the receptor pentamer. Incorporation of the α5 subunit in either an α4β2α5-nAChR or an α3β4α5-nAChR markedly affects the physiological and pharmacological properties of the receptors without the α5 subunit [31–33]. Consistent with altered pharmacology, α5 null mutant mice are significantly less sensitive to the effects of an acute nicotine administration on locomotor activity and body temperature, as well as nicotine action as an antinociceptive [34]. Inasmuch as the α5 subunit can coassemble with both α4β2- and α3β4-nAChR subtypes, the effects of α5 subunit deletion cannot be unambiguously ascribed to either major subtype. However, the observation that deletion of either the α4 or β2 subunit decreases the hypomotive, hypothermic and antinociceptive [17] [35] [12] properties of nicotine suggests that the α4β2α5-nAChR mediates at least some of these responses.
4. Voluntary Oral Nicotine Consumption
The propensity of mice to self-administer nicotine is likely to be an important indicator of potential for nicotine abuse. Establishing models for intravenous nicotine self-administration in the mouse has, until recently, be extremely challenging [36]. We used oral self-selection of nicotine using the two-bottle choice paradigm to examine genetic influences on nicotine intake [37]. The six inbred mouse strains used in this study (A, BUB,C57BL/6, C3H, DBA/2, and ST/b) differ significantly in their responses to acute nicotine administration [10, 38]. These six strains also differed markedly in oral nicotine intake from water or 0.2% saccharin; total fluid consumption for saccharin solutions tended to be higher. The vehicle made little difference in the pattern of oral intake of nicotine for all strains other than ST/b mice. C57BL/6 mice consumed the most nicotine, while A, C3H and ST/b mice had low nicotine intake.
5. Conditioned Place Preference
Conditioned place preference has been successfully used to evaluatethe condition reinforcing effects of environmental stimuli associated with drug administration. Unlike the genetic analyses of the effects of nicotine on many other responses, much of the research on conditioned place preference has been conducted using genetically modified mice, rather than inbred strains. However, one study did note that C57BL/6 mice acquired conditioned place preference while DBA/2 mice did not [39]. In contrast to the relative paucity of data from strain comparisons, studies with knockout and knockin mice have helped to define the nAChR subtypes contributing to the development of conditioned place preference. Deletion of the β2 subunit eliminated conditioned place preference, while deletion of the α7 subunit did not, supporting the role of β2*-nAChR [40]. Subsequently, targeted deletion of the α4 subunit in dopaminergic neurons was found to eliminate conditioned place preference, supporting the role of α4β2*-nAChR on the reward pathway [41]. However, in a different study little effect of a global deletion of the α4 subunit was noted, but mice expressing the α4 S6’F gain of function mutation achieved conditioned place preference at lower nicotine doses than did wild-type mice [42]. Mice differing in the α4 A529T polymorphism differed significantly in nicotine conditioned place preference; mice expressing the T variant developed conditioned place preference, while those with the A variant did not [43]. Interestingly, DBA mice express the A variant and C57BL/6 express the T variant a result consistent with the difference in conditioned place preference reported for these inbred strains [39]. The α5 subunit also contributes to nicotine-induced conditioned place preference [34]. Wild-type mice and α5 knockout mice showed similar dose-response curves for conditioned place preference in the lower range of nicotine doses. However, after conditioning with higher nicotine doses, α5 knockouts continued to exhibit conditioned place preference while wild-type mice did not. This pattern of response is reminiscent of the effect of the deletion of the α5 subunit on intravenous nicotine self-administration where α5 knockout mice self-administer significantly more nicotine than wild-type mice [44]. Pharmacological studies have implicated the α6 subunit in conditioned place preference, as well [45]. Treatment of mice with the selective α6β2*-nAChR antagonist α-conotoxin MII (H9A, L15A) resulted in a dose dependent inhibition of nicotine-induced conditioned place preference. Overall, several different nAChR subtypes participate in nicotine-induced conditioned place preference and appear to participate both in the rewarding aspects (α4, α6 and β2) as well as the adversive aspects (α5) of this complex behavioral response.
6. Seizure Activity
Administration of relatively high doses of nicotine causes convulsions. Sensitivity to nicotine-induced seizures varies markedly among inbred strains administered nicotine either intraperitoneally or intravenously [38]. A greater than 2.5-fold difference in both ED50 and seizure latency were determined with ST/b mice being the most sensitive for both measures and DBA/2 among the most resistant. Subsequent comparison of seizure sensitivity to the density of α-bungarotoxin binding sites (now known to measure α7-nAChR) revealed a significant inverse correlation. This result suggested that mice expressing higher levels of α7-nAChR were more prone to nicotine –induced seizures. This potential relationship between seizure sensitivity and α-bungarotoxin binding was consistent with the results of previous studies in which the inheritance of these measures was examined with a classical genetic cross between C3H and DBA/2 mice that are relatively sensitive or resistant to nicotine-induced seizures, respectively [46–47]. These studies revealed that resistance to seizures was dominant as was expression of lower levels of α-bungarotoxin binding sites. These results are also consistent with the role for α7-nAChR in mediating nicotine-induced clonic seizures. However, in the segregating F2 population the relationship between seizure sensitivity and α-bungarotoxin binding sites was not observed. No significant correlation was also reported when seizure sensitivity was compared to α-bungarotoxin binding as well as polymorphisms in the Chrna5 and Chrna7 genes [48]. A relatively small proportion of the F2 generation from an independent DBA by C3H cross mice was seizure sensitive. This result is consistent with the previous observation of dominance toward seizure resistance for these F2 mice. Although higher seizure sensitivity was noted in mice expressing the C3H polymorphism in the Chrna7 gene, the effect was more pronounced for the Chrna5 polymorphism, suggesting a possible role for α5*-nAChR in modulating nicotine-induced seizures.
