Abstract
