Summary
The biogenesis of mitochondria requires the import of a large number of proteins from the cytosol [1, 2]. While numerous studies have defined the proteinaceous machineries that mediate mitochondrial protein sorting, little is known about the role of lipids in mitochondrial protein import. Cardiolipin, the signature phospholipid of the mitochondrial inner membrane [3–5], affects the stability of many inner membrane protein complexes [6–12]. Perturbation of cardiolipin metabolism leads to the X-linked cardioskeletal myopathy, Barth syndrome [13–18]. We report that cardiolipin affects the preprotein translocases of the mitochondrial outer membrane. Cardiolipin mutants genetically interact with mutants of outer membrane translocases. Mitochondria from cardiolipin yeast mutants, as well as Barth syndrome patients, are impaired in the biogenesis of outer membrane proteins. Our findings reveal a new role for cardiolipin in protein sorting at the mitochondrial outer membrane and bear implications for the pathogenesis of Barth syndrome.
Results and Discussion
Mitochondria play crucial roles in cellular bioenergetics, programmed cell death, and the metabolism of amino acids, lipids, heme and iron [1, 2, 19]. To fulfill these tasks, mitochondria contain ~1,000 different proteins, 99% of which have to be imported from the cytosol [1, 2]. Of the mitochondrial lipids, the dimeric phospholipid cardiolipin is characteristic for this organelle [3–5]. Cardiolipin is synthesized in the inner membrane and affects the stability of various inner membrane protein complexes, including respiratory chain complexes and metabolite carriers [6–12]. Mitochondria from mutants defective in cardiolipin biosynthesis have a reduced inner membrane potential Δψ, leading to a reduction of the Δψ-dependent protein translocation into the inner membrane [6, 11, 15, 20]. Mutations of the phospholipid transacylase tafazzin, which remodels the fatty acyl side chains of cardiolipin, cause the severe human disease Barth syndrome [13, 14, 17]. The mitochondrial defects of tafazzin mutants have been attributed to defects of inner membrane protein complexes [15–18]. Interestingly, tafazzin was not only found in the inner membrane but also in the outer membrane [15, 21]. However, different views have been reported on the presence of cardiolipin in the outer membrane [3, 22–24] and little has been known on a possible function of outer membrane cardiolipin, the only exception being a controversial discussion on the role of cardiolipin in outer membrane permeabilization in apoptosis [23–26]. Since apoptotic defects have not been found in Barth syndrome [26], experimental evidence for a functional relation of Barth syndrome and the outer mitochondrial membrane has been lacking.
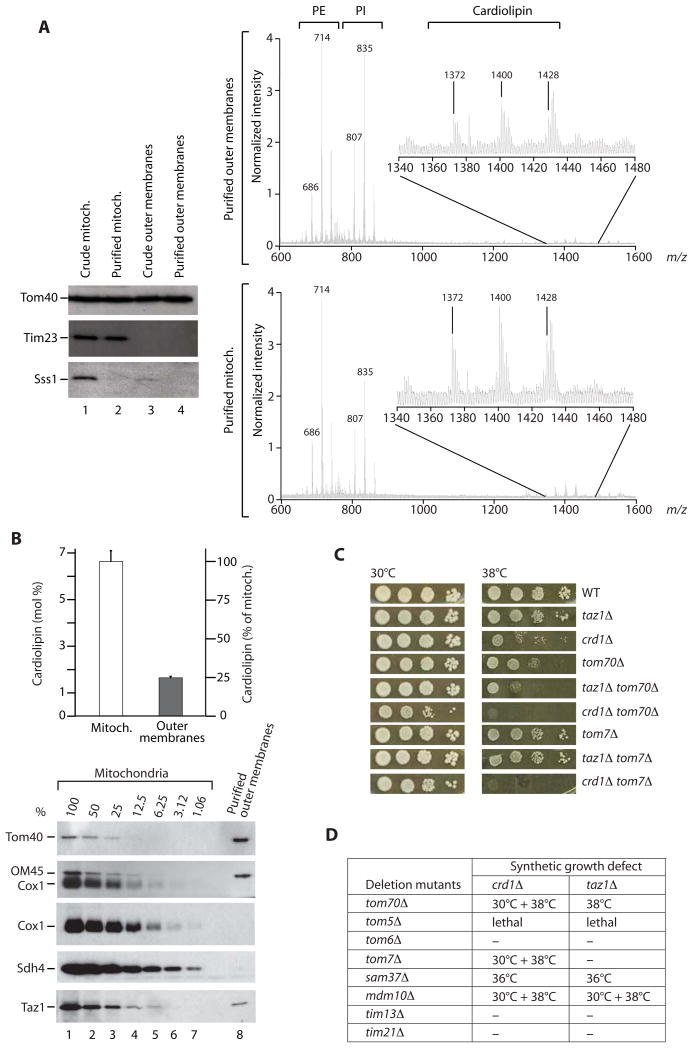
To determine the presence of cardiolipin in the outer membrane of Saccharomyces cerevisiae mitochondria, we prepared highly pure outer membrane vesicles from isolated yeast mitochondria. The liquid chromatography/mass spectrometry profile demonstrated the presence of cardiolipin in the outer membrane (Figure 1A, upper panel; total mitochondrial membranes are shown for comparison, lower panel). A quantitative determination revealed that the concentration of cardiolipin in the outer membrane was 25% of the cardiolipin concentration of total mitochondrial membranes (Figure 1B). To determine the purity of the outer membrane preparation, we performed a Western blot titration from 100-1%, demonstrating that a contamination with inner membrane marker proteins (Cox1, Sdh4) was below the detection limit and thus far below 1% (Figure 1B). In contrast, a fraction of tafazzin (Taz1) was clearly present in the purified outer membranes. Using an antiserum against the authentic (untagged) protein, we found Taz1 at a 16% level in the outer membrane (Figure 1B).