The contribution of α7-nAChR to nicotine-induced seizures was subsequently examined using null mutant mice. Deletion of the α7 subunit did not alter the seizures elicited by nicotine, strongly indicating that the wild-type α7-nAChR did not mediate this response [49]. However, heterozygotic mice harboring a hyperactive α7-nAChR are indeed seizure sensitive, suggesting that stimulating a hyperactive α7-nAChR can indeed elicit seizure activity [50]. The appearance of nicotine-induced seizures in mice expressing hyperactive receptors has also been observed for the α4 subunit [51–52] and the β2 subunit [53]. This general result suggests that gain of function mutations can recruit nAChR subtypes to mediate nicotine-induced seizures, subtypes that may not mediate this response without altered receptor sensitivity.
As mentioned above, a polymorphism in the Chrna5 gene was implicated in mediating nicotine-induced seizures in the C3H×DBA F2 population [48]. The observation that deletion of the α5 nAChR subunit substantially reduced sensitivity to nicotine-induced seizures confirmed this prediction [54]. Furthermore, demonstration that deletion of the β4 subunit or reduced expression of the α3 subunit both significantly reduced seizures elicited by nicotine [55]strongly argues that an α3β4α5-nAChR subtype mediates this response.
6. Auditory Gating
Although the α7-nAChR may not be the primary mediator of nicotine-induced seizures, pharmacological studies have implicated this subtype in auditory gating [56]. A subsequent study compared auditory gating and α-bungarotoxin binding in hippocampus of eight different mouse strains. A significant inverse correlation was seen between the degree of auditory gating and the density of hippocampal α-bungarotoxin sites (α7-nAChR) but not nicotine sites (α4β2*-nAChR) [57], indicating that mice with relatively low α7-nAChR displayed poor auditory gating. In order to test this relationship, gating was investigated using C3H wild-type and α7 heterozygotes, which express significantly fewer α-bungarotoxin binding sites [58]. Indeed, the C3H α7+/− mice displayed much less auditory gating than did C3H+/+ mice, which except for the difference in α7 expression have virtually the same genetic background. Additional support for the role of α7-nAChR in modulating auditory gating is supplied by pharmacological studies demonstrating improvement in gating in DBA/2 mice (a poor gaiter) following administration of α7-nAChR selective nicotinic drugs [59].
7. Chronic Nicotine Treatment
Chronic exposure to nicotine results in the development of tolerance to the effects of nicotine and changes in the expression of nAChR. The increase in nAChR with high affinity for agonists was initially observed in rats [60] and mice [61]. Importantly, the nicotine-induced increase in these receptors also occurs in human tobacco users [62–64]. We have investigated the effects of genetic factors on development of tolerance to and regulation of nAChR expression following chronic nicotine treatment in mice.
Initially the four strains that had been tested for differences in response to acute nicotine treatment (BALB, C3H, C57BL/6 and C3H) were chronically treated with a single dose of nicotine (3 mg/kg/hr for 10 days) [65]. Tolerance to nicotine effects on locomotor activity and body temperature was noted in three of the strains, but C3H mice developed little tolerance following this treatment. A follow-up study in which DBA and C3H mice were treated with one of four nicotine doses (0, 2, 4 or 6 mg/kg/hr) confirmed that DBA mice develop increasing tolerance with increasing chronic treatment dose, while C3H mice develop very little tolerance [66].
These studies revealed significant genetic influences on the development of nicotine tolerance and suggested that mice that are initially less sensitive to the effects of nicotine (C3H) developed less tolerance following chronic nicotine treatment than mice that exhibited greater acute effects. The screen of 19 inbred strains [10] identified additional strains that were very sensitive (A) and very resistant (BUB) to acute nicotine administration. Mice from five inbred strains (most sensitive to least sensitive: C57BL/6 > A > DBA > C3H >BUB) chronically treated with nicotine (0, 0.5, 1, 2, 4 or 6 mg/kg/hr) were tested for tolerance in order to further examine genetic influences on tolerance development. As was the case with the previous studies, mice that were more sensitive to the acute effects of nicotine developed tolerance after treatment with lower nicotine doses than mice that were less sensitive to the acute effects (C57BL/6 > A > DBA > C3H >BUB).
