Abstract
Purpose.
To investigate preoperative and intraoperative factors associated with persistent subfoveal fluid in surgically closed macular holes (MHs).
Methods.
This was a prospective consecutive case series of eyes undergoing surgical repair for full-thickness MH in the PIONEER study, a prospective intraoperative optical coherence tomography (OCT) multisurgeon single-center study. Thirty-seven eyes (36 patients) with surgically closed MH were studied. Quantitative OCT analysis was performed including intraoperative MH area, volume, ellipsoid zone to retinal pigment epithelium (EZ-RPE) height, extent of subretinal hyporeflectivity (SRHR), and the amount of postoperative subfoveal fluid.
Results.
Persistent subfoveal fluid was identified in 58% of eyes at 2 weeks following surgery. The mean time to two-line improvement in visual acuity was greater in eyes with persistent subfoveal fluid (P = 0.03). Final visual acuity did not correlate with the initial presence of fluid. Two intraoperative factors following internal limiting membrane (ILM) peeling were associated with the formation of persistent subfoveal fluid: EZ-RPE height and SRHR width (P < 0.01). These were both negatively correlated with amount of postoperative subfoveal fluid (P = 0.028 and 0.04, respectively).
Conclusions.
Persistent subfoveal fluid following MH surgery is a common finding that appears to delay visual recovery but not effect final visual outcome. The incidence of persistent subfoveal fluid appears to be related to intraoperative alterations after ILM peeling in the outer retinal architecture (e.g., increased EZ-RPE height and SRHR width). This finding suggests a novel mechanism for facilitating MH closure through ILM peeling (e.g., altering photoreceptor/RPE adherence and increasing retinal mobility that allows for complete hole closure).
Keywords: macular hole, subfoveal fluid, iOCT, intraoperative OCT, surgery
Persistent subfoveal fluid and incomplete outer retinal closure are associated with outer retinal alterations that occur during ILM peeling identified with intraoperative OCT, suggesting a novel mechanism that ILM peeling may promote closure (e.g., increased outer retinal mobility).
Introduction
Surgical vitrectomy has been the mainstay treatment for macular holes (MHs) since the first description of the technique by Kelly and Wendel in 1991.1 Since then, surgical techniques, such as internal limiting membrane (ILM) peeling, have increased MH closure rates to more than 90%.2,3 Optical coherence tomography (OCT) has allowed us to identify detailed anatomical configurations in the retina before, during, and after MH surgery to delineate possible factors in achieving successful hole closure and optimizing visual acuity recovery. Previous studies have described the presence of persistent outer retinal defects after successful MH closure.4 Numerous terms have been used to describe this entity, including outer foveal defects, subfoveal hyporeflectance, outer foveal hyporeflective defects, and subfoveal fluid.5–9 An additional report described these terms as slightly distinct entities, with outer foveal defects to mean a small, hyporeflective disruption in the normally hyperreflective ellipsoid zone (EZ) and a persistent foveal detachment to represent elevated foveal photoreceptor segments off the retinal pigment epithelium (RPE) but with no change in the normal foveal inner contour.10 For the purpose of this article, the term “persistent subfoveal fluid” is used to describe any area of hyporeflective area in the subfoveal space that persists after the surgical closure of MHs (Fig. 1).
Figure 1.