Figure 1. Outer membrane lipids and genetic interaction of cardiolipin mutants with tom/sam mutants.
(A) Purified yeast mitochondrial outer membrane vesicles were analyzed by immunoblotting (left panel) and liquid chromatography/mass spectrometry (right panels). PE, phosphatidylethanolamine; PI, phosphatidylinositol; Sss1, endoplasmic reticulum protein.
(B) Cardiolipin was quantified [11] using tetramyristoyl cardiolipin as internal standard. Data from two independent outer membrane preparations are represented as mean +/− range. The purity of outer membrane vesicles was determined by a Western blot titration of outer membrane proteins (Tom40, OM45) and inner membrane proteins (Cox1, Sdh4).
(C and D) Genetic interactions, synthetic growth defects. Cells were grown at 30°C in liquid YPD to the early stationary phase, serially diluted, spotted on YPD plates and incubated at the indicated temperatures. Synthetic growth defects of double deletion mutants are indicated; ‘-‘ no synthetic growth defect.
To study a possible role of cardiolipin in the outer membrane, we used yeast mutants that lacked either cardiolipin synthase (crd1Δ) or tafazzin (taz1Δ). crd1Δ cells lack cardiolipin, whereas in taz1Δ cells, the cardiolipin levels are lower and immature cardiolipin species (lacking unsaturated fatty acids) and monolysocardiolipin are observed [4, 5, 17]. We generated double deletion mutants with genes encoding components of the two protein transport machineries of the outer membrane: the translocase of the outer membrane (TOM complex) and the sorting and assembly machinery (SAM complex). The majority of tom/sam deletion mutants showed synthetic growth defects with the cardiolipin mutants, including a synthetic lethality of tom5Δ crd1Δ and tom5Δ taz1Δ (Figures 1C and 1D). These results indicate a genetic interaction of cardiolipin mutants with components of outer membrane protein import.
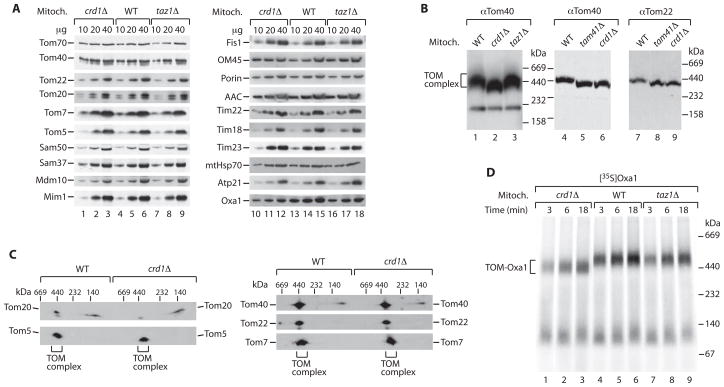
To define the molecular steps affected in protein import, we analyzed the translocases and import capability of isolated mitochondria. Since cardiolipin mutants are temperature-sensitive for growth [6, 11], we searched for conditions that minimized indirect, pleiotropic effects. We found that growth of the cells at very low temperature (21°C) yielded crd1Δ and taz1Δ mitochondria that contained the components of the protein import machineries of outer and inner membranes in amounts close to that of wild-type mitochondria (Figure 2A). Thus, alterations of steady-state protein levels, which would indirectly affect mitochondrial biogenesis and function, were minimized (facilitating the identification of primary mutant defects by a kinetic analysis of protein import as outlined below). The isolated mutant mitochondria contained an intact outer membrane as the intermembrane space-exposed Tim23 subunit of the translocase of the inner membrane was protected against externally added proteinase K (unless the outer membrane was disrupted by swelling) (Figure S1A available online). To analyze the TOM complex, we lysed mitochondria with digitonin and separated the protein complexes by blue native electrophoresis. The mobility of the TOM complex was altered in crd1Δ and tam41Δ mitochondria that lack cardiolipin (Figure 2B) (Tam41 is required for an early step of cardiolipin biosynthesis [11]). For comparison, the mobility of the inner membrane TIM22 complex, but not of the Tim9-Tim10 complex of the intermembrane space, was also altered in crd1Δ mitochondria as reported (Figure S1B) [11]. In taz1Δ mitochondria, the mobility of TOM complex and TIM22 complex was close to that of wild-type mitochondria (Figures 2B and S1B). These