Increases in nicotine binding were noted in the six brain regions assayed. The extent of the increase varied among the regions, but the responses were similar among the strains [65–67]. Chronic nicotine treatment did not affect the KD for nicotine. The generally similar response of binding sites measuring α4β2*-nAChR among the strains that differ markedly in tolerance development indicates that changes in the expression of these receptors do not adequately explain the differences in the development of tolerance following chronic nicotine treatment among these strains. Alternative or additional mechanisms are required to define the strain differences.
Chrna and Chrnb knockout and knock-in are being used to investigate various aspects of nicotine dependence including their roles in reinforcement and withdrawal. is The results of these studies demonstrate important roles for specific subunits including α4, α5, α6, β2 and β4 nAChR subunits and are the subject to several recent reviews and will not be discussed here [68–70].
8. Comparison of Patterns of Response Across Genotypes
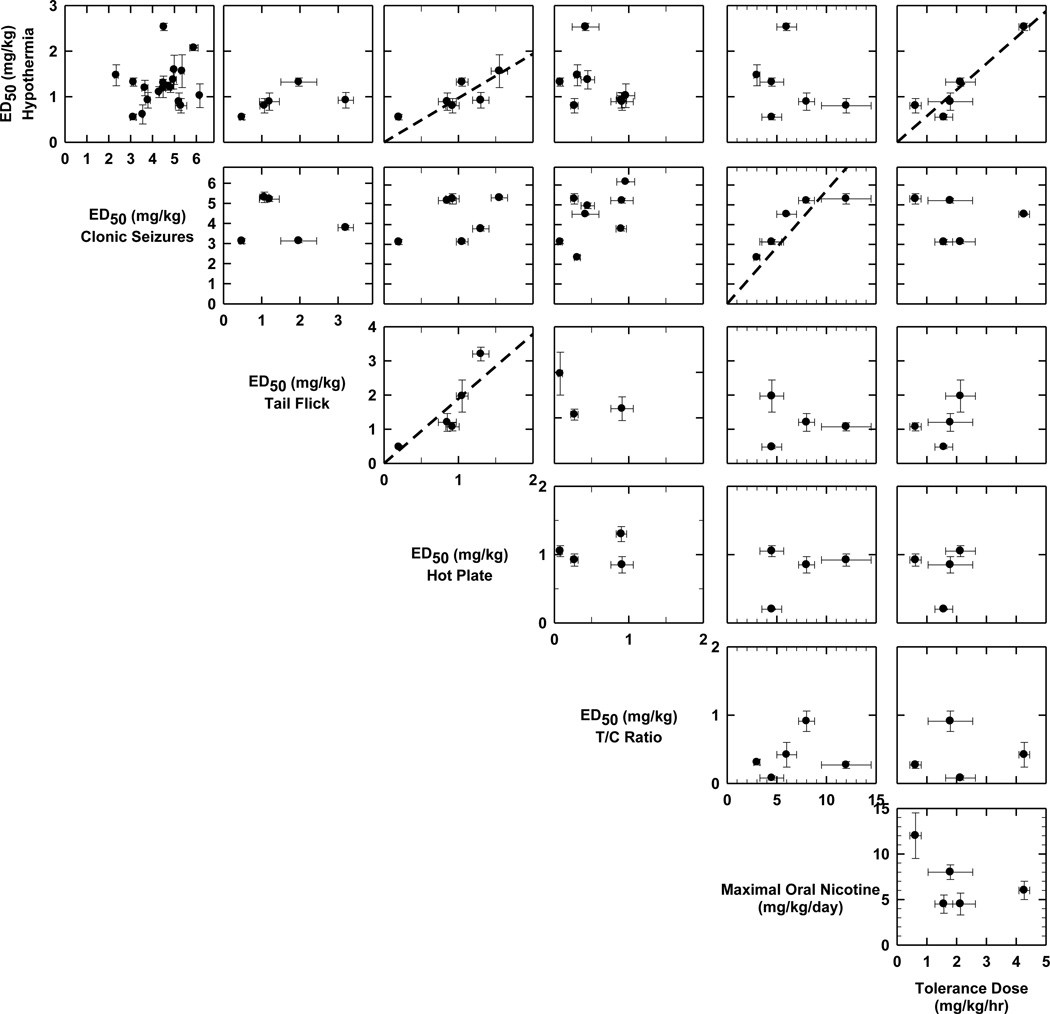
The scattergrams shown in Figure 2 compare the responses to nicotine measured during the screens for the inbred mouse strains. All responses were not measured in every strain, so the number of points on the scattergrams varies among the tests. In general, correlations among the various tests were not significant, indicating either that these responses either do not have a common genetic basis or too few strains have been tested. However, several responses for which regression lines are included in the panels were correlated: ED50 for antinociception measured by tail flick to that measured by hotplate (r = 0.89), ED50 for hypothermia and ED50 for antinociception measured with the hot plate (r = 0.82), ED50 for hypothermia and threshold tolerance dose for hypothermia (r = 0.93), and ED50 for seizures and maximum oral nicotine intake (r = 0.89). These significant correlations may indicate some shared genetic factors mediate the correlated behaviors.
Figure 2.
