Abstract
Background
Microsomal enzyme P450 (CYP450) plays an important role in metabolism of most xenobiotics. The activity of CYP3A decreases in patients with liver dysfunction. However, whether serum concentrations of liver enzymes reflect the activity of CYP3A is unclear. We aimed to search for a new clue to predict the activity of CYP3A and guide clinical medication.
Material/Methods
Forty-five patients undergoing surgery under general anesthesia were enrolled in the study, including 15 cases with normal liver function (Group N), 15 cases with moderate fatty liver according to both the results of ultrasonic diagnosis of moderate fatty liver and the laboratory results of elevated alanine transaminase less than 3 times the normal (Group M), and 15 cases with end-stage liver disease (Group S). Each patient received a single dose of 5 mg midazolam intravenously. CYP3A activity was measured by plasma 1′hydroxymidsazolam/midazolam (1′-OH-MDZ/MDZ) ratio at 2 h after administration of midazolam.
Results
They was no significant difference in CYP3A activity between the patients with normal liver function and moderate fatty liver (P=0.332). The activity of CYP3A in Group S was lower than in Group N and Group M (P=0.000). Multiple linear regression analysis showed a statistically significant linear relationship between the activity of CYP3A and alanine transaminase (ALT, R2=0.682, P=0.000), and total bilirubin (TB, R2=0.519, P=0.002). There were no other factors, including albumin (ALB, P=0.881) and alkaline phosphatase (ALP, P=0.497), correlated with the activity of CYP3A.
Conclusions
We conclude that the activity of CYP3A in patients with end-stage liver disease decreased. The decrease in the activity of CYP3A was determined by the increase in the serum concentration of ALT and TB and not by patient’s age or body weight. ALT and TB therefore might have predictive value for the activity of CYP3A. An abnormal liver function test likely gives the clinician a hint about dosage adjustment.
MeSH Keywords: Cytochrome P-450 CYP3A, Liver Function Tests, Midazolam
Background
Microsomal enzyme P450 (CYP450), encoded by the CYP3A gene family, accounts for most in the hepatic cytochrome oxidase, which is mainly expressed in the adult liver and small intestine. CYP3A plays an important role in metabolism of most xenobiotics, including more than 120 different kinds of drug metabolism [1,2], and relatively high CYP3A levels – about 50% hepatic levels and 70% total CYP protein – are also presented in small intestinal epithelium [3]. The term CYP3A is usually understood to reflect the collective activity of all the isoforms [4].
Previous liver cirrhosis animal models and human studies have suggested that CYP3A activity was reduced, which was inversely proportional to the degree of liver cirrhosis [5–7]. CYP3A activity was also significantly reduced in patients with fatty liver of hepatitis and was negatively correlated with the degree of fat accumulation [8,9].
Northern blot, RT-PCR, and bDNA have commonly been used in detecting the induction and expression of CYP3A, while Western blot can determine the expression of CYP3A protein. Currently, probe substrates such as testosterone or midazolam are usually used to evaluate the activity of CYP3A [10]. Midazolam (MDZ) is metabolized in the liver by CYP3A4 to 2 active metabolites: 1′-hydroxymidazolam (1′-OH-MDZ) and 4′-hydroxymidazolam (4′-OH-MDZ) [11–13]. The 1′-OH-MDZ is catalyzed almost exclusively by CYP3A [11], and is thus also proposed for use as a probe for CYP3A activity in vitro [14] and in vivo [15]. The erythromycin breath test (ERMT) and the formation of monoethylglycinexylidide (MEGX) can be also used as markers for hepatic CYP3A activity in vivo [16]. MEGX test has been demonstrated to correlate with Child-Pugh score [17]. At present, the usefulness of detecting the marker for hepatic CYP3A activity for dosage adjustment for patient with liver function abnormality is rather limited.
To measure the activity of CYP3A in the current study we chose patients with normal liver function, moderate fatty liver patients, and patients with end-stage liver disease, using the one-point midazolam method. We aimed to investigate the relationship between hepatic CYP3A activity and serum concentration of liver enzymes, and to explore the role of liver function indices in predicting the hepatic CYP3A activity in patients with liver dysfunction. We hope that our results will provide the clinician with a readily available method for determining the dosage regimen for liver patients.