results indicated that the lack of cardiolipin affected the organization of the TOM complex. We thus performed a 2D-electrophoresis (blue native followed by SDS-PAGE) to determine if the lack of cardiolipin influenced the association of Tom subunits. The core components Tom40, Tom22 and the small Tom subunits were present in the TOM complex independently of the presence or absence of cardiolipin (Figure 2C). However, the association of the receptor Tom20 with the TOM complex was clearly impaired (Figure 2C). Tom20 is more loosely associated with the TOM complex and thus only a fraction of this receptor migrated with the TOM complex under wild-type conditions. In crd1Δ mitochondria, this fraction was considerably reduced (Figure 2C), indicating that cardiolipin influences the interaction of Tom20 with the TOM complex.
Figure 2. Cardiolipin mutant mitochondria are impaired in precursor accumulation at the outer membrane.
(A) Steady-state protein levels. Mitochondria (Mitoch., μg protein) from wild-type (WT), crd1Δ and taz1Δ yeast grown at 21°C in liquid YPG were analyzed by SDS-PAGE and immunoblotting.
(B) Isolated mitochondria were analyzed by blue native electrophoresis and immunoblotting. The band at ~230 kDa in the Tom40 Western blot is only visible after long exposure.
(C) 2D-analysis. Isolated mitochondria were separated by blue native electrophoresis, followed by SDS-PAGE and immunoblotting.
(D) Oxa1 was imported into isolated BY mitochondria at 24°C in the absence of a Δψ. Samples were analyzed by blue native electrophoresis and autoradiography.
To monitor protein import, we synthesized and radiolabeled the precursor proteins in a cell-free system and incubated them with isolated mitochondria. The Δψ-dependent import of hydrophilic precursor proteins across the inner membrane (F1β, Su9-DHFR) was not or only mildly affected in mitochondria lacking Crd1 or Taz1 (Figure S2A), in agreement with the observation that cardiolipin-deficient cells grown at low temperature are able to generate a Δψ [11]. (It has been shown that at higher temperature, cardiolipin-deficient mitochondria are partially impaired in protein import across the inner membrane concomitant with a decrease of Δψ [6, 11].) However, import of the multispanning inner membrane protein Oxa1 was of lower efficiency in crd1Δ mitochondria compared to wild-type mitochondria, and also partially impaired in taz1Δ mitochondria (Figure S2B). The steady-state levels of Oxa1 were also moderately reduced in the mutant mitochondria (Figure 2A). It has been shown that the Oxa1 precursor accumulates as a translocation intermediate at the TOM complex in the absence of a Δψ (the intermediate can be directly visualized by blue native electrophoresis [27, 28]), whereas hydrophilic precursor proteins destined for the matrix pass through the TOM complex and do not form stable TOM intermediates on blue native gels. We thus asked if the alteration of the TOM complex in cardiolipin mutants affected the accumulation of the Oxa1 intermediate. The amount of TOM-Oxa1 intermediate was considerably decreased in crd1Δ mitochondria (and the blue native mobility was altered as expected) (Figure 2D). Interestingly, the amount of TOM-Oxa1 was also reduced in taz1Δ mitochondria (Figure 2D). Here the mobility of the TOM complex was close to that of wild-type mitochondria (Figures 2B and 2D) as cardiolipin was still present, yet the altered cardiolipin species partially impaired the accumulation of the Oxa1 intermediate. Thus, lack or alteration of cardiolipin impair the import of Oxa1 at the level of the TOM complex, i.e. at an early step before the Δψ-dependent insertion into the inner membrane.
