Abstract
Background
The aim of this study was to investigate the relationship between plasma fatty acid binding protein 4 (FABP4), phosphatase and tensin homolog (PTEN), and insulin resistance in patients with gestational diabetes mellitus (GDM).
Material/Methods
Plasma FABP4 and PTEN were determined by ELISA in GDM patients (GDM group, n=30) and in euglycemic pregnant women (control group, n=30). The clinical features, body mass index (BMI), homeostasis model assessment of insulin resistance (HOMA-IR), and lipid profiles were compared between the 2 groups. The influence of risk factors on insulin resistance, including BMI, lipid profiles, FABP4, and PTEN, were further investigated by multiple-factor stepwise regression analysis.
Results
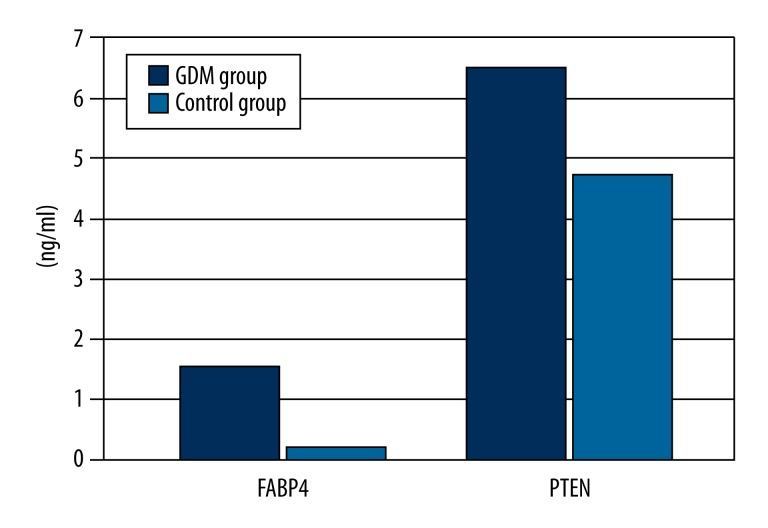
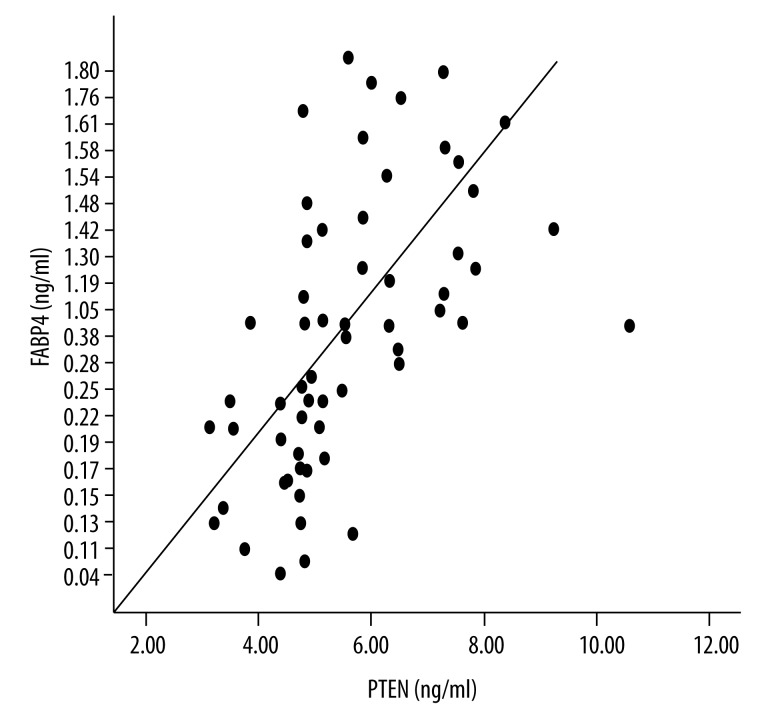
Higher levels of BMI, ΔBMI, triglyceride (TG), fasting plasma glucose (FPG), 2-hour plasma glucose (2hPG), fasting insulin, HOMA-IR, FABP4, PTEN, and lower level of high-density lipoprotein cholesterol (HDL-C) were found in the GDM patients than in the controls (all P<0.005). The plasma FABP4 was 1.47±0.25 vs. 0.20±0.07 ng/ml in the GDM and control group, respectively (P<0.0001). Plasma PTEN was 6.46±1.57 vs. 4.72±0.82 ng/ml in the GDM and control group, respectively (P<0.0001). There was a positive relation between plasma FABP4 and PTEN when all blood samples, including GDM and control groups, were analyzed (P<0.05). The multiple-factor regression analysis revealed that plasma FABP4, TG, and PTEN were independent risk factors for increased insulin resistance.
Conclusions
GDM patients have more severe insulin resistance compared to euglycemic pregnant women. Higher levels of plasma FABP4 and PTEN are associated with increased insulin resistance and may participate in the pathogenesis of insulin resistance during gestation.
MeSH Keywords: Diabetes, Gestational; Fatty Acid-Binding Proteins; Insulin Resistance; PTEN Phosphohydrolase
Background
Gestational diabetes mellitus (GDM) may give rise to higher risks of multiple complications for mother and fetus, including miscarriage, preterm delivery, pregnancy hypertension, embryonic abnormalities, macrosomia, and even fetal death [1,2]. Prevalence of GDM in China is increasing, from 3.7% in 1995 [3] to 5.1% in 2008 [4].
Increased insulin resistance is one of the main mechanisms for the development of GDM. Previous studies indicate that there are 2 types of insulin resistance in GDM. One is called as “physiological insulin resistance”, which is mediated by pregnancy-related hormones and will aggravate during the third trimester. The other is called “chronic insulin resistance”, which already existed before pregnancy [5]. Insulin sensitivity in pregnant women will gradually decrease as the gestational weeks increase. Inadequate compensatory secretion of insulin can result in gestational diabetes mellitus. Hyperglycemia will reverse to normal after delivery for most GDM patients. However, in some patients it may extend to postpartum. Near half of GDM patients will develop to type 2 diabetes and obesity in the future [6]. Furthermore, the risk of type 2 diabetes in their offspring is also increased [7–9].
Adiposity fatty acid-binding protein 4 (FABP4) is a member of the lipid-binding protein super-family. As an important intracellular fatty acid carrier protein, it is widely involved in fatty acid uptake, transport, and metabolism. The roles of FABP4 in the development of metabolic syndrome, type 2 diabetes mellitus, cardiovascular disease [10,11], preeclampsia and GDM [12] are of great concern.
Phosphatase and tensin homolog (PTEN) was first discovered as a tumor suppressor gene. Deletion or inactivation of this gene can lead to a variety of cancers. Recent findings indicate that it plays a role in the regulation of the insulin signal transduction pathway. The higher expression of this gene may contribute to more severe insulin resistance. A complex of FABP4 and PTEN was recently detected and is presumed to play a role in the pathogenesis of insulin resistance [13].
Therefore, we hypothesized that increased levels of plasma FABP4 and PTEN are associated with GDM. The relationship between FABP4, PTEN, HOMA-IR, and other biochemical parameters in GDM were further investigated.
Material and Methods
Participants
A total of 30 pregnant women with GDM (GDM group) and 30 euglycemic pregnant women (control group) were recruited. The mean age of the GDM patients and the control group was 31.83±3.91 and 26.53±1.91 years (P>0.05). Patients with renal diseases, preeclampsia, or systemic inflammatory diseases were excluded. The study protocol was approved by Inner Mongolia Medical University ethics committee. The written informed consents were obtained from all participants.
Definition of our research
GDM was diagnosed if 1 or more plasma glucose levels were elevated during an oral glucose tolerance test with 75 g glucose, according to the criteria of Standards of Medical Care in Diabetes 2011 [14]. The following plasma glucose threshold was used: fasting blood glucose ≥5.1 mmol/l; 1-h blood glucose ≥10.0 mmol/l; 2-h blood glucose ≥8.5 mmol/l.
BMI was calculated as weight divided by squared height before pregnancy. ΔBMI is the net increment of BMI during pregnancy. HOMA-IR was calculated as previously described: HOMA-IR=(FPG × FINS)/22.5 (FINS, fasting insulin) [7].
Biochemistry and hormonal assays
Maternal blood samples were collected at 24–28 weeks of gestation. Blood samples were taken after an overnight fast. At the time of blood sampling, none of the women were in labor. Serum insulin was determined by 2-site chemiluminescent enzyme immunometric assay in an Immulite automated analyzer (DPC, Siemens, Marburg, Germany). FABP4 and PTEN were measured by ELISA according to the manufacturers’ instructions (R&D Systems China, Shanghai). TG, total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), FPG, and 2-hPG were measured by standard laboratory methods in a certificated laboratory.
Statistical analysis
SPSS software version 19.0 was used for statistical analysis. The differences between the GDM and control groups were assessed by t test. Correlations were conducted by using the Pearson correlation analysis method. To evaluate the effects of covariates on insulin resistance, multivariate linear regression analyses were undertaken. Before performing multivariate analyses, non-normally distributed parameters were logarithmically transformed. A P-value <0.05 was considered as statistically significant.
Results
The GDM group had higher levels of BMI, ΔBMI, TG, FPG, 2-hPG, FINS, HOMA-IR, FABP4, and PTEN than the control group (Table 1). Serum HDL-C was lower in the GDM group compared to controls. No significant differences in TC and LDL-C were found between the 2 groups.
Table 1.
Baseline characteristics of the study population.
| GDM N=30 |
Controls N=30 |
t | P | |
|---|---|---|---|---|
| Age | 31.83±3.91 | 26.53±1.91 | 6.68 | >0.05 |
| Body Mass Index (Kg/m2) | 21.80±1.02 | 19.18±0.68 | 11.663 | <0.0001* |
| ΔBMI (Kg/m2) | 7.38±1.14 | 6.19±0.91 | 4.4439 | <0.0001* |
| FPG (mmol/L) | 5.53±0.64 | 4.18±0.45 | 9.49 | <0.0001* |
| 2hPG (mmol/L) | 10.81±2.30 | 9.16±2.20 | 2.844 | 0.006* |
| Fating insulin (mU/L) | 11.19±1.84 | 7.88±0.91 | 8.859 | <0.0001* |
| Triglycerides (mmol/L) | 2.80±0.85 | 2.23±0.55 | 3.068 | 0.003* |
| Total cholesterol (mmol/L) | 5.44±0.84 | 5.65±0.63 | −1.071 | >0.05 |
| LDL-C (mmol/L) | 2.88±0.68 | 2.54±0.73 | 1.901 | >0.05 |
| HDL-C (mmol/L) | 1.46±0.41 | 1.81±0.42 | −3.287 | 0.002* |
| HOMA-IR | 2.74±0.51 | 1.47±0.25 | 12.388 | <0.0001* |
| FABP4 (ng/ml) | 1.47±0.25 | 0.20±0.07 | 27.094 | <0.0001* |
| PTEN (ng/ml) | 6.46±1.57 | 4.72±0.82 | 5.685 | <0.0001* |
Indicates that the difference between two groups is of significance.
GDM – gestational diabetes mellitus; ΔBMI – BMI before delivery abstracts Pre-pregnancy BMI; FABP4 – adipocyte fatty acid binding proteins; PTEN – phosphatase and tensin homolog deleted on chromosome ten; HOMA-IR – homeostasis model assessment- insulin resistance; FPG – fasting plasma glucose; 2hPG – 2 hour plasma glucose.
The plasma FABP4 in the GDM and control group was 1.47±0.25 vs. 0.20±0.07 ng/ml (P<0.0001), respectively; the PTEN in the GDM and control group was 6.46±1.57 vs. 4.72±0.82 ng/ml, P<0.0001, respectively (Figure 1). When all samples (including the GDM and control groups) were statistically analyzed, there was a positive relationship between PTEN and FABP4 (r=0.64, P<0.0001) (Figure 2). In the GDM group, there was positive relation between plasma FABP4 and HOMA-IR (r=0.566, P=0.001). A similar relation were also found between PTEN and HOMA-IR (r=0.542, P=0.002) in the GDM group.
Figure 1.
Plasma levels of FABP4 and PTEN in the GDM and control groups. FABP4: Plasma fatty acid-binding protein-4; PTEN: Phosphatase and tensin homolog.
Figure 2.
Positive relationship between plasma FABP4 and PTEN. FABP4: Plasma fatty acid-binding protein-4; PTEN: Phosphatase and tensin homolog.
In the GDM group, the influence of parameters such as BMI, ΔBMI, TG, TC, LDL-C, HDL-C, 2-hPG, and FINS on HOMA-IR was further investigated by multiple linear regression analysis, which showed that FABP4 and TG are 2 significant independent factors influencing HOMA-IR, while the effect of PTEN on HOMA-IR was nearly significance (P=0.053, Table 2). The multiple linear regression equation was Y=0.484+0.807x1+0.224x2+0.07x3 (x1: FABP4; x2: TG; x3: PTEN; Y: HOMA-IR), R=0.931, R2=0.867, F=121.882 (P<0.001).
Table 2.
The influence of factors on HOMA-IR by multiple regression analysis.
| Model | Unstandardized Coefficients | Standardized coefficients | t | Sig. | |
|---|---|---|---|---|---|
| B | Std. eroor | Beta | |||
| (Constant) | 0.484 | 0.163 | 2.971 | 0.004 | |
| FABP4 | 0.807 | 0.072 | 0.712 | 11.200 | 0.000 |
| TG | 0.224 | 0.057 | 0.227 | 3.888 | 0.000 |
| PTEN | 0.070 | 0.035 | 0.138 | 1.976 | 0.053 |
| BMI | 0.119 | 1.397 | 0.168 | ||
| ΔBMI | −0.080 | −1.444 | 0.154 | ||
| 2hPG | −0.045 | −0.888 | 0.378 | ||
| TC | −0.014 | −0.271 | 0.787 | ||
| LDL | 0.009 | 0.180 | 0.858 | ||
| HDL-C | 0.029 | 0.531 | 0.598 | ||
Dependent Variable: HOMA-IR.
Discussion
We found that the GDM group had a higher HOMA-IR than the control group, which is consistent with previous studies [15–17]. Several case-control studies found that increased insulin resistance is associated with abnormalities in body weight, blood glucose, lipids, and lipoprotein. For example, higher pre-pregnant BMI is coupled with increased risk of GDM [18]. Another study showed that the FPG, 2-hPG, FINS, 2-hFINS, TG, LDL-C, and HOMA-IR in GDM patients were significantly higher than that of euglycemic pregnant women and a positive relation was found between pregnant age, pre-pregnant BMI, and HOMA-IR [19].
Our study also found that the GDM patients had a higher concentration of FABP4 compared to the controls. Furthermore, the multiple regression analysis revealed that plasma FABP4 is an independent risk factor for insulin resistance. In a study by Ortega et al., the GDM group (n=98) had a higher FABP4 level than the control group (n=86), 19.9±1.0 vs. 17.7±0.8 ng/mL, P=0.0493 [20]. These results consistently indicate that a higher level of FABP4 is associated with increased insulin resistance and may play a significant role in the pathogenesis of GDM [20–22].
As an important intracellular fatty acids carrier protein, FABP4, is released from adipocytes and plays an important biologic role in fatty acid uptake, transport, and metabolism [23]. FABP4 may influence insulin sensitivity and energy metabolism by regulation of fatty acid metabolism. Increased FABP4 can promote the accumulation of short-chain fatty acids in cells and decrease PI3K-AKT protein activity, thereby inhibiting glucose oxidation and glycolysis and significantly reducing glucose uptake and utilization in muscle and liver [24–27]. Therefore, the pathway from glucose to triglyceride is disturbed and the increased insulin resistance may lead to GDM [24–27]. However, why adipocytes in GDM patients produce higher levels of FABP4 is still unknown.
The relation between GDM and the plasma levels of PTEN has not been investigated. Our study found for the first time that GDM patients had a significantly higher level of PTEN than normal controls and that this is positively associated with HOMA-IR, indicating that the increased PTEN levels may also contribute to the pathogenesis of increased insulin resistance.
PTEN, as a tumor-suppressive protein, has been widely investigated in recent years, but its role in insulin sensitivity and glucose metabolism is largely unknown. Increased expression of PTEN may result in more severe insulin resistance by blocking the intracellular insulin-signaling pathway [22], catalyzing phosphatidylinositol-3,4,5-triphosphate (PIP3) degradation [28], and inhibiting the transportation of glucose into the cells. In PTEN knockout experimental animals, insulin sensitivity is increased in liver, muscle, and adipose tissue [29–32]. Cowden disease, caused by a PTEN gene inactive mutation, also shows improved insulin sensitivity [33]. All these findings strongly suggest that the lost function of PTEN can increase insulin sensitivity.
Our study showed that there is a positive correlation between plasma FABP4 and PTEN, indicating that the interaction of these two factors may cause more severe insulin resistance. A recent study revealed that a complex of FABP4 and PTEN was found in differentiated adipocytes, showing that the complex may enhance the potential activity of PTEN and result in more severe insulin resistance [13], but the underlying mechanisms need further investigation.
Our study had some limitations. As a cross-sectional study, we could not make a cause-and-effect conclusion between higher levels of FABP4, PTEN, and increased insulin resistance. Furthermore, we did not know whether the higher levels of FABP4 and PTEN already existed before the pregnancy or whether they extend after delivery. Prospective longitudinal studies are required to answer these questions.
Conclusions
GDM patients had more severe insulin resistance than the control group, partially contributed to by elevated plasma FABP4 and PTEN. The net increment of body weight during pregnancy and dyslipidemia were independent factors for insulin resistance.
Footnotes
Source of support: This work was supported by the Applied Technology Research and Development Funding Program of Science and Technology Plan, Inner Mongolia Autonomous Region, China, 2014
References
- 1.Dudzik D, Zorawski M, Skotnicki M, et al. Metabolic fingerprint of Gestational Diabetes Mellitus. J Proteomics. 2014;103:57–71. doi: 10.1016/j.jprot.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Li HP, Chen X, Li MQ. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. 2013;6(4):650–59. [PMC free article] [PubMed] [Google Scholar]
- 3.Shi CY, Yang HX, Dong Y, et al. [Study of 8665 cases of the 50 goral glucose challenge test to screen the gestational diabetes mellitus]. Zhonghua Fu Chan Ke Za Zhi. 2003;38(03):136–39. [in Chinese] [PubMed] [Google Scholar]
- 4.Yang H, Wei Y, Gao X, et al. Risk factors for gestational diabetes mellitus in Chinese women: a prospective study of 16,286 pregnant women in China. Diabet Med. 2009;26(11):1099–104. doi: 10.1111/j.1464-5491.2009.02845.x. [DOI] [PubMed] [Google Scholar]
- 5.Winn VD, Haimov-Kochman R, Paquet AC, et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148(3):1059–79. doi: 10.1210/en.2006-0683. [DOI] [PubMed] [Google Scholar]
- 6.Yun S, Kabeer NH, Zhu BP, Brownson RC. Modifiable risk factors for developing diabetes among women with previous gestational diabetes. Prev Chronic Dis. 2007;4(1):A07. [PMC free article] [PubMed] [Google Scholar]
- 7.Boerschmann H, Pflüger M, Henneberger L, et al. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care. 2010;33(8):1845–49. doi: 10.2337/dc10-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isganaitis E, Woo M, Ma H, et al. Developmental programming by maternal insulin resistance: hyperinsulinemia, glucose intolerance, and dysregulated lipid metabolism in male offspring of insulin-resistant mice. Diabetes. 2014;63(2):688–700. doi: 10.2337/db13-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega-Gonzalez C, Ballesteros A, Casanueva E, et al. Searching for alternative methods of diagnosing gestational diabetes mellitus in a Mexican urban population. Med Sci Monit. 2008;14(12):CR598–603. [PubMed] [Google Scholar]
- 10.Cabré A, Lázaro I, Girona J, et al. Plasma fatty acid binding protein 4 is associated with atherogenic dyslipidemia in diabetes. J Lipid Res. 2008;49(8):1746–51. doi: 10.1194/jlr.M800102-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Rhee EJ, Lee WY, Park CY, et al. The association of serum adipocyte fatty acid-binding protein with coronary artery disease in Korean adults. Eur J Endocrinol. 2009;160(2):165–72. doi: 10.1530/EJE-08-0665. [DOI] [PubMed] [Google Scholar]
- 12.Wang XJ, Yan JY. Relationship between adipocyte fatty acid-binding protein and pregnancy-associated diseases. J Int Obstet Gynecol. 2010;37(03):168–70. [Google Scholar]
- 13.Gorbenko O, Panayotou G, Zhyvoloup A, et al. Identification of novel PTEN-binding partners: PTEN interaction with fatty acid binding protein FABP4. Mol Cell Biochem. 2010;337(1–2):299–305. doi: 10.1007/s11010-009-0312-1. [DOI] [PubMed] [Google Scholar]
- 14.Executive summary: Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(Suppl 1):S4–10. doi: 10.2337/dc13-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colomiere M, Permezel M, Lappas M. Diabetes and obesity during pregnancy alter insulin signalling and glucose transporter expression in maternal skeletal muscle and subcutaneous adipose tissue. J Mol Endocrinol. 2010;44(4):213–23. doi: 10.1677/JME-09-0091. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Zhang S, Liu H, et al. Different associations of diabetes with beta-cell dysfunction and insulin resistance among obese and non-obese Chinese women with prior gestational diabetes. Diabetes Care. 2014;37(9):2533–39. doi: 10.2337/dc14-0573. [DOI] [PubMed] [Google Scholar]
- 17.Niu JM, et al. The study on the relationship between the serum leptin and insulin resistance and pancreatic β-cell function in the pregnancies with different glucose tolerance. Journal of practical medicine China. 2004;20(09):1012–14. [Google Scholar]
- 18.Berkowitz GS, Lapinski RH, Wein R, Lee D. Race/ethnicity and other risk factors for gestational diabetes. Am J Epidemiol. 1992;135(9):965–73. doi: 10.1093/oxfordjournals.aje.a116408. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Relationship between insulin resistance and metabolic disorder in patients with gestational diabetes mellitus. Medical Journal of National Defending Forces in southern China. 2009;23(03):40–41. [Google Scholar]
- 20.Ortega-Senovilla H, Schaefer-Graf U, Meitzner K, et al. Gestational diabetes mellitus causes changes in the concentrations of adipocyte fatty acid-binding protein and other adipocytokines in cord blood. Diabetes Care. 2011;34(9):2061–66. doi: 10.2337/dc11-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kralisch S, Stepan H, Kratzsch J, et al. Serum levels of adipocyte fatty acid binding protein are increased in gestational diabetes mellitus. Eur J Endocrinol. 2009;160(1):33–38. doi: 10.1530/EJE-08-0540. [DOI] [PubMed] [Google Scholar]
- 22.Tang X, Powelka AM, Soriano NA, et al. PTEN, but not SHIP2, suppresses insulin signaling through the phosphatidylinositol 3-kinase/Akt pathway in 3T3-L1 adipocytes. J Biol Chem. 2005;280(23):22523–29. doi: 10.1074/jbc.M501949200. [DOI] [PubMed] [Google Scholar]
- 23.Fasshauer M, Seeger J, Waldeyer T, et al. Serum levels of the adipokine adipocyte fatty acid-binding protein are increased in preeclampsia. Am J Hypertens. 2008;21(5):582–86. doi: 10.1038/ajh.2008.23. [DOI] [PubMed] [Google Scholar]
- 24.Tso AW, Xu A, Sham PC, et al. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30(10):2667–72. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- 25.Boord JB, Maeda K, Makowski L, et al. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2002;22(10):1686–91. doi: 10.1161/01.atv.0000033090.81345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baar RA, Dingfelder CS, Smith LA, et al. Investigation of in vivo fatty acid metabolism in AFABP/aP2(−/−) mice. Am J Physiol Endocrinol Metab. 2005;288(1):E187–93. doi: 10.1152/ajpendo.00256.2004. [DOI] [PubMed] [Google Scholar]
- 27.Uysal KT, Scheja L, Wiesbrock SM, et al. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000;141(9):3388–96. doi: 10.1210/endo.141.9.7637. [DOI] [PubMed] [Google Scholar]
- 28.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70(6):247–79. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 29.Kurlawalla-Martinez C, Stiles B, Wang Y, et al. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol. 2005;25(6):2498–510. doi: 10.1128/MCB.25.6.2498-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wijesekara N, Konrad D, Eweida M, et al. Muscle-specific pten deletion protects against insulin resistance and diabetes. Mol Cell Biol. 2005;25(3):1135–45. doi: 10.1128/MCB.25.3.1135-1145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stiles B, Wang Y, Stahl A, et al. Liver-specific deletion of negative regulator pten results in fatty liver and insulin hypersensitivity [corrected] Proc Natl Acad Sci USA. 2004;101(7):2082–87. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komazawa N, Matsuda M, Kondoh G, et al. Enhanced insulin sensitivity, energy expenditure and thermogenesis in adipose-specific pten suppression in mice. Nat Med. 2004;10(11):1208–15. doi: 10.1038/nm1117. [DOI] [PubMed] [Google Scholar]
- 33.Iida S, Ono A, Sayama K, et al. Accelerated decline of blood glucose after intravenous glucose injection in a patient with Cowden disease having a heterozygous germline mutation of the PTEN/MMAC1 gene. Anticancer Res. 2000;20(3B):1901–4. [PubMed] [Google Scholar]