Correlations of relative sensitivity of inbred strains for seven measures of nicotine-induced changes. Scattergrams were constructed to compare measures of relative sensitivity of inbred mouse strains to each of seven independently measured responses to nicotine. Each individual panel presents the correlations between the measures of sensitivity to nicotine ± S.E.M for two tests (ED50 or similar value) for the mouse strains for which each measurement was conducted. Note that the numbers of points on each scattergram differ owing to variation in the number of strains measured for each test. The ordinate and abscissa for the responses on the diagonal in the figure change for the various comparisons. The axis label for each measurement is provided near the vertex of the panels in the horizontal and vertical directions and is read upwards as the abscissa for figures above the label and to the right as the ordinate for figures to the right of the label. For responses that were significantly correlated, regression lines passing through the origin are shown. Correlation coefficients for these measures were: ED50 hypothermia vs. ED50 hot plate, r = 0.82; ED50, hot plate vs. ED50 tail flick, r = 0.89; ED50 hypothermia vs. minimal tolerance dose, r = 0.93; ED50 clonic seizures vs. Maximal oral nicotine intake, r = 0.89. All other correlation coefficients were less than 0.55. Note that relatively few points are included for several pairs of tests reflecting limited number of strains tested for both measures.
The correlation between the two measures of thermal pain may not be surprising since both of these responses are significantly reduced in both α4 and β2 knockout mice [12], indicating the involvement of α4β2*-nAChR in mediating both responses. However, the effect of these gene deletions is not identical. A larger effect on the hot plate test was noted in both the α4 and β2 knockout mice as well as for the α4 L9’S knock-in heterozygote.
The positive correlation between ED50 values for hot plate antinociception and hypothermia was not quite as robust has that for the two measures of thermal pain. However, the fact that deletion of the α4 and β2 subunits significantly reduced sensitivity to nicotine for both of these measures, albeit to a different degree, is consistent with some shared mechanism of action.
The relationship between sensitivity to acute responsiveness to nicotine and the chronic treatment dose that elicits tolerance has been noted previously [67]. This observation indicates that tolerance develops to the effects of nicotine. That is mice that are initially more sensitive to the effects of the drug (such as C57BL/6) develop tolerance after exposure to lower nicotine doses than mice that are less responsive to an acute dose of nicotine (such as BUB). This differential tolerance development is not directly related to the up-regulation of nicotine binding sites since the pattern of change in these binding sites is generally similar for each of the five strains tested.
The significant correlation between oral nicotine intake and ED50 values for nicotine-induced seizures has also been noted previously [37]. Mice that are more sensitive to this adverse effect of nicotine consumed less nicotine. This result suggests that the perception of an unpleasant effect of nicotine, perhaps indicated by sensitivity to nicotine-induced seizures, limits voluntary oral intake of nicotine. Adverse effects of the drug may be the limiting factor in oral nicotine consumption. The observation that α5 knockout mice, which are resistant to nicotine-induced seizures [54], self-administer significantly more nicotine that wild-type mice [44] also indicates that reduction in adverse responses to nicotine facilitates drug intake.
9. Summary and Discussion
Genetic factors clearly influence several different physiological and behavioral responses to nicotine administered either acutely or chronically. Investigations using genetically modified mice have identified the importance of several different nAChR subunits, and consequently different nAChR subtypes, in mediating many of the responses in mice. With the advent of genome wide association studies, it has been demonstrated that variation among CHRN genes in either translated or untranslated regions (particularly CHRNA5, which encodes the α5 nAChR subunit) contribute to several different aspects of human tobacco use [71–73]. However, these variations in CHRN genes account for a relatively small amount of the genetic variance clearly indicating that other factors exist. One of these factors is differences in nicotine metabolism [74]. Several genetic factors are now known to influence nAChR function and/or expression. Probably most obvious changes are mutations leading to changes in primary sequence of a nAChR subunit and subsequently to functional change. Single point mutations, frequently leading to a gain of function of the mutant nAChR, contribute to the relatively rare syndrome, autosomal dominant frontal lobe epilepsy (reviewed in [75–77]). Animal models that express the mutant subunits have been developed [78]. Studies with mice engineered to express these mutations demonstrate that the gain of function mutations increase sensitivity to nicotine for locomotor activity and body temperature [18, 79]. In addition, gain of function mutations also alter sensitivity to nicotine-induced convulsions such that activation of nAChR subtypes containing α4, α7 or β2 subunits that do not normally mediate this response elicit seizure activity [50–51, 53, 80–81]. In addition to the profound effect of the hypersensitive nAChR subunits, more subtle changes in expression can alter drug responses. A naturally occurring mutation of the α4 subunit of mice also seems to elicit changes in stoichiometry and modifies responses to nicotine for several behaviors including hypothermia and open field activity [43]. Changes in the expression of α4 and β2 nAChR subunits alter the expression of two stoichiometric forms of α4β2-nAChR that are differentially sensitive to activation by agonists, including nicotine [27]. Stoichiometric changes were also observed for α4 transcripts lacking a 3’ untranslated region [29]. Relatively subtle structural changes can also markedly affect receptor function. Inclusion of the α5 subunit in α4β2α5-nAChR markedly alters physiological activity [32] and deletion of the α5 subunit dramatically reduces sensitivity to nicotine in vivo [34, 54], illustrating an important role for this auxiliary subunit consistent with the well established role of the α5 subunit in human smoking [82–83]. These examples illustrate that differences in nAChR expression and function are important factors in mediating response to nicotine.