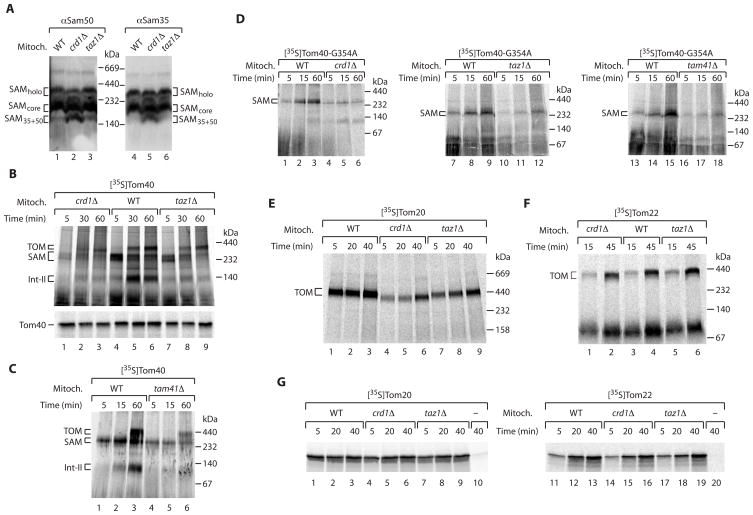
We thus asked if further steps of protein biogenesis of the outer membrane were affected by cardiolipin defects. The outer membrane contains two main classes of proteins: α-helical proteins and the characteristic β-barrel proteins [29, 30]. The biogenesis pathway of β-barrel proteins involves both the TOM complex and the SAM complex. Blue native electrophoresis resolves several forms of the SAM complex [30, 31]: the SAMcore complex consisting of Sam35, Sam37 and Sam50; the SAMholo complex that additionally contains Mdm10; and a smaller form containing Sam35 and Sam50 (Figure 3A). In crd1Δ mitochondria, more of the smaller complex was observed (Figure 3A), indicating that the lack of cardiolipin affected the organization of the SAM complex. With the radiolabeled precursor of the β-barrel protein Tom40, the biogenesis steps can be monitored by blue native electrophoresis and dissected into three steps [30, 31]: first, an intermediate of ~250 kDa, representing the association of the precursor with the SAM complex; followed by a second, smaller intermediate (Int-II); and third, the assembly into the mature TOM complex of ~400 kDa. crd1Δ mitochondria were strongly inhibited in forming the assembly steps of Tom40, yet also taz1Δ mitochondria were impaired (Figure 3B, upper panel). Similarly, tam41Δ mitochondria were impaired in the assembly pathway of Tom40 (Figure 3C). The initial targeting of the precursor to mitochondria was not blocked as the total amount of Tom40 precursor bound to mitochondria was only moderately affected in crd1Δ and taz1Δ mitochondria (Figure 3B, lower panel). To analyze the step of interaction with the SAM complex in more detail, we used a mutant form of the Tom40 precursor where one conserved glycine residue was replaced by alanine, leading to an accumulation of the precursor at the SAM complex in wild-type mitochondria [30]. All three cardiolipin mutant mitochondria showed a strong defect in precursor accumulation at the SAM complex (Figure 3D), demonstrating that the lack or alteration of cardiolipin impaired the assembly pathway of the β-barrel precursor already at the level of the SAM complex.
Figure 3. Cardiolipin mutant mitochondria are impaired in the assembly of outer membrane proteins.
(A) Isolated yeast mitochondria were separated by blue native electrophoresis and analyzed by immunoblotting.
(B) [35S]Tom40 precursor was incubated with WT, crd1Δ and taz1Δ mitochondria (min). Mitochondria were reisolated and analyzed by blue native electrophoresis (upper panel) or SDS-PAGE (lower panel) and autoradiography.
(C) Tom40 precursor was imported and analyzed by blue native electrophoresis.
(D) Tom40-G354A precursor was imported in isolated mitochondria.
(E) [35S]Tom20 was incubated with WT, crd1Δ and taz1Δ mitochondria at 24°C (min). Samples were analyzed by blue native electrophoresis and autoradiography.
(F) Tom22 precursor was imported and analyzed as in E.
(G) After import of [35S]Tom20 (lane 1–10) or Tom22 (lane 11–20), mitochondria were incubated with 0.1 M Na2CO3 for 30 min. Pellets were analyzed by SDS-PAGE and autoradiography. Samples 10 and 20 did not contain mitochondria.