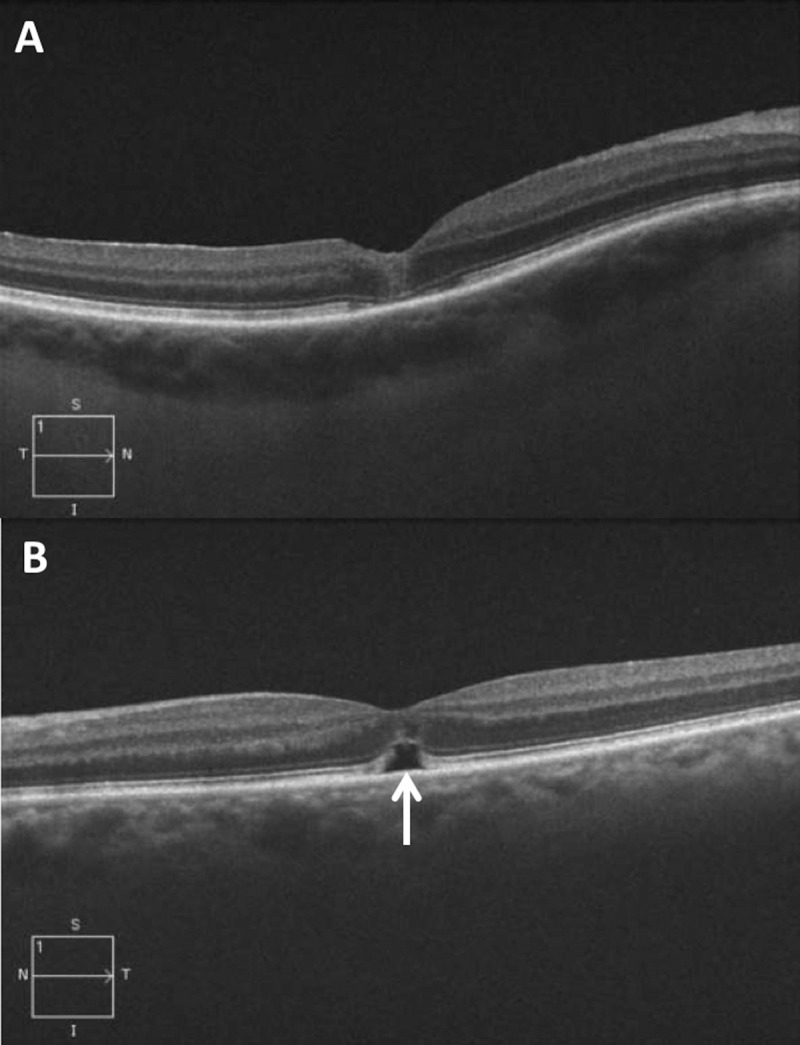
Optical coherence tomography B-scans 2 weeks after successful MH repair. (A) Case example without subfoveal fluid after surgery. (B) Case example with persistent subfoveal fluid (white arrow).
Persistent subfoveal fluid has been postulated to be involved in MH closure and possibly a result in the process of achieving normal foveal configuration. Takahashi and Kishi11 described a bridge formation of inner retina tissue over the RPE that mimicked a foveal retinal detachment as a pattern of MH closure. Christensen et al.9 note that most MHs begin closing with contraction of the inner retina that forms a roof over the subfoveal fluid, most likely due to the larger basal diameter of the holes. The subfoveal fluid eventually is resorbed and a more normal foveal contour is restored.9 Persistent subfoveal fluid appears to have a significant effect on rate of visual acuity recovery but not on final visual acuity.9 It is still unclear what factors are associated with the development of persistent subfoveal fluid.
Intraoperative OCT (iOCT) has allowed us to study the anatomical changes that occur during surgery.6,12–14 Preliminary studies have identified immediate changes in MH architecture after membrane peeling.6,15 Subretinal hyporeflectance between the EZ and RPE has been observed using iOCT at various surgical time points.6 It has been shown that the width of subretinal hyporeflectance measured intraoperatively after ILM peeling is correlated with postoperative visual acuity.6 Additionally, novel algorithms have been described that allow for volumetric segmentation of MH geometry providing unique insights into intraoperative dynamics following surgical manipulation.6,16 The multisurgeon, single-site Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) study is investigating the role of iOCT in ophthalmic surgery.17
In this report, we provide an analysis of factors associated with persistent subfoveal fluid development and the postoperative subfoveal fluid dynamics following successful MH closure in the PIONEER study, examining both preoperative and intraoperative factors.
Methods
The PIONEER study is a single-site, multisurgeon, prospective, institutional review board (IRB)-approved study examining the feasibility, safety, and utility of iOCT in the surgical management of ophthalmic disease.17 The study was approved by the Cleveland Clinic IRB and adhered to all the tenets of the Declaration of Helsinki. All patients gave written informed consent after an explanation of the nature and possible consequences of the study before enrolling in it. For this report, the primary inclusion criterion was enrollment in PIONEER for MH repair with successful MH closure. Exclusion criteria included traumatic MH, insufficient OCT data, iOCT data of insufficient quality of quantitative analysis, concurrent macular disease (e.g., diabetic macular edema, neovascular AMD), high myopia, lack of ILM peeling, and previous vitrectomy. Demographic and clinical data, including preoperative MH classification (e.g., size), axial length, and Snellen visual acuity, were collected from the medical record.