Nevertheless, the importance of genetic factors, in addition to variation in nAChR subtype [34, 40, 45], distribution [41], and primary sequence [43] that also influence response to nicotine can be illustrated for conditioned place preference. Nicotine conditioned place preference is modified by changes in the expression of cannabinoid receptors [84–85], NMDA receptors [86], μ-opioid receptors [87], δ-opioid receptors [88], galanin [89], protein kinase C ε activity [90], and CREB expression [91]. Furthermore, nAChR are known to mediate the release of neurotransmitters dopamine, GABA, glutamate, and serotonin [92–93] and hormones such as corticosterone [94]. Thus, genetic variation in any of these processes can affect responses to nicotine. Consequently, while the genetic influences on nicotine mediated behaviors are surely affected by the complex array of and variations in nAChR, themselves, genetic diversity of the myriad processes that are mediated by or interact with nAChR no doubt contribute significantly to the complex phenotypes observed in response to nicotine exposure. The complexity of the genetic influences on nicotine response is illustrated to some extent by the observation that the inbred BUB/Bn mouse strain, which expresses relatively normal levels of α4β2*nAChR, [10] is less affected by acute nicotine administration than the β2 null mutant [17].
These genetic studies illustrate the complexity of the physiological, biochemical and behavioral responses observed following exposure to nicotine. Indeed, 30 years of research has progressed from the time before central nervous system nAChR were recognized as real until a diverse array of differentially distributed subtypes have been identified [95–100]. Further study of the role of these diverse nAChR and their interactions with the myriad of neuronal pathways will no doubt demonstrate additional levels of complexity underlying responses to acute and chronic nicotine and may reveal the basis for the persistence of tobacco use.
Acknowledgments
This work was supported by the following NIH grants: R01 DA003194, R01 DA012242, P30 DA15663 and U19 DA019375.
The author wishes to extend special thanks to Allan C. Collins, who introduced me to the power of genetic analysis of nicotine responses throughout our long and rewarding collaborations. The author would also like to thank the following people who performed much of the testing at the Institute for Behavioral Genetics, University of Colorado: Sharon Grady, Jerry Stitzel, Tristan McClure-Begley, Cristian Zambrano, Heidi O’Neill, Theresa Tritto, Outi Salminen, Sarah McCallum, Amy Bullock, Paul Whiteaker, James Pauly, Ratan Bhat, Christopher Butt, Peter Dobelis, David Fulker, Jeanne Wehner, Tammy Booker, Scott Robinson, Linda Artman, Douglas Patinkin, James Burch, Elena Romm, Lucinda Miner, Scott Bealer, Stephen Campbell, Sandra Selvaag, Stephan Gross, Douglas Farnham, Elizabeth Grun, Brian Slobe, Kimberly Smith, Melissa Jimenez, Natalie Meinerz, Karen Murphy, Satori Waddle, Scott Hutton, Robert Brown, Duncan Laverty, Erin Meyers, Esteban Loetz, Charles Wageman.
In addition the author would like to acknowledge our collaborators: Karen Stevens, Sherry Leonard and Robert Freedman at the Anschutz Medical Campus, University of Colorado Medical School, Aurora, CO., Marina Picciotto and colleagues at Yale University, New Haven, Ct; Henry Lester and colleagues, California Institute of Technology, Pasadena, CA; Cecilia Gotti and colleagues in the School of Medical Pharmacology, CNR, University of Milan, Italy; M. Imad Damaj and colleagues, Virginia Commonwealth University, Richmond, VA; Jon Lindstrom and colleagues, University of Pennsylvania, Philadelphia; J. Michael McIntosh and colleagues, University of Utah, Salt Lake City; Paul Kenny and colleagues, Scripps Florida, Jupiter, FL; John Drago, University of Melbourne, Australia; Stephan Heinemann and colleagues, Salk Institute, LaJolla, CA; Maryka Quik, SRI, Palo Alto, CA; Susan Wonnacott and colleagues, University of Bath, UK.