To determine a possible influence of cardiolipin on the biogenesis of α-helical outer membrane proteins, we used two precursors that are imported by different pathways: Tom20, which is imported via the outer membrane protein Mim1 and subsequently assembles onto the TOM complex [32–34]; and Tom22, which uses the SAM complex for membrane insertion and with the help of Mdm10, assembles into the TOM complex [35, 36]. The assembly of Tom20 with the TOM complex was impaired in both crd1Δ and taz1Δ mitochondria (Figure 3E), whereas the assembly of the precursor of Tom22 was not or only mildly affected (Figure 3F) (β-barrel precursors and Tom22 show different characteristics of SAM-dependence, including access to SAM from the intermembrane space or cytosolic side, respectively [35, 36]). The initial insertion of Tom20 and Tom22 into the outer membrane, as analyzed by treatment of the membranes at alkaline pH, was not inhibited by the lack of cardiolipin (Figure 3G). Thus the lack of cardiolipin does not impair the Mim1-mediated membrane insertion of the Tom20 precursor but inhibits the subsequent assembly step. Since the precursor of Tom22 is correctly imported and assembled in cardiolipin mutant mitochondria, we conclude that the outer membrane and the SAM complex are not generally (unspecifically) damaged in the mutant mitochondria.
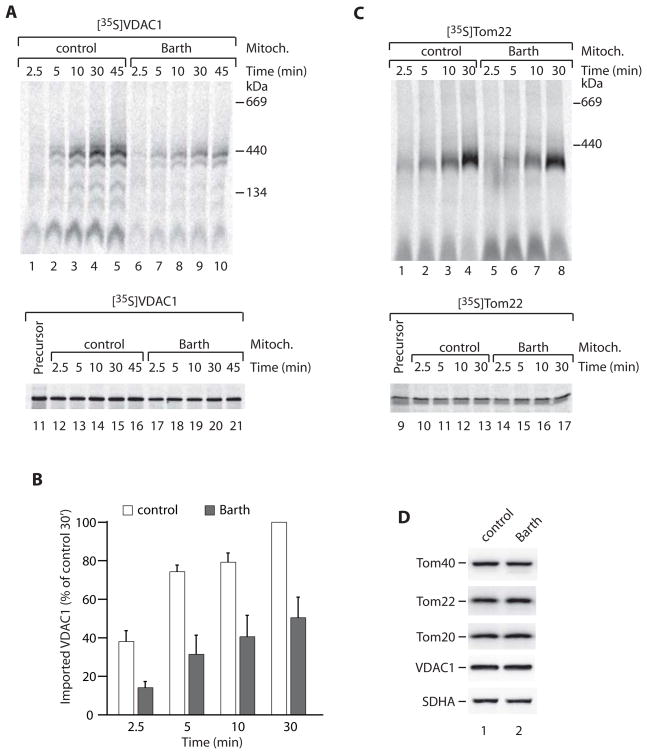
We asked if these observations with yeast mitochondria bear relevance for the biogenesis of outer membrane proteins in Barth syndrome mitochondria. We isolated mitochondria from a human lymphoblast cell line derived from a Barth syndrome patient with a TAZ splicing defect that is expected to result in no production of tafazzin protein [16]. We used precursors for which the assembly kinetics in lymphoblast mitochondria could be monitored by blue native electrophoresis: the major β-barrel protein VDAC1 and α-helical Tom22. The 35S-labeled precursors were imported into isolated Barth syndrome and control mitochondria. The assembly kinetics of the β-barrel precursor was significantly delayed in the mutant mitochondria (Figures 4A and 4B) (the total binding of the precursor to the mutant mitochondria was only mildly affected; Figure 4A, lower panel). The assembly of Tom22 into the mutant mitochondria was similar to control mitochondria (Figure 4C). Steady-state protein levels, including Tom proteins, were comparable between mutant and control mitochondria (Figure 4D). Together with the efficient import of Tom22, this indicates that the impaired import kinetics of VDAC1 import was not caused by a general (unspecific) damage of the outer membrane. We conclude that Barth syndrome mitochondria show a similar defect in biogenesis of outer membrane proteins as cardiolipin mutant mitochondria from yeast.
Figure 4. Assembly of outer membrane proteins in Barth syndrome mitochondria.
(A–C) Mitochondria isolated from control or Barth syndrome patient lymphoblasts were incubated at 37°C (min) in the presence of [35S]VDAC1 or [35S]Tom22. Following import, samples were analyzed by blue native electrophoresis (upper panel) and SDS-PAGE (lower panel) and autoradiography. A sample of reticulocyte lysate (representing 50% of added precursor/import) is also shown on the SDS-PAGE gel. For B, assembled VDAC1 was quantified from four independent experiments (three for 2.5 min). Data are represented as mean +/− SEM.
(D) Control and Barth syndrome mitochondria were analyzed by SDS-PAGE and immunoblotting. SDHA, 70 kDa subunit of succinate dehydrogenase.