Surgical Procedure
Macular hole repair was performed by one of the four surgeons (JPE, PKK, RPS, SKS) using standard three-port small-gauge pars plana vitrectomy. In phakic eyes, combined vitrectomy with phacoemulsification was performed at the discretion of the operating surgeon. Dilute indocyanine green was used to stain the ILM. The ILM was then was peeled in a circumferential pattern from the edge of the MH, either by immediately engaging with vitreoretinal forceps or initiating with a diamond-dusted membrane scraper followed by peeling with vitreoretinal forceps. After air–fluid exchange, perfluoropropane (C3F8, n = 1) or sulfur hexafluoride (SF6, n = 36) was used as tamponade.
Intraoperative OCT Protocol
Intraoperative OCT was performed using the Bioptigen SDOIS system (Bioptigen, Inc., Research Triangle Park, NC, USA), which was attached to the surgical microscope with a custom microscope-mounting system to allow for rapid and reproducible scanning, as previously described.6,17 Imaging was analyzed at two surgical time points: immediately before initiation of pars plana vitrectomy (preincision scan) and after ILM peeling (postpeel scan). A standardized imaging protocol was used, which included a 10 × 10-mm volumetric cube with 100 B-scans per volume at both 0 and 90 degrees, a 5 × 10-mm volumetric cube with 7 B-scans per frame for averaging and a total of 175 B-scans per volume at both 0 and 90 degrees.
Quantitative assessment was performed by two independent readers using a proprietary OCT review software package that allowed for linear, area, and volumetric measurements of the areas of interest. Several key features were measured at each time point, including MH width, area, and volume, as previously described.6,16 Additionally, outer retinal alterations also were analyzed, including the extent of subretinal hyporeflectivity (SRHR) expansion and the EZ-RPE height, as previously described (Fig. 2).6
Figure 2.
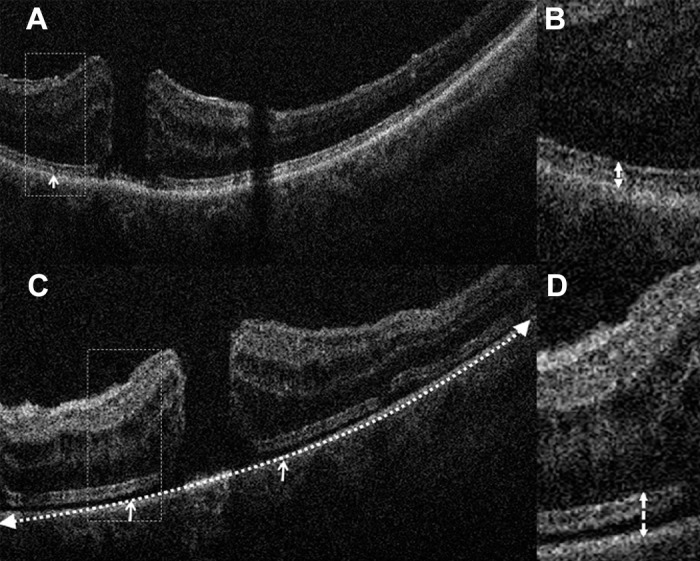
Intraoperative outer retinal dynamics after ILM peeling. (A) Preincision iOCT B-scan revealing baseline EZ-RPE height (white arrows). (B) Inset (dotted rectangle) reveals high magnification of outer retina and EZ-RPE relationship (dotted arrow). (C) Post-ILM peel iOCT scan revealing expansion of the EZ-RPE height (solid arrows) and SRHR width extension (dotted arrow). (D) Inset (dotted rectangle) reveals high magnification of outer retina with increased EZ-RPE height (dotted arrow).