Abbreviations
- nAChR
nicotinic acetylcholine receptor
- LS
long sleep mice
- SS
Short sleep mice
- Chrna
genes encoding α subunits of the nAChR
- Chrnb
genes encoding β subunits of the nAChR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher RA. Cancer and smoking. Nature. 1958;182:596. doi: 10.1038/182596a0. [DOI] [PubMed] [Google Scholar]
- 2.Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, et al. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify "connectivity constellation" and drug target genes with pleiotropic effects. Ann N Y Acad Sci. 2008;1141:318–381. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrieze SI, McGue M, Iacono WG. The interplay of genes and adolescent development in substance use disorders: leveraging findings from GWAS meta-analyses to test developmental hypotheses about nicotine consumption. Hum Genet. 2012;131:791–801. doi: 10.1007/s00439-012-1167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- 5.Tyndale RF. Genetics of alcohol and tobacco use in humans. Ann Med. 2003;35:94–121. doi: 10.1080/07853890310010014. [DOI] [PubMed] [Google Scholar]
- 6.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 7.Marks MJ, Burch JB, Collins AC. Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther. 1983;226:291–302. [PubMed] [Google Scholar]
- 8.Marks MJ, Miner L, Burch JB, Fulker DW, Collins AC. A diallel analysis of nicotine-induced hypothermia. Pharmacol Biochem Behav. 1984;21:953–959. doi: 10.1016/s0091-3057(84)80079-9. [DOI] [PubMed] [Google Scholar]
- 9.Marks MJ, Miner LL, Cole-Harding S, Burch JB, Collins AC. A genetic analysis of nicotine effects on open field activity. Pharmacol Biochem Behav. 1986;24:743–749. doi: 10.1016/0091-3057(86)90584-8. [DOI] [PubMed] [Google Scholar]
- 10.Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacol Biochem Behav. 1989;33:667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- 11.Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- 12.Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d'Exaerde A, et al. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- 13.Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- 14.Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, et al. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks MJ, Romm E, Campbell SM, Collins AC. Variation of nicotinic binding sites among inbred strains. Pharmacol Biochem Behav. 1989;33:679–689. doi: 10.1016/0091-3057(89)90407-3. [DOI] [PubMed] [Google Scholar]
- 16.Tammimaki A, Horton WJ, Stitzel JA. Recent advances in gene manipulation and nicotinic acetylcholine receptor biology. Biochem Pharmacol. 2011;82:808–819. doi: 10.1016/j.bcp.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, et al. Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob Res. 2004;6:145–158. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- 18.O'Neill HC, Laverty DC, Patzlaff NE, Cohen BN, Fonck C, McKinney S, et al. Mice expressing the ADNFLE valine 287 leucine mutation of the Beta2 nicotinic acetylcholine receptor subunit display increased sensitivity to acute nicotine administration and altered presynaptic nicotinic receptor function. Pharmacol Biochem Behav. 2013;103:603–621. doi: 10.1016/j.pbb.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobelis P, Marks MJ, Whiteaker P, Balogh SA, Collins AC, Stitzel JA. A polymorphism in the mouse neuronal alpha4 nicotinic receptor subunit results in an alteration in receptor function. Mol Pharmacol. 2002;62:334–342. doi: 10.1124/mol.62.2.334. [DOI] [PubMed] [Google Scholar]
- 20.De Fiebre CM, Medhurst LJ, Collins AC. Nicotine response and nicotinic receptors in long-sleep and short-sleep mice. Alcohol. 1987;4:493–501. doi: 10.1016/0741-8329(87)90092-9. [DOI] [PubMed] [Google Scholar]
- 21.Tritto T, Stitzel JA, Marks MJ, Romm E, Collins AC. Variability in response to nicotine in the LS×SS RI strains: potential role of polymorphisms in alpha4 and alpha6 nicotinic receptor genes. Pharmacogenetics. 2002;12:197–208. doi: 10.1097/00008571-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]
- 25.Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- 26.Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, et al. Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the beta2 subunit. J Pharmacol Exp Ther. 1999;289:1090–1103. [PubMed] [Google Scholar]
- 27.Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, et al. Partial deletion of the nicotinic cholinergic receptor alpha 4 or beta 2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of alpha 4 and beta 2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Flanagin BA, Qin C, Macdonald RL, Stitzel JA. The mouse Chrna4 A529T polymorphism alters the ratio of high to low affinity alpha 4 beta 2 nAChRs. Neuropharmacology. 2003;45:345–354. doi: 10.1016/s0028-3908(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 29.Briggs CA, Gubbins EJ, Marks MJ, Putman CB, Thimmapaya R, Meyer MD, et al. Untranslated region-dependent exclusive expression of high-sensitivity subforms of alpha4beta2 and alpha3beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2006;70:227–240. doi: 10.1124/mol.105.020198. [DOI] [PubMed] [Google Scholar]