Our results suggest a new role of cardiolipin for the biogenesis of mitochondrial outer membrane proteins. By a quantitative analysis of highly pure outer membranes, we demonstrate that a fraction of cardiolipin as well as tafazzin is present in the outer membrane. In yeast mitochondria isolated from cardiolipin mutants and human mitochondria isolated from a Barth syndrome patient, the assembly of β-barrel proteins is delayed. How does the lack or alteration of cardiolipin affect the mitochondrial outer membrane? The outer membrane was not generally damaged in the cardiolipin mutants since the initial targeting of precursor proteins to mitochondria still took place and different types of α-helical precursors exhibited selective defects (impaired assembly of Tom20 but not of Tom22). These findings suggest a more specific role of the non-bilayer forming phospholipid cardiolipin for protein sorting and influencing the activity of preprotein translocases. Studies with inner membrane protein complexes revealed a specific interaction of cardiolipin with respiratory chain complexes, metabolite carriers and further proteins; in several cases, alteration of the complexes in cardiolipin mutants could be visualized by blue native gels [6, 10–12, 15, 37]. Indeed, the blue native pattern of the TOM and SAM complexes was altered in cardiolipin-deficient mitochondria, indicating that cardiolipin is involved in the organization of the translocase complexes. In tafazzin mutant mitochondria, cardiolipin is not absent but altered species are observed and thus the defects are not as strong as in cardiolipin-deficient mitochondria [14–18]. The functional assays of precursor binding to TOM and SAM indeed revealed that the altered cardiolipin led to a partial impairment of precursor binding. We conclude that the outer membranes of cardiolipin mutant mitochondria are not unspecifically damaged but cardiolipin is involved in the assembly pathways of β barrel precursors and some α-helical precursors. Outer membrane protein assembly pathways are delayed but not blocked in cardiolipin mutants as a block of TOM or SAM functions would be lethal even to yeast cells. We suggest that mitochondrial defects of Barth syndrome patients are not only caused by inner membrane defects but also involve the role of cardiolipin in the biogenesis of outer membrane proteins. In summary, we conclude that loss or alteration of cardiolipin impairs the assembly pathways of several mitochondrial outer membrane proteins.
Experimental Procedures
Yeast strains, growth and analysis
The S. cerevisiae strains used in this work are derived from YPH499, BY4741 and BY4742 (Euroscarf). crd1Δ, taz1Δ and tam41Δ strains have been described [11, 15] (Euroscarf). The strains used for import studies are listed in Table S1; cells were grown on non-fermentable medium (liquid YPG or YPLac [11]) at 21°C. Mutants of TOM, SAM or TIM genes were screened for genetic interactions with crd1Δ and taz1Δ. The crd1Δ::URA3 and taz1Δ::URA3 mutants in the Matα background were crossed to Mata deletion mutants of TOM or SAM genes disrupted with KanMX4. Diploids were selected on double dropout synthetic medium lacking methionine and lysine; the resulting heterozygous diploids were sporulated and subjected to meiotic tetrad analysis. Double mutants were identified by growth on Ura− and G418 (200 μg/ml) medium. The lethality observed in double mutants was not due to defective spore germination, as a few tiny colonies from the spores inferred to be the double mutants could be seen under the microscope.
Import of precursor proteins into mitochondria
Yeast mitochondria were isolated by differential centrifugation. Precursor proteins were synthesized in rabbit reticulocyte lysate in the presence of [35S]methionine. Import into isolated mitochondria was performed in import buffer (3 % (w/v) BSA, 250 mM sucrose, 80 mM KCl, 5 mM methionine, 5 mM MgCl2, 2 mM KH2PO4, 10 mM MOPS-KOH, pH 7.2, 2 mM NADH, 4 mM ATP, 20 mM creatine phosphate, 0.1 mg/ml creatine kinase) at 24°C. The import reaction was stopped on ice or by addition of AVO (8 μM antimycin A, 20 μM oligomycin, 1 μM valinomycin). To dissipate Δψ, AVO was added before the import experiment. Were indicated, samples were treated with 50 μg/ml proteinase K for 15 min on ice. For native analysis, mitochondria were solubilized in digitonin-containing buffer (0.4–1% digitonin, 20 mM Tris/HCl, pH 7.4, 0.1 mM EDTA, 50 mM NaCl, 10% glycerol) and resolved by blue native electrophoresis.
Lymphoblast cell lines were derived from a control subject and Barth Syndrome patient with a TAZ splicing defect (c.527-1G>A) [16]. Cells were grown at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented with 10% (v/v) fetal bovine serum (FBS, Invitrogen) and 50 μg/ml uridine. In vitro import and assembly of radiolabeled human Tom22 and mouse VDAC1 into freshly isolated lymphoblast mitochondria was performed as previously described [38]. Following import, samples were solubilized in digitonin and aggregates were removed by a clarifying spin. Samples were split and analyzed by blue native electrophoresis or SDS-PAGE.