Clinical OCT Imaging
All patients were scanned with a Cirrus HD-OCT (Model 5000; Carl Zeiss Meditec, Dublin, CA, USA) preoperatively, at 2 weeks (if the gas bubble was still present in front of the fovea, the OCT was obtained across the bubble [n = 1]), 1 month, and 3 months postoperatively. Scans from all postoperative time points were analyzed for presence of subfoveal fluid. The DICOM file was exported from Cirrus and analyzed using the same proprietary OCT analysis software. Preoperative OCT scans were analyzed for MH width, area, and volume. Postoperatively, the area and volume of subfoveal fluid was calculated. Visual acuity was measured at all postoperative time points until final follow-up visit. Significant visual improvement was defined by a two-line increase in vision.
Statistical Analyses
Categorical variables were described as percentages, and continuous variables were described as mean ± SD. Best corrected Snellen visual acuity was converted to logMAR for statistical manipulation. Due to the smaller sample size, the nonparametric Mann-Whitney U test was used to compare subfoveal fluid area between postoperative time points, and between the visual acuity recovery period in patients with subfoveal fluid to the recovery period in patients without subfoveal fluid. Due to unequal variances between the groups, Welch's t-test was used to compare preincision EZ-RPE height and SRHR width to postpeel measures. Spearman's rank correlation coefficient test was used to calculate the correlation between EZ-RPE height and SRHR width to subfoveal fluid area.
Results
Clinical Demographics and Characteristics
Fifty-five eyes in the PIONEER study were identified that underwent successful MH repair. Thirteen eyes were excluded due to concurrent macular disease, one was excluded for a traumatic MH, one was excluded for lack of ILM peeling during surgery, and three were excluded for insufficient imaging data. Thus, 37 eyes from 36 patients were included in the analysis. The baseline characteristics are presented in Table 1.
Table 1.
Baseline Clinical Characteristics
| No. of eyes (patients) | 37 (36) |
| Age, y, mean ± SD | 68.3 ± 7.5 |
| Sex, n (%) | |
| Men | 11 (31) |
| Women | 25 (69) |
| Eye, n (%) | |
| Right | 22 (59) |
| Left | 15 (41) |
| Axial length, mm, mean ± SD | 23.8 ± 1.1 |
| Preoperative mean BCVA (logMAR ± SD) | 20/162 (0.94 ± 0.5) |
| Symptom duration, mo, mean ± SD | 3.4 ± 2.9 |
| Preoperative Gass stage, n (%) | |
| Stage 2 | 8 (22) |
| Stage 3 | 26 (70) |
| Stage 4 | 3 (8) |
| Combined cataract surgery, n (%) | 29 (78) |
Persistent Subfoveal Fluid
The incidence of persistent subfoveal fluid at 2 weeks, 1 month, 3 months, and 6 months postoperatively are shown in Figure 3. Two weeks postoperatively, 58% of eyes (n = 26) had subfoveal fluid. The incidence decreased to 49% (n = 33), 39% (n = 26), and 37% (n = 19) at 1 month, 3 months, and 6 months, respectively. The area of subfoveal fluid also decreased in the postoperatively period (Fig. 3). The average area at 2 weeks, 1 month, 3 months, and 6 months was 9831 ± 12,745 μm2, 5582 ± 11,654 μm2, 2535 ± 7331 μm2, and 1823 ± 3897 μm2, respectively. The area of persistent subfoveal fluid at 3 months and 6 months was significantly less than the area at 2 weeks (P = 0.02 and 0.03, respectively).
Figure 3.