- 30.Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, et al. Genetic approaches identify differential roles for alpha4beta2* nicotinic receptors in acute models of antinociception in mice. J Pharmacol Exp Ther. 2007;321:1161–1169. doi: 10.1124/jpet.106.112649. [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, et al. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J Biol Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- 32.Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- 33.Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- 34.Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, et al. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, et al. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fowler CD, Kenny PJ. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology. 2011;61:687–698. doi: 10.1016/j.neuropharm.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson SF, Marks MJ, Collins AC. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl) 1996;124:332–339. doi: 10.1007/BF02247438. [DOI] [PubMed] [Google Scholar]
- 38.Miner LL, Collins AC. Strain comparison of nicotine-induced seizure sensitivity and nicotinic receptors. Pharmacol Biochem Behav. 1989;33:469–475. doi: 10.1016/0091-3057(89)90532-7. [DOI] [PubMed] [Google Scholar]
- 39.Jackson KJ, Walters CL, Miles MF, Martin BR, Damaj MI. Characterization of pharmacological and behavioral differences to nicotine in C57Bl/6 and DBA/2 mice. Neuropharmacology. 2009;57:347–355. doi: 10.1016/j.neuropharm.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- 41.McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. {alpha}4{beta}2 Nicotinic Acetylcholine Receptors on Dopaminergic Neurons Mediate Nicotine Reward and Anxiety Relief. J Neurosci. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cahir E, Pillidge K, Drago J, Lawrence AJ. The necessity of alpha4* nicotinic receptors in nicotinedriven behaviors: dissociation between reinforcing and motor effects of nicotine. Neuropsychopharmacology. 2011;36:1505–1517. doi: 10.1038/npp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilking JA, Hesterberg KG, Crouch EL, Homanics GE, Stitzel JA. Chrna4 A529 knock-in mice exhibit altered nicotine sensitivity. Pharmacogenet Genomics. 2010;20:121–130. doi: 10.1097/FPC.0b013e3283369347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009;331:547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miner LL, Marks MJ, Collins AC. Relationship between nicotine-induced seizures and hippocampal nicotinic receptors. Life Sci. 1985;37:75–83. doi: 10.1016/0024-3205(85)90628-9. [DOI] [PubMed] [Google Scholar]
- 47.Miner LL, Marks MJ, Collins AC. Genetic analysis of nicotine-induced seizures and hippocampal nicotinic receptors in the mouse. J Pharmacol Exp Ther. 1986;239:853–860. [PubMed] [Google Scholar]
- 48.Stitzel JA, Blanchette JM, Collins AC. Sensitivity to the seizure-inducing effects of nicotine is associated with strain-specific variants of the alpha 5 and alpha 7 nicotinic receptor subunit genes. J Pharmacol Exp Ther. 1998;284:1104–1111. [PubMed] [Google Scholar]
- 49.Franceschini D, Paylor R, Broide R, Salas R, Bassetto L, Gotti C, et al. Absence of alpha7-containing neuronal nicotinic acetylcholine receptors does not prevent nicotine-induced seizures. Brain Res Mol Brain Res. 2002;98:29–40. doi: 10.1016/s0169-328x(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 50.Broide RS, Salas R, Ji D, Paylor R, Patrick JW, Dani JA, et al. Increased sensitivity to nicotine-induced seizures in mice expressing the L250T alpha 7 nicotinic acetylcholine receptor mutation. Mol Pharmacol. 2002;61:695–705. doi: 10.1124/mol.61.3.695. [DOI] [PubMed] [Google Scholar]
- 51.Fonck C, Nashmi R, Deshpande P, Damaj MI, Marks MJ, Riedel A, et al. Increased sensitivity to agonist-induced seizures, straub tail, and hippocampal theta rhythm in knock-in mice carrying hypersensitive alpha 4 nicotinic receptors. J Neurosci. 2003;23:2582–2590. doi: 10.1523/JNEUROSCI.23-07-02582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teper Y, Whyte D, Cahir E, Lester HA, Grady SR, Marks MJ, et al. Nicotine-induced dystonic arousal complex in a mouse line harboring a human autosomal-dominant nocturnal frontal lobe epilepsy mutation. J Neurosci. 2007;27:10128–10142. doi: 10.1523/JNEUROSCI.3042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Cohen BN, Zhu Y, Dziewczapolski G, Panda S, Lester HA, et al. Altered activity-rest patterns in mice with a human autosomal-dominant nocturnal frontal lobe epilepsy mutation in the beta2 nicotinic receptor. Mol Psychiatry. 2011;16:1048–1061. doi: 10.1038/mp.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- 55.Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- 57.Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, et al. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- 58.Adams CE, Yonchek JC, Zheng L, Collins AC, Stevens KE. Altered hippocampal circuit function in C3H alpha7 null mutant heterozygous mice. Brain Res. 2008;1194:138–145. doi: 10.1016/j.brainres.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- 60.Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- 61.Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- 62.Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 63.Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, et al. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- 64.Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- 65.Marks MJ, Romm E, Gaffney DK, Collins AC. Nicotine-induced tolerance and receptor changes in four mouse strains. J Pharmacol Exp Ther. 1986;237:809–819. [PubMed] [Google Scholar]
- 66.Marks MJ, Stitzel JA, Collins AC. Dose-response analysis of nicotine tolerance and receptor changes in two inbred mouse strains. J Pharmacol Exp Ther. 1986;239:358–364. [PubMed] [Google Scholar]
- 67.Marks MJ, Campbell SM, Romm E, Collins AC. Genotype influences the development of tolerance to nicotine in the mouse. J Pharmacol Exp Ther. 1991;259:392–402. [PubMed] [Google Scholar]
- 68.Stoker AK, Markou A. Unraveling the neurobiology of nicotine dependence using genetically engineered mice. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol. 2008;19:461–484. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fowler CD, Kenny PJ. Utility of genetically modified mice for understanding the neurobiology of substance use disorders. Hum Genet. 2012;131:941–957. doi: 10.1007/s00439-011-1129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bierut LJ. Genetic vulnerability and susceptibility to substance dependence. Neuron. 2011;69:618–627. doi: 10.1016/j.neuron.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schlaepfer IR, Hoft NR, Ehringer MA. The genetic components of alcohol and nicotine co-addiction: from genes to behavior. Curr Drug Abuse Rev. 2008;1:124–134. doi: 10.2174/1874473710801020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Portugal GS, Gould TJ. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behav Brain Res. 2008;193:1–16. doi: 10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature. 1998;393:750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- 75.Mann EO, Mody I. The multifaceted role of inhibition in epilepsy: seizure-genesis through excessive GABAergic inhibition in autosomal dominant nocturnal frontal lobe epilepsy. Curr Opin Neurol. 2008;21:155–160. doi: 10.1097/WCO.0b013e3282f52f5f. [DOI] [PubMed] [Google Scholar]
- 76.Ferini-Strambi L, Sansoni V, Combi R. Nocturnal frontal lobe epilepsy and the acetylcholine receptor. Neurologist. 2012;18:343–349. doi: 10.1097/NRL.0b013e31826a99b8. [DOI] [PubMed] [Google Scholar]
- 77.Steinlein OK, Bertrand D. Nicotinic receptor channelopathies and epilepsy. Pflugers Arch. 2009 doi: 10.1007/s00424-009-0766-8. [DOI] [PubMed] [Google Scholar]
- 78.Steinlein OK. Animal models for autosomal dominant frontal lobe epilepsy: on the origin of seizures. Expert Rev Neurother. 2010;10:1859–1867. doi: 10.1586/ern.10.130. [DOI] [PubMed] [Google Scholar]
- 79.Tapper AR, McKinney SL, Marks MJ, Lester HA. Nicotine responses in hypersensitive and knockout alpha 4 mice account for tolerance to both hypothermia and locomotor suppression in wild-type mice. Physiol Genomics. 2007;31:422–428. doi: 10.1152/physiolgenomics.00063.2007. [DOI] [PubMed] [Google Scholar]
- 80.Lester HA, Fonck C, Tapper AR, McKinney S, Damaj MI, Balogh S, et al. Hypersensitive knockin mouse strains identify receptors and pathways for nicotine action. Curr Opin Drug Discov Devel. 2003;6:633–639. [PubMed] [Google Scholar]
- 81.Fonck C, Cohen BN, Nashmi R, Whiteaker P, Wagenaar DA, Rodrigues-Pinguet N, et al. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive alpha 4* nicotinic receptors. J Neurosci. 2005;25:11396–11411. doi: 10.1523/JNEUROSCI.3597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bierut LJ. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24-25. Trends Pharmacol Sci. 2010;31:46–51. doi: 10.1016/j.tips.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326:483–492. doi: 10.1124/jpet.108.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 86.Wang LP, Li F, Shen X, Tsien JZ. Conditional knockout of NMDA receptors in dopamine neurons prevents nicotine-conditioned place preference. PLoS One. 2010;5:e8616. doi: 10.1371/journal.pone.0008616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, Zimmer A, et al. Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J Neurosci. 2005;25:1103–1112. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berrendero F, Plaza-Zabala A, Galeote L, Flores A, Bura SA, Kieffer BL, et al. Influence of delta-opioid receptors in the behavioral effects of nicotine. Neuropsychopharmacology. 2012;37:2332–2344. doi: 10.1038/npp.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neugebauer NM, Henehan RM, Hales CA, Picciotto MR. Mice lacking the galanin gene show decreased sensitivity to nicotine conditioned place preference. Pharmacol Biochem Behav. 2011;98:87–93. doi: 10.1016/j.pbb.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee AM, Messing RO. Protein kinase C epsilon modulates nicotine consumption and dopamine reward signals in the nucleus accumbens. Proc Natl Acad Sci U S A. 2011;108:16080–16085. doi: 10.1073/pnas.1106277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J Mol Neurosci. 2006;30:137–140. doi: 10.1385/JMN:30:1:137. [DOI] [PubMed] [Google Scholar]
- 93.Barik J, Wonnacott S. Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol. 2009:173–207. doi: 10.1007/978-3-540-69248-5_7. [DOI] [PubMed] [Google Scholar]
- 94.Pauly JR, Ullman EA, Collins AC. Adrenocortical hormone regulation of nicotine sensitivity in mice. Physiol Behav. 1988;44:109–116. doi: 10.1016/0031-9384(88)90353-8. [DOI] [PubMed] [Google Scholar]
- 95.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 96.Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987;84:7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marubio LM, Changeux J. Nicotinic acetylcholine receptor knockout mice as animal models for studying receptor function. Eur J Pharmacol. 2000;393:113–121. doi: 10.1016/s0014-2999(00)00007-8. [DOI] [PubMed] [Google Scholar]
- 98.Boulter J, O'Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, et al. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990;265:4472–4482. [PubMed] [Google Scholar]
- 99.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.le Novere N, Zoli M, Lena C, Ferrari R, Picciotto MR, Merlo-Pich E, et al. Involvement of alpha6 nicotinic receptor subunit in nicotine-elicited locomotion, demonstrated by in vivo antisense oligonucleotide infusion. Neuroreport. 1999;10:2497–2501. doi: 10.1097/00001756-199908200-00012. [DOI] [PubMed] [Google Scholar]