Miscellaneous
The preparation of highly pure mitochondria and isolation of outer membrane vesicles via sucrose gradient centrifugation were performed as described [39]. Lipid analysis by liquid chromatography/mass spectrometry was performed as reported [11, 40].
Supplementary Material
Acknowledgments
We thank Birgit Schönfisch for expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (P.R., C.M., N.P.), Sonderforschungsbereich 746, Excellence Initiative of the German Federal & State Governments (EXC 294), Gottfried Wilhelm Leibniz Program, Landesforschungspreis Baden-Wurttemberg, Fonds der Chemischen Industrie (N.P.), grant HL084218 from the National Institutes of Health (M.L.G), Barth Syndrome Foundation (M.L.G), Australian Research Council, National Health and Medical Research Council of Australia (M.T.R.), the Singapore National Research Foundation CRP Award No. 2007-04 (M.R.W.), Landesstiftung Baden-Wurttemberg (T.B.), Trinational Research Training Group GRK 1478 (C.M., fellowship to D.A.S.), and a Boehringer Ingelheim Fonds predoctoral fellowship (S.K.).
Footnotes
Supplemental Data
Supplemental Data include two figures and one table and can be found with this article online.
References
- 1.Dolezal P, Likic V, Tachezy J, Lithgow T. Evolution of the molecular machines for protein import into mitochondria. Science. 2006;313:314–318. doi: 10.1126/science.1127895. [DOI] [PubMed] [Google Scholar]
- 2.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 3.Daum G, Vance JE. Import of lipids into mitochondria. Prog Lipid Res. 1997;36:103–130. doi: 10.1016/s0163-7827(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 4.Mileykovskaya E, Zhang M, Dowhan W. Cardiolipin in energy transducing membranes. Biochemistry (Mosc) 2005;70:154–158. doi: 10.1007/s10541-005-0095-2. [DOI] [PubMed] [Google Scholar]
- 5.Joshi AS, Zhou J, Gohil VM, Chen S, Greenberg ML. Cellular functions of cardiolipin in yeast. Biochim Biophys Acta. 2009;1793:212–218. doi: 10.1016/j.bbamcr.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg ML. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem. 2000;275:22387–22394. doi: 10.1074/jbc.M909868199. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- 8.Nury H, Dahout-Gonzalez C, Trezeguet V, Lauquin G, Brandolin G, Pebay-Peyroula E. Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett. 2005;579:6031–6036. doi: 10.1016/j.febslet.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Mileykovskaya E, Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J Biol Chem. 2005;280:29403–29408. doi: 10.1074/jbc.M504955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutik S, Rissler M, Guan XL, Guiard B, Shui G, Gebert N, Heacock PN, Rehling P, Dowhan W, Wenk MR, et al. The translocator maintenance protein Tam41 is required for mitochondrial cardiolipin biosynthesis. J Cell Biol. 2008;183:1213–1221. doi: 10.1083/jcb.200806048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenz T, Hielscher R, Hellwig P, Schägger H, Richers S, Hunte C. Role of phospholipids in respiratory cytochrome bc1 complex catalysis and supercomplex formation. Biochim Biophys Acta. 2009;1787:609–616. doi: 10.1016/j.bbabio.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Bione S, D’Adamo P, Maestrini E, Gedeon AK, Bolhuis PA, Toniolo D. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat Genet. 1996;12:385–389. doi: 10.1038/ng0496-385. [DOI] [PubMed] [Google Scholar]
- 14.Barth PG, Valianpour F, Bowen VM, Lam J, Duran M, Vaz FM, Wanders RJ. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am J Med Genet A. 2004;126A:349–354. doi: 10.1002/ajmg.a.20660. [DOI] [PubMed] [Google Scholar]
- 15.Brandner K, Mick DU, Frazier AE, Taylor RD, Meisinger C, Rehling P. Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth Syndrome. Mol Biol Cell. 2005;16:5202–5214. doi: 10.1091/mbc.E05-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol. 2006;361:462–469. doi: 10.1016/j.jmb.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 17.Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Claypool SM, Boontheung P, McCaffery JM, Loo JA, Koehler CM. The cardiolipin transacylase, tafazzin, associates with two distinct respiratory components providing insight into Barth syndrome. Mol Biol Cell. 2008;19:5143–5155. doi: 10.1091/mbc.E08-09-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lill R, Muhlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Sutachan JJ, Plesken H, Kelley RI, Schlame M. Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab Invest. 2005;85:823–830. doi: 10.1038/labinvest.3700274. [DOI] [PubMed] [Google Scholar]