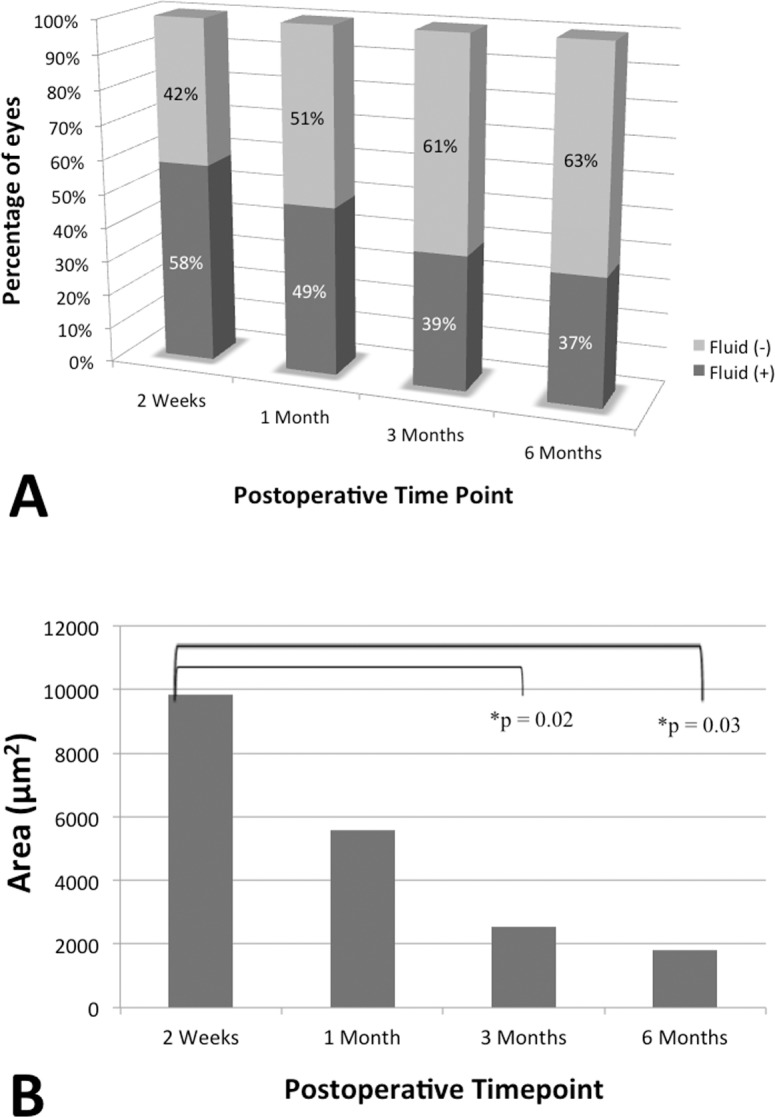
Persistent subfoveal fluid dynamics. (A) Prevalence of persistent subfoveal fluid postoperatively. Percentage of eyes with persistent subfoveal fluid (dark gray) and percentage of eyes without persistent subfoveal fluid (light gray) at 2 weeks, 1 month, 3 months, and 6 months postoperatively. (B) Area of persistent subfoveal fluid postoperatively.
Visual Acuity and Persistent Subfoveal Fluid
The mean preoperative vision was 20/162. Mean visual acuity was 20/67 at 1 month, 20/50 at 3 months, and 20/43 at 6 months. The visual acuity recovery period (i.e., time to gain two or more lines compared to preoperative vision) in patients with persistent subfoveal fluid was 4.3 ± 4.2 months, whereas the visual acuity recovery period patients without subfoveal fluid was 0.9 ± 0.7 months (P = 0.03). The absolute area of subfoveal fluid was not significantly correlated to best-corrected visual acuity (BCVA) at any time point. Final visual acuity was also not associated with the presence or absence of persistent subfoveal fluid.
Factors Associated With Persistent Subfoveal Fluid
Preoperative factors, including MH area, MH volume, symptom duration, axial length, and MH classification were not significantly correlated with the area of persistent subfoveal fluid postoperatively (Table 2). Quantitative OCT analysis of the EZ-RPE height and SRHR width revealed that there was a significant increase in these variables following ILM peeling (P < 0.01, Table 3). Both increased EZ-RPE height and increased SRHR were significantly correlated (correlation coefficients = −0.47, −0.37; P = 0.028 and 0.040, respectively) with persistent subfoveal fluid at 2 weeks with an inverse relationship (e.g., as EZ-RPE height increased or SRHR increased there was a corresponding decrease in subfoveal fluid). Intraoperative measurements of MH geometry (e.g., volume, area) before and after ILM peeling were not correlated with the area of subfoveal fluid (correlation coefficients = −0.29, −0.018; P = 0.10, 0.43, respectively; Table 2).
Table 2.
Correlation of Preoperative and Intraoperative Factors With Area of Persistent Subfoveal Fluid
|
Factor |
Correlation Coefficient |
P |
| Preoperative Factors | ||
| MH area | −0.31 | 0.12 |
| Symptom duration | 0.16 | 0.49 |
| Axial length | −0.056 | 0.60 |
| MH stage | 0.026 | 0.43 |
| Intraoperative Factors | ||
| MH volume before ILM peel | −0.29 | 0.10 |
| MH volume after ILM peel | −0.018 | 0.43 |
| Preincision EZ-RPE height | −0.16 | 0.42 |
| Preincision SRHR width | −0.28 | 0.079 |
| Post-ILM peel EZ-RPE height | −0.46 | 0.028* |
| Post-ILM peel SRHR width | −0.37 | 0.04* |
Significant P values (<0.05).
Table 3.
Intraoperative Quantitative Assessment of EZ-RPE Height and Subretinal Hyporeflectivity Width Before and After ILM Peeling
|
Preincision |
Post-ILM Peel |
|||
|
EZ-RPE Height |
SRHR Width |
EZ-RPE Height |
SRHR Width |
|
| Mean ± SD, μm | 41.6 ± 4.5 | 1.3 ± 0.4 | 49.7 ± 6.2* | 3.4 ± 1.1* |
P ≤ 0.01.
Intraoperative Architectural Dynamics and Visual Acuity Recovery
The correlation between change in visual acuity and intraoperative measurements (e.g., EZ-RPE height, SRHR before and after ILM peeling) were assessed and are summarized in Table 4. The pre-ILM peel EZ-RPE height and change in visual acuity were significantly correlated at 1, 3, and 6 months (P = 0.00098, 0.00069, and 0.0019, respectively). The length of SRHR before ILM peeling and vision change at 1 month was significantly correlated (P = 0.033), but not correlated at 3 months and 6 months (P = 0.091 and 0.079, respectively). The EZ-RPE height after ILM peeling was also significantly correlated to the vision change at 1 month and 3 months after surgery (P = 0.014 and 0.00059, respectively). The length of SRHR after ILM peeling was significantly correlated to vision change at 3 months (P = 0.0068, Table 4).
Table 4.
Correlation Intraoperative Outer Retinal Measurements With Postoperative Change in Visual Acuity From Baseline
|
1 Month |
3 Months |
6 Months |
||||
|
Correlation Coefficient |
P |
Correlation Coefficient |
P |
Correlation Coefficient |
P |
|
| Pre-ILM peel | ||||||
| EZ-RPE height | 0.42 | 0.00098* | 0.48 | 0.00069* | 0.62 | 0.0019* |
| SRHR | 0.35 | 0.033* | 0.31 | 0.091 | 0.38 | 0.079 |
| Post-ILM peel | ||||||
| EZ-RPE height | 0.44 | 0.014* | 0.65 | 0.00059* | 0.2 | 0.42 |
| SRHR | 0.22 | 0.24 | 0.53 | 0.0068* | 0.29 | 0.23 |
Significant P values (<0.05).
Discussion
In this report, we describe the incidence and overall fluid dynamics associated with persistent subfoveal fluid following successful MH closure. Our study confirms previous findings that the percentage of eyes with persistent subfoveal fluid postoperatively decreases over time.5,9,18,19 In this study, we report that most eyes had a persistent subfoveal fluid at 2 weeks (59%). This number decreased to 50% at 1 month, similar to the reported literature of 43% to 49%.5,18 At 3 months, the percentage of persistent subfoveal fluid decreased to 40%, consistent the with other reports of 37%,9 and slightly lower than a report of 50%.5 One study found only an 8% incidence of “foveal detachment” at 3 months.19 This may be due to differences in the sample population and/or various definitions of subfoveal fluid versus foveal detachment versus outer foveal defect, as other studies have found the percentage of subfoveal fluid to be 25% to 47% after 6 months.5,18 The etiology of the persistent subfoveal fluid remains unclear. However, this study suggests that perhaps there may be a mechanical factor involved in the incidence and persistence of subfoveal fluid.
With a larger sample size, our study confirms a previous report of dynamic changes in subfoveal measurements during MH surgery.6,15,20 The analysis confirms an increase in both EZ-RPE height and SRHR width following ILM peeling. The most likely etiology for this expansion is the anterior tractional force that occurs during ILM peeling. One previous study postulated that the subretinal hyporeflectance may represent a subclinical neurosensory retinal detachment or a stretching of the outer retina leading to decreased reflectance.15 Other potential explanations that have been suggested for this increased subretinal hyporeflectivity include stretching of the photoreceptors or disinsertion of photoreceptors from the RPE.6
Various theories exist for the mechanisms behind persistent subfoveal fluid, including “drawbridge” theory of MH closure, tissue defects from previous operculum, RPE dysfunction, and potentially decreased mobility of the outer retina compared with the inner retina.9 The current study represents novel information that identifies factors associated with persistent subfoveal fluid. Interestingly, the variables that were associated with the development of persistent subfoveal fluid were surgical factors that were identified only with iOCT. Christensen et al.9 note that most MHs begin closing with contraction of the inner retina that forms a roof over the subfoveal fluid and hypothesizes that this was most likely due to the large basal diameter of the holes. However, in this study, the size of hole, both preoperatively and intraoperatively after surgical maneuvers, was not associated with the area of persistent subfoveal fluid. In fact, no preoperative factors in this study, including symptom duration, MH area, axial length, or MH stage, were shown to be correlated with persistent subfoveal fluid. Rather, this study demonstrates that increased EZ-RPE height and increased SRHR width following ILM peeling is significantly correlated with reduction in persistent subfoveal fluid area postoperatively. This study demonstrates that both EZ-RPE height and SRHR width increase significantly after ILM peeling but this is variable from patient to patient.
Traditionally, ILM peeling is thought to increase flexibility and reduce tangential traction at the inner retinal surface, thus facilitating MH closure. The data in this study support an additional mechanism that ILM peeling also may facilitate complete hole closure. The anterior tractional force, which results in increased subretinal hyporeflectivity, may perhaps reduce outer retinal/RPE adherence, facilitating complete hole closure.
The relationship of postoperative visual acuity and persistent subfoveal fluid has been previously studied showing patients with subfoveal fluid at 3 months have worse visual acuity than those without.9 However, at 12 months, there was no significant difference between the two groups.9,19 Other studies also have found that persistent subfoveal fluid was not correlated with final visual outcomes at 6 months postoperatively.18 The current study results confirm previous studies that visual acuity recovery is delayed in patients with persistent subfoveal fluid than those who did not (i.e., a mean visual recovery period of approximately 5 vs. 1 month). In fact, intraoperative EZ-RPE height and SRHR width measured with iOCT also correlated with visual recovery, particularly in the early postoperative period (e.g., 3 months). The mere presence of subfoveal fluid appeared to affect visual recovery rate, as the area of subfoveal fluid did not correlate with visual recovery. This indicates that the presence or absence of subfoveal fluid is more important than its size with regard to visual acuity.
This study has some limitations that should be acknowledged. Our study is limited by the relatively small sample size and lack of a control group. All eyes received ILM peeling, which also limited comparison between those eyes with peeling and those eyes without. This study also includes multiple peeling techniques between surgeons and did not include microscope-integrated OCT visualization.
In conclusion, the present study supports previous findings that persistent subfoveal fluid after surgical closure of MHs decreases over time,5,9,18,19 and that dynamic changes exist in the subretinal hyporeflectance area intraoperatively associated with ILM peeling.5,6,9,15,18–20 This study represents one of the first studies to identify a potential mechanism for postoperative anatomic configurations that are visible only with iOCT, as the changes in SRHR are subclinical and can be identified only intraoperatively. Further research is needed to validate these findings and to better elucidate the role of these outer retinal changes in rate of complete MH closure, as well as potentially at rate of hole closure. These findings may have implications for duration and/or need for postoperative positioning in MH surgery. This study demonstrates the role of iOCT in studying immediate changes in retinal anatomy after surgical maneuvers and the impact of these changes on postoperative macular architecture. Further investigations are needed to determine the factors that affect SRHR intraoperatively and their relationship to persistent subfoveal fluid, including peel technique and staining techniques.
Acknowledgments
Supported by National Institutes of Health/National Eye Institute Grant K23-EY022947-01A1 (JPE), Ohio Department of Development Grant TECH-13-059 (JPE, SKS); Machemer Foundation Scholarship (YI); and Research to Prevent Blindness (PKK).
Disclosure: J.P. Ehlers, Leica (C), Bioptigen (C), Carl Zeiss Meditec (C), Thrombogenics (C, F), Genentech (F), P; Y. Itoh, None; L.T. Xu, Thrombogenics (R); P.K. Kaiser, Carl Zeiss Meditec (C), Alcon (C), Topcon (C), Bausch and Lomb (C), Novartis (C); R.P. Singh, Carl Zeiss Meditec (C); S.K. Srivastava, Leica (C), Zeiss (C), Bausch and Lomb (C, F), Allergan (F), P
Footnotes
JPE and YI are joint first authors.
References
- 1. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991; 109: 654–659. [DOI] [PubMed] [Google Scholar]
- 2. Lois N, Burr J, Norrie J, et al. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: a pragmatic randomized controlled trial. Invest Ophthalmol Vis Sci. 2011; 52: 1586–1592. [DOI] [PubMed] [Google Scholar]
- 3. SpiteriCornish K, Lois N, Scott NW, et al. Vitrectomy with internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole. Ophthalmology. 2014; 121: 649–655. [DOI] [PubMed] [Google Scholar]
- 4. Moshfeghi AA, Flynn HW Jr, Elner SG, Puliafito CA, Gass JD. Persistent outer retinal defect after successful macular hole repair. Am J Ophthalmol. 2005; 139: 183–184. [DOI] [PubMed] [Google Scholar]
- 5. Kawano H, Uemura A, Sakamoto T. Incidence of outer foveal defect after macular hole surgery. Am J Ophthalmol. 2011; 151: 318–322. [DOI] [PubMed] [Google Scholar]
- 6. Ehlers JP, Xu D, Kaiser PK, Singh RP, Srivastava SK. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina. 2014; 34: 213–221. [DOI] [PubMed] [Google Scholar]
- 7. Bottoni F, De Angelis S, Luccarelli S, Cigada M, Staurenghi G. The dynamic healing process of idiopathic macular holes after surgical repair: a spectral-domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2011; 52: 4439–4446. [DOI] [PubMed] [Google Scholar]
- 8. Hasler PW, Prunte C. Early foveal recovery after macular hole surgery. Br J Ophthalmol. 2008; 92: 645–649. [DOI] [PubMed] [Google Scholar]
- 9. Christensen UC, Kroyer K, Sander B, Larsen M, la Cour M. Prognostic significance of delayed structural recovery after macular hole surgery. Ophthalmology. 2009; 116: 2430–2436. [DOI] [PubMed] [Google Scholar]
- 10. Ko TH, Witkin AJ, Fujimoto JG, et al. Ultrahigh-resolution optical coherence tomography of surgically closed macular holes. Arch Ophthalmol. 2006; 124: 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takahashi H, Kishi S. Tomographic features of early macular hole closure after vitreous surgery. Am J Ophthalmol. 2000; 130: 192–196. [DOI] [PubMed] [Google Scholar]
- 12. Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013; 33: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 13. Ehlers JP, Tam T, Kaiser PK, Martin DF, Smith GM, Srivastava SK. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014; 34: 1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hahn P, Migacz J, O'Connell R, Maldonado RS, Izatt JA, Toth CA. The use of optical coherence tomography in intraoperative ophthalmic imaging. Ophthalmic Surg Lasers Imaging. 2011; 42: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ray R, Baranano DE, Fortun JA, et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology. 2011; 118: 2212–2217. [DOI] [PubMed] [Google Scholar]
- 16. Xu D, Yuan A, Kaiser PK, et al. A novel segmentation algorithm for volumetric analysis of macular hole boundaries identified with optical coherence tomography. Invest Ophthalmol Vis Sci. 2013; 54: 163–169. [DOI] [PubMed] [Google Scholar]
- 17. Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am J Ophthalmol. 2014; 158: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sano M, Shimoda Y, Hashimoto H, Kishi S. Restored photoreceptor outer segment and visual recovery after macular hole closure. Am J Ophthalmol. 2009; 147: 313–318.e1. [DOI] [PubMed] [Google Scholar]
- 19. Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S, Oshima Y. Foveal microstructure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010; 117: 1815–1824. [DOI] [PubMed] [Google Scholar]
- 20. Pichi F, Alkabes M, Nucci P, Ciardella AP. Intraoperative SD-OCT in macular surgery. Ophthalmic Surg Lasers Imaging. 2012; 43: S54–S60. [DOI] [PubMed] [Google Scholar]