Material and Methods
Drugs and reagents
Midazolam injection was provided by Enhua Pharmaceutical Co, Ltd. (Xuzhou, China, batch number 20010807). Midazolam standard was purchased from Roche Pharmaceuticals Co, Ltd. (Shanghai, China, batch number 5081114, purity: 100%), and estazolam was purchased from Sichuan Province Food and Drug Inspection (Chengdu, China). Methanol and acetonitrile of HPLC (pure grade) was from Fisher Company (Pittsburgh, PA). Ammonium acetate (Sigma Company, USA), triethylamine (Sigma Company, USA), disodium hydrogen phosphate (Sigma Company, USA), and ethyl ether (Sigma Company, USA) were analytically pure grade.
Instruments
A multifunctional monitor (Detex-ohmeda, Holland), an 1100 high performance liquid chromatography with diode array detector (Agilent Company, USA), a Biofuge primo centrifuge (Hreaeus Company, USA), a Milli-Q pure water machine (Millipore Company), and an ESJ120-4 electronic scale (Shengyang Longteng Electronic Scale Co, Ltd, China) were purchased from a domestic agency.
Experimental design
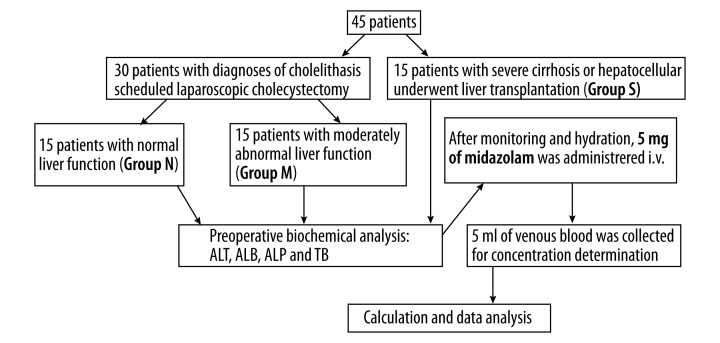
The protocol (reference number 20090150) was approved on May 15, 2009 by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, China). Forty-five patients participated in this study after having given their written informed consent. Thirty of these patients with diagnoses of cholelithiasis were scheduled for laparoscopic cholecystectomy, including 15 patients with normal liver function (Group N) and 15 patients with moderately abnormal liver function, according to both the results of ultrasonic diagnosis of moderate fatty liver and the laboratory results of elevated alanine transaminase less than 3 times normal (Group M). The other 15 patients with end-stage liver disease (Group S) underwent liver transplantation. The patients in Group S had severe cirrhosis or hepatocellular carcinoma (Figure 1).
Figure 1.
Flow chart of patient inclusion and methods.
Patients were excluded from the study if they had serious impairment of respiratory, cardiovascular, renal, or endocrine function; or they received medication likely to influence the course of the study. All patients abstained from medication, including alcohol, caffeine, or grapefruit juice, for at least 7 days before the study. All subjects were non-smokers. All patients underwent preoperative blood biochemical analyses, including alanine transaminase (ALT), albumin (ALB), alkaline phosphatase (ALP), and total bilirubin (TB).
Participants were not premedicated before the operation. After prehydration with 500 ml lactated Ringer solution and using standard monitors, as well as taking into account the previous study [18] and our preliminary studies, 5 mg of midazolam was given as a bolus injection over a period of 30 s into a peripheral vein of the upper arm. Venous blood samples (5 ml) were collected into lithium-heparin tubes 2 h after intravenous administration of midazolam. Plasma was separated and stored at −20°C until analysis within 4 weeks. When a patient had dyspnea, appropriate respiratory support was supplied. Hypotension was defined as a reduction of systolic blood pressure by more than 30% of the pre-anesthetic value or a systolic blood pressure less than 90 mmHg. Patients with hypotension received treatment of 6 mg of i.v. ephedrine and 300 ml of crystalloid fluids. Bradycardia was defined as HR less than 55 beats per min and was treated by administering 0.5 mg of i.v. atropine. On completion of the experimental evaluation, the experiment ended and anesthesia started (Figure 1).
Analysis of midazolam and 1′-hydroxymidazolam
The plasma concentration of midazolam and 1′-hydroxymidazolam were determined using a specific HPLC method with u.v. detection based on Carrillo et al. with maximum u.v. absorption peak at 220 nm [19]. The analytes were separated on an 1100 high-performance liquid chromatography device with diode array detector. Gradients elution was performed on an Extend C18 analytical column (250×4.0 mm, size 5 um). The column oven temperature was set at 30°C. The mobile phase consisted of methanol/acetonitrile/acetic acid (50: 5: 45, v/v). The flow rate was 1.0/min.
Data analysis
Demographic data are presented as means ±SD. Statistical calculations were done using one-way analysis of variance (ANOVA). Correlations between parameters were evaluated by simple and multiple linear regression analysis. Analyses were performed by using the following software: Excel 2003 (Microsoft, Redmond, WA), and SPSS 10.5 (SPSS, Inc., Chicago, IL). Statistical significance was defined for overall α error at the 0.05 level. All P values were 2-sided. P<0.05 was considered statistically significant. Sample size estimation was based on type I statistical error of 0.05 and type II statistical error of 0.2 to detect a 20% reduction in metabolic ratio of 1′-OH-MDZ/MDZ. It was estimated that 15 patients would be necessary for each of 3 groups.
Results
The study included 22 men and 23 women; demographic information is summarized in Table 1. There was no statistically significant difference between the 3 groups in terms of age, body weight, or height (P>0.05, Table 1). The serum concentration of ALT was higher in Group M and Group S than Group N (P<0.01) and higher in Group S than in Group M. ALB, ALP, and TB levels in Group N were comparable with Group M (P>0.05), but these 3 liver function indices in Group S were higher than in Group N and M (P<0.01, Table 1). Changes in arterial blood pressure, heart rate, and SpO2 in all the patients remained within normal limits throughout the study.
Table 1.
Demographic data, preoperative liver function and the activity of CYP3A in patients with different liver function test.
| Group N (n=15) | Group M (n=15) | Group S (n=15) | |
|---|---|---|---|
| Age (yr) | 39±9 | 42±11 | 43±11 |
| Weight (kg) | 61±8 | 62±10 | 64±7 |
| Height (m) | 1.66±0.08 | 1.68±0.09 | 1.67±0.08 |
| Sex (male/female) | 7/8 | 8/7 | 7/8 |
| Diagnosis (number of patients) | Cholelithiasis (15) | Cholelithiasis (15) | Cirrhosis (11) Hepatocellular carcinoma (4) |
| ALT (U/L) | 20±7b,c | 114±12c | 404±108 |
| ALB (g/L) | 44±2c | 42±5c | 33±3 |
| ALP (U/L) | 61±12c | 65±9c | 211±45 |
| TB (μmol/L) | 14±5c | 22±4c | 295±104 |
| Activity of CYP3A [lg(1′-OH-MDZ/MDZ)] | −0.38±0.11c | −0.42± 0.10c | −0.60± 0.12 |
ALT – alanine transaminase; ALB – albumin; ALP – alkaline phosphatase; TB – total bilirubin. All values, except n and sex, are expressed as mean ±SD.
Compared with Group M, P<0.05,
compared with Group S, P<0.05.
Using the metabolism of midazolam to its 1′-hydroxylation metabolite as a marker of activity, CYP3A activities were detected in all participants. The difference in metabolic ratio between the 3 groups was statistically significant (P<0.05). The activity of CYP3A in Group S was the lowest, and the activity of CYP3A in Group N was the highest (Table 1). The mean metabolic ratio was significantly higher for patients in Group M versus patients in Group S (P<0.05), but there was no significant difference in metabolic ratio between patients in Group N and Group M (P>0.05).
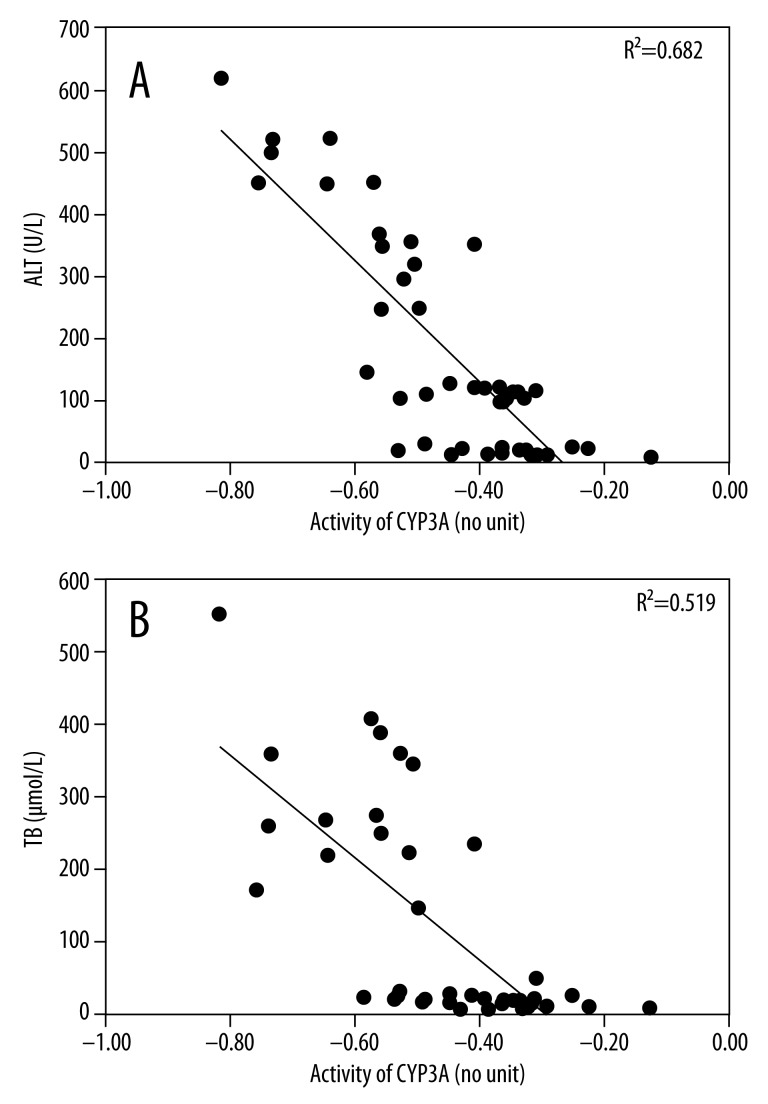
We searched for factors correlating with the activity of CYP3A, and found a statistically significant linear relationship between the activity of CYP3A and ALT (R2=0.682, P=0.000) (Figure 2A), and between TB (R2=0.519, P=0.002) (Figure 2B). Multiple regression analysis confirmed that no other factors, including ALB (P=0.881) and ALP (P=0.497), correlated with the activity of CYP3A. There was no correlation between body weight, age, height, or activity of CYP3A (Table 2).
Figure 2.
Association between the activity of CYP3A ([lg(1′-OH-MDZ/MDZ)]) in patients with different liver function test results and serum concentration of ALT (A) and TB (B). The symbol (·) represents the data from all patients included. The solid line is a linear-regression analysis of data in all patients (R2=0.682; P=0.000 for activity of CYP3A vs. ALT; R2=0.519; P=0.002 for activity of CYP3A vs. TB). ALT: alanine transaminase; TB: total bilirubin; MDZ: midazolam.
Table 2.
Linear regression analysis showing relationships between several variables and the activity of CYP3A.
| R2 | Coefficient β | P value | |
|---|---|---|---|
| Age | 0.203 | 0.005 | 0.091 |
| Weight | 0.184 | 0.007 | 0.110 |
| Height | 0.248 | 0.363 | 0.373 |
| ALT | 0.682 | 0.247 | 0.000 |
| ALB | 0.002 | 0.002 | 0.881 |
| ALP | 0.036 | 0.000 | 0.497 |
| TB | 0.519 | 0.001 | 0.002 |
ALT – alanine transaminase; ALB – albumin; ALP – alkaline phosphatase; TB – total bilirubin.
The regression equations for MDZ and 1′-OH-MDZ were y=132.15x+0.3245 (R2=0.9994; n=9), and y=134.43x–0.1578 (R2=0.9998; n=9), respectively. The limit of quantification was 15 ng/ml for both MDZ and 1′-OH-MDZ. The inter-assay coefficient of variation (CV) was <10% at concentrations of 20 ng/ml (n=9) and 500 ng/ml (n=9) for both MDZ and 1′-OH-MDZ. The mean relative recoveries at high, medium, and low concentration were 86.2%, 97.6%, and 96.3%, respectively, for MDZ, and 87.4%, 98.1%, and 95.8% for 1′-OH-MDZ, respectively.
Discussion
The main purpose of this study was to determine whether the hepatic function indices influenced the activity of CYP3A. Our results indicated that reduction in CYP3A activity was associated with severity of liver disease. Generally, patients with end-stage advanced liver disease (Group S) had significantly lower CYP3A activity compared with patients with normal liver function or less severe liver disease. Indeed, there was no significant difference in CYP3A activity between moderate liver diseases (Group M) and the control group (Group N).
The liver plays a central role in the pharmacokinetics of most drugs. Liver dysfunction was associated with reductions in drug-metabolizing and an extensive literature indicates that the metabolism of drugs is impaired in patients with chronic liver disease [20–26]. Chronic liver diseases are associated with variable and non-uniform reductions in drug-metabolizing activities. George et al. [27] reported that drug metabolism reduced (especially CYP450-mediated) drug metabolism in patients with liver disease, and the extent of the reduction was associated with the severity of liver disease. It was also suggested that there may be different patterns of altered hepatic P450 expression according to the cause of liver disease. In samples from subjects with cirrhosis, McConn et al. [5] determined that duodenal CYP3A expression and midazolam hydroxylation effect were reduced by 47% and 34%, respectively, as compared with samples from controls. Greater decreases in CYP3A expression were seen in subjects with increasing severity of cirrhosis. Similarly, using midazolam as a CYP3A probe, Chalasani et al. [6] used a pharmacokinetic modeling approach to evaluate the effect of cirrhosis on intestinal and hepatic CYP3A activity, reporting that hepatic CYP3A activity was reduced in cirrhosis patients compared with healthy controls. In addition, Yang et al. reevaluated difference in CYP3A4 enzyme and its gene expression between patients with hepatic cirrhosis and obstructive jaundice, and found that CYP3A4 isoenzyme and its activity declined in patients with hepatic cirrhosis as the expression of CYP3A4 gene was significantly reduced [7]. In contrast, patients with hepatic steatosis exhibited a marked reduction in CYP3A activity, and there was a significantly negative relationship between severity of steatosis and hepatic CYP3A activity [8]. Further research confirmed that forced-feeding animals resulting in a 235% increased liver fat content and was associated a decrease in total CYP (55%) activity, as well as in CYP3A (70%) protein content. Furthermore, the decreases in both CYP content and related activity were correlated with degree of liver fat content [9]. However, not all results of studies of CYP3A activity in patients with liver disease are fully consistent with these observations. Conflicting findings have also been reported that alcohol-induced liver damage was associated with a generalized induction of CYP450, which also occurred in patients with non-alcoholic steatosis. It has been shown that the induction of CYP450 may play a role in cytoprotection in response to hepatocellular damage and endoplasmic reticulum stress [28,29]. The present study selected cases of moderate fatty liver with slight hepatocellular damage. The activity of CYP3A declined, with no statistical significance. How different degrees of liver steatosis affect the activity of CYP 450 remains uncertain and requires further investigation.
It has been confirmed that there was a significant correlation between plasma MDZ clearance and the 1′-OH-MDZ/MDZ plasma ratio, assessed at 1 h after 7.5-mg MDZ intake in the volunteers [30]. The finding provided a simpler estimate for measuring liver and intestinal CYP3A activity, with a single blood measurement in a population study. Subsequent research further supported that the single-point approach offered efficient means to assess in-vivo phenotyping for CYP3A [31–33]. Thummel et al. found a high correlation (r=0.93, P<0.01) between hepatic CYP3A content and plasma 1′-hydroxy-MDZ/MDZ ratio of 30-min post-dose intravenously [34]. Villeneuve et al. [15] assessed CYP3A probe from single-point blood measurement 1 h after intravenous administration of MDZ. Lin et al. [31] showed that the concentration at the mean residence time (MRT) should be the best predictor of the total AUC. They concluded that a single 4-h midazolam concentration following i.v. administration is an accurate marker of CYP3A phenotype. Furthermore, Chaobal et al. [32] found the optimal plasma sample at 5–6 h post-dose accurately predicted midazolam AUC after a single-dose i.v. dose. Frupka et al. [33] found a moderate correlation between MDZ clearance and the 1′-OH-MDZ/MDZ ratio at 9 h post-dosing (r=0.81, P=0.04), and an even better correlation (r=0.99, P<0.009) was found between MDZ AUC and MDZ plasma concentration at 6 h post-dosing. Thus it can be seen that different studies collected 1 sample at different times post-dose. According to the pharmacokinetics of midazolam: t1/2 α=6–15 min, t1/2 β=1.7–4 h, the sampling times mentioned above were nearly within 1–2 t1/2 β of MDZ [18]. Therefore, in this study, we used the plasma 1′-OH-MDZ/MDZ ratio at 2 h after intravenous administration of midazolam as the CYP3A activity index.
To the best of our knowledge, there have been no published reports about liver function index related to the activity of CYP3A. The second purpose of the present study was to determine which liver function index was the predictor of the activity of CYP3A. In this study, serum concentrations of both ALT and TB were found to be associated with the activity of CYP3A, and these 2 items of the liver function index might have a predictive value for the activity of CYP3A in patients with liver dysfunction. Several mechanisms have been used in this associate linking ALT and TB with the activity of CYP3A. The elevation of serum ALT reflects the hepatocellular damage, which may contribute to the decrease in number of hepatic microsomes and the reduction in amount of liver microsomal enzymes. Serum concentration of ALT increased when there is a small amount of liver cell damage, then, but the amount of liver microsomal enzymes remained unchanged, as indicated in this study. Patients with moderate liver dysfunction are probably more dependent on the intestine for metabolism of CYP3A substrates [35]. We also found that serum TB was another independent factor in CYP3A activity. Liver cell damage and biliary obstruction are likely to lead to an elevated serum TB, which might reflect a general decline in hepatic biliary transport of endogenous molecules. The absence of endogenous factors that are positive regulators of duodenal CYP3A might result in a reduction in CYP3A protein expression [36]. Liver microsomal enzymes play a very important role in jaundice metabolism; therefore, elevated serum concentration of TB is thought to be the result of the CYP3A activity reduction.
In addition to the clinically commonly used and readily available liver function assessment such as Child-Pugh and model for end-stage liver disease (MELD) for liver patients, dynamic liver function tests such as ERM, 1′-OH-MDZ/MDZ ratio, and MEGX [16], which can be used as measurement of in vivo of hepatic CYP3A activity, have been developed to guide drug dosage adjustment for patients with hepatic abnormality. Huang et al. [17] showed that there was a high correlation between MEGX and Child-Pugh score. Hooker et al. [37] evaluated the relationship between CYP3A activity and docetaxel clearance in patients with varying degrees of liver function. The results indicated that the population pharmacokinetic model based on ERMT can predict docetaxel clearance and provide a guide to drug dosage in patients with liver dysfunction. From their results we can infer that abnormal liver function test likely give the clinician data indicating that drug clearance may decrease in patients with liver dysfunction and that the dosage regimen should be modified.
The present study had some limitations. First, the number of enrolled patients was too small to draw definite conclusions from. There were different mechanisms of liver damage in patients with end-stage liver disease, which made the impact on hepatic microsomal enzymes more complex. Future studies will require much larger populations to verify these preliminary results. Second, the absence of measurements of content of hepatic microsomal CYP3A was also a limitation. Third, whether a single plasma 1′-OH-MDZ and MDZ quantification ratio is valid for subjects with various degrees of liver insufficiency is currently not known. Further studies may measure 1′-OH-MDZ/MDZ concentration-time profiles for 1, 2, 4, and 9 h after i.v. MDZ for intra-group comparison to validate the single-plasma sampling technique.
Patients with unusually high or low CYP3A activity should be at increased risk for subtherapeutic or toxic responses [1]. Because CYP3A is involved in the metabolism of many medications and environmental contaminants, there might be ethic difference in toxic responses to some medications and risk of some environmental diseases. We conclude that patients with severely impaired liver function have decreased CYP3A activity as compared with both healthy controls and patients with moderate liver dysfunction. In patients with liver dysfunction, serum concentrations of ALT and TB might have a predictive value for the activity of CYP3A, and these biomarkers might be useful in defining the phenotype of the activity of CYP3A.
Conclusions
The results of the present study, as well as results of our companion study [38] about effects of hepatic function on EC50 and BIS50 of midazolam for consciousness in patients, suggest that reduced dosage of midazolam or other CYP3A-metabolized drugs in patients with advanced cirrhosis of the liver is recommended on the basis of both pharmacokinetics and pharmacodynamics.
Acknowledgements
The authors thank Fang-Ping Bao who assisted during the experiment.
Footnotes
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Source of support: This study was supported by a grant from the Qianjiang Talents Project of the Technology Office of Zhejiang Province (No. 2012R10033)
References
- 1.Watkins PB. Noninvasive tests of CYP3A enzymes. Pharmacogentics. 1994;4:171–84. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxical. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 3.Thummel RE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP3A. Annu Rev Pharmacol Toxicoal. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 4.Du J, Yu L, Wang L, et al. Differences in CYP3A41G genotype distribution and haplotypes of CYP3A4, CYP3A5 and CYP3A7 in 3 Chinese populations. Clin Chim Acta. 2007;383:172–74. doi: 10.1016/j.cca.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 5.McConn DJ, II, Lin YS, Mathisen TL, et al. Reduced Duodenal Cytochrome P450 3A Protein Expression and Catalytic Activity in Patients with Cirrhosis. Clin Pharmacol Ther. 2009;85:387–93. doi: 10.1038/clpt.2008.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalasani N, Gorski JC, Patel NH, et al. Hepatic and intestinal cytochrome P450 3A activity in cirrhosis: effects of transjugular intrahepatic portosystemic shunts. Hepatology. 2001;34:1103–8. doi: 10.1053/jhep.2001.29306. [DOI] [PubMed] [Google Scholar]
- 7.Yang LQ, Li SJ, Cao YF, et al. Different alterations of cytochrome P450 3A4 isoform and its gene expression in livers of patients with chronic liver diseases. World J Gastroenterol. 2003;9:359–63. doi: 10.3748/wjg.v9.i2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolwankar D, Vuppalanchi R, Ethell B, et al. Association Between Nonalcoholic Hepatic Steatosis and Hepatic Cytochrome P-450 3A Activity. Clin Gastroenterol Hepatol. 2007;5:388–93. doi: 10.1016/j.cgh.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Leclercq I, Horsmans Y, Desager JP, et al. Reduction in hepatic cytochrome P-450 is correlated to the degree of liver fat content in animal models of steatosis in the absence of inflammation. J Hepatol. 1998;28:410–16. doi: 10.1016/s0168-8278(98)80314-0. [DOI] [PubMed] [Google Scholar]
- 10.Czewrinski M, Opdam P, Madan A, et al. Analysis of CYP mRNA expression by branched DNA technology. Methods Enzymol. 2002;357:170–79. doi: 10.1016/s0076-6879(02)57676-x. [DOI] [PubMed] [Google Scholar]
- 11.Kronbach T, Mathys D, Umeno M, et al. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol. 1989;36:89–96. [PubMed] [Google Scholar]
- 12.Gorski JC, Hall SD, Jones DR, et al. Regioselective biotransformation of midazolam by members of the human cytochrome P4503A (CYP3A) subfamily. Biochem Pharmacol. 1994;47:1643–53. doi: 10.1016/0006-2952(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 13.Mandema JW, Tuk B, van Stevenick AL, et al. Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite alpha-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther. 1992;51:715–28. doi: 10.1038/clpt.1992.84. [DOI] [PubMed] [Google Scholar]
- 14.von Moltke LL, Greenblatt DJ, Schmider J, et al. Midazolam hydroxylation by human liver microsomes in vitro: inhibition by fluoxetine, norfluoxetine, and by azole antifungal agents. J Clin Pharmacol. 1996;36:783–91. doi: 10.1002/j.1552-4604.1996.tb04251.x. [DOI] [PubMed] [Google Scholar]
- 15.Villeneuve JP, L’Ecuyer L, De Maeght S, et al. prediction of cyclosporine clearance in liver transplant recipients by the use of midazolam as cytochrome P450 3A probe. Clin Pharmacol Ther. 2000;67:242–48. doi: 10.1067/mcp.2000.104642. [DOI] [PubMed] [Google Scholar]
- 16.Burra P, Masier A. Dynamic tests to study liver function. Eur Rev Med Pharmacol Sci. 2004;8:19–21. [PubMed] [Google Scholar]
- 17.Huang YS, Lee SD, Deng JF, et al. Measuring lidocaine metabolite-monoethylglycinexylidide as a quantitative index of hepatic function in adults with chronic hepatitis and cirrhosis. J Hepatol. 1993;19:140–47. doi: 10.1016/s0168-8278(05)80187-4. [DOI] [PubMed] [Google Scholar]
- 18.Reves JG, Fragen RJ, Vinik HR, et al. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310–24. [PubMed] [Google Scholar]
- 19.Carrillo JA, Ramos SI, Agundez JAG, et al. Analysis of midazolam and metabolites in plasma by high-performance liquid chromatography: probe of CYP3A. Ther Drug Monit. 1998;20:319–24. doi: 10.1097/00007691-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Williams RL, Mamelok RD. Hepatic disease and drug pharmacokinetics. Clin Pharmacokinet. 1980;5:528–47. doi: 10.2165/00003088-198005060-00002. [DOI] [PubMed] [Google Scholar]
- 21.Westphal JF, Brogard JM. Drug administration in chronic liver disease. Drug Saf. 1997;17:47–73. doi: 10.2165/00002018-199717010-00004. [DOI] [PubMed] [Google Scholar]
- 22.Verbeeck RK, Horsmans Y. Effect of hepatic insufficiency on pharmacokinetics and drug dosing. Pharm World Sci. 1998;20:183–92. doi: 10.1023/a:1008656930082. [DOI] [PubMed] [Google Scholar]
- 23.Delcò F, Tchambaz L, Schlienger R, et al. Dose adjustment in patients with liver disease. Drug Saf. 2005;28:529–45. doi: 10.2165/00002018-200528060-00005. [DOI] [PubMed] [Google Scholar]
- 24.Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147–61. doi: 10.1007/s00228-008-0553-z. [DOI] [PubMed] [Google Scholar]
- 25.Periáñez-Párraga L, Martínez-López I, Ventayol-Bosch P, et al. Drug dosage recommendations in patients with chronic liver disease. Rev Esp Enferm Dig. 2012;104:165–84. doi: 10.4321/s1130-01082012000400002. [DOI] [PubMed] [Google Scholar]
- 26.Ali S, Fonseca V. Saxagliptin overview: special focus on safety and adverse effects. Expert Opin Drug Saf. 2013;12:103–9. doi: 10.1517/14740338.2013.741584. [DOI] [PubMed] [Google Scholar]
- 27.George J, Murray M, Byth K, et al. Differential alterations of cytochrome P450 proteins in livers from patients with severe chronic liver disease. Hepatology. 1995;21:120–28. [PubMed] [Google Scholar]
- 28.Gilmore WJ, Kirby GM. Endoplasmic reticulum stress due to altered cellular redox status positively regulates murine hepatic CYP2A5 expression. J Pharmacol Exp Ther. 2004;308:600–8. doi: 10.1124/jpet.103.060111. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, Zhang XH, Cederbaum AI. Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway. Toxicol Sci. 2012;128:427–38. doi: 10.1093/toxsci/kfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu B, Ou-Yang DS, Cheng ZN, et al. Single plasma sampling to predict oral clearance of the CYP3A probe midzolam. Acta Phamacol Sin. 2001;22:634–38. [PubMed] [Google Scholar]
- 31.Lin YS, Lockwood GF, Graham MA, et al. In-vivo phenotyping for CYP3A by a single-point determination of midazolam plasma concentration. Pharmacogenetics. 2001;11:781–91. doi: 10.1097/00008571-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Chaobal HN, Kharasch ED. Single-point sampling for assessment of constitutive, induced, and inhibited cytochrome P450 3A activity with alfentanil or midazolam. Clin Pharmacol Ther. 2005;78:529–39. doi: 10.1016/j.clpt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Krupka E, Venisse N, Lafay C, et al. Probe of CYP3A by a single-point blood measurement after oral administration of midazolam in healthy elderly volunteers. Eur J Clin Pharmacol. 2006;62:635–39. doi: 10.1007/s00228-006-0159-2. [DOI] [PubMed] [Google Scholar]
- 34.Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: I. In vitro-in vivo correlations in liver transplant patients. J Pharmacol Exp Ther. 1994;271:549–56. [PubMed] [Google Scholar]
- 35.Andersen V, Pedersen N, Larsen NE, et al. Intestinal first pass metabolism of midazolam in liver cirrhosis – effect of grapefruit juice. Br J Clin Pharmacol. 2002;54:120–24. doi: 10.1046/j.1365-2125.2002.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConn DJ, II, Lin YS, Mathisen TL, et al. Reduced duodenal cytochrome P450 3A protein expression and catalytic activity in patients with cirrhosis. Clin Pharmacol Ther. 2009;85:387–93. doi: 10.1038/clpt.2008.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooker AC, Ten Tije AJ, Carducci MA, et al. Population pharmacokinetic model for docetaxel in patients with varying degrees of liver function: incorporating cytochrome P4503A activity measurements. Clin Pharmacol Ther. 2008;84:111–18. doi: 10.1038/sj.clpt.6100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YH, He R, Ruan JG. Effect of hepatic function on the EC50 of midazolam and the BIS50 at the time of loss of consciousness. J Zhejiang Univ Sci B. 2014;15:743–49. doi: 10.1631/jzus.B1300242. [DOI] [PMC free article] [PubMed] [Google Scholar]