- 21.Claypool SM, McCaffery JM, Koehler CM. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J Cell Biol. 2006;174:379–390. doi: 10.1083/jcb.200605043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Kroon AI, Dolis D, Mayer A, Lill R, de Kruijff B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa: is cardiolipin present in the mitochondrial outer membrane? Biochim Biophys Acta. 1997;1325:108–116. doi: 10.1016/s0005-2736(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 23.Kim TH, Zhao Y, Ding WX, Shin JN, He X, Seo YW, Chen J, Rabinowich H, Amoscato AA, Yin XM. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome c release. Mol Biol Cell. 2004;15:3061–3072. doi: 10.1091/mbc.E03-12-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer B, Quispe J, Choudhary V, Chipuk JE, Ajero TG, Du H, Schneiter R, Kuwana T. Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. Mol Biol Cell. 2009;20:2276–2285. doi: 10.1091/mbc.E08-10-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RS, Petit PX, Vaz FM, Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frazier AE, Chacinska A, Truscott KN, Guiard B, Pfanner N, Rehling P. Mitochondria use different mechanisms for transport of multispanning membrane proteins through the intermembrane space. Mol Cell Biol. 2003;23:7818–7828. doi: 10.1128/MCB.23.21.7818-7828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chacinska A, Lind M, Frazier AE, Dudek J, Meisinger C, Geissler A, Sickmann A, Meyer HE, Truscott KN, Guiard B, et al. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell. 2005;120:817–829. doi: 10.1016/j.cell.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Matouschek A, Glick BS. Barreling through the outer membrane. Nat Struct Biol. 2001;8:284–286. doi: 10.1038/86140. [DOI] [PubMed] [Google Scholar]
- 30.Kutik S, Stojanovski D, Becker L, Becker T, Meinecke M, Krüger V, Prinz C, Meisinger C, Guiard B, Wagner R, et al. Dissecting membrane insertion of mitochondrial β-barrel proteins. Cell. 2008;132:1011–1024. doi: 10.1016/j.cell.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Wiedemann N, Kozjak V, Chacinska A, Schönfisch B, Rospert S, Ryan MT, Pfanner N, Meisinger C. Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature. 2003;424:565–571. doi: 10.1038/nature01753. [DOI] [PubMed] [Google Scholar]
- 32.Becker T, Pfannschmidt S, Guiard B, Stojanovski D, Milenkovic D, Kutik S, Pfanner N, Meisinger C, Wiedemann N. Biogenesis of the mitochondrial TOM complex: Mim1 promotes insertion and assembly of signal-anchored receptors. J Biol Chem. 2008;283:120–127. doi: 10.1074/jbc.M706997200. [DOI] [PubMed] [Google Scholar]
- 33.Hulett JM, Lueder F, Chan NC, Perry AJ, Wolynec P, Likic VA, Gooley PR, Lithgow T. The transmembrane segment of Tom20 is recognized by Mim1 for docking to the mitochondrial TOM complex. J Mol Biol. 2008;376:694–704. doi: 10.1016/j.jmb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Popov-Celeketic J, Waizenegger T, Rapaport D. Mim1 functions in an oligomeric form to facilitate the integration of Tom20 into the mitochondrial outer membrane. J Mol Biol. 2008;376:671–680. doi: 10.1016/j.jmb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Meisinger C, Rissler M, Chacinska A, Szklarz LK, Milenkovic D, Kozjak V, Schonfisch B, Lohaus C, Meyer HE, Yaffe MP, et al. The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev Cell. 2004;7:61–71. doi: 10.1016/j.devcel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Stojanovski D, Guiard B, Kozjak-Pavlovic V, Pfanner N, Meisinger C. Alternative function for the mitochondrial SAM complex in biogenesis of α-helical TOM proteins. J Cell Biol. 2007;179:881–893. doi: 10.1083/jcb.200706043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osman C, Haag M, Potting C, Rodenfels J, Dip PV, Wieland FT, Brugger B, Westermann B, Langer T. The genetic interactome of prohibitins: coordinated control of cardiolipin and phosphatidylethanolamine by conserved regulators in mitochondria. J Cell Biol. 2009;184:583–596. doi: 10.1083/jcb.200810189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazarou M, McKenzie M, Ohtake A, Thorburn DR, Ryan MT. Analysis of the assembly profiles for mitochondrial- and nuclear-DNA-encoded subunits into complex I. Mol Cell Biol. 2007;27:4228–4237. doi: 10.1128/MCB.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zahedi RP, Sickmann A, Boehm AM, Winkler C, Zufall N, Schonfisch B, Guiard B, Pfanner N, Meisinger C. Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol Biol Cell. 2006;17:1436–1450. doi: 10.1091/mbc.E05-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shui G, Bendt AK, Pethe K, Dick T, Wenk MR. Sensitive profiling of chemically diverse bioactive lipids. J Lipid Res. 2007;48:1976–1984. doi: 10.1194/jlr.M700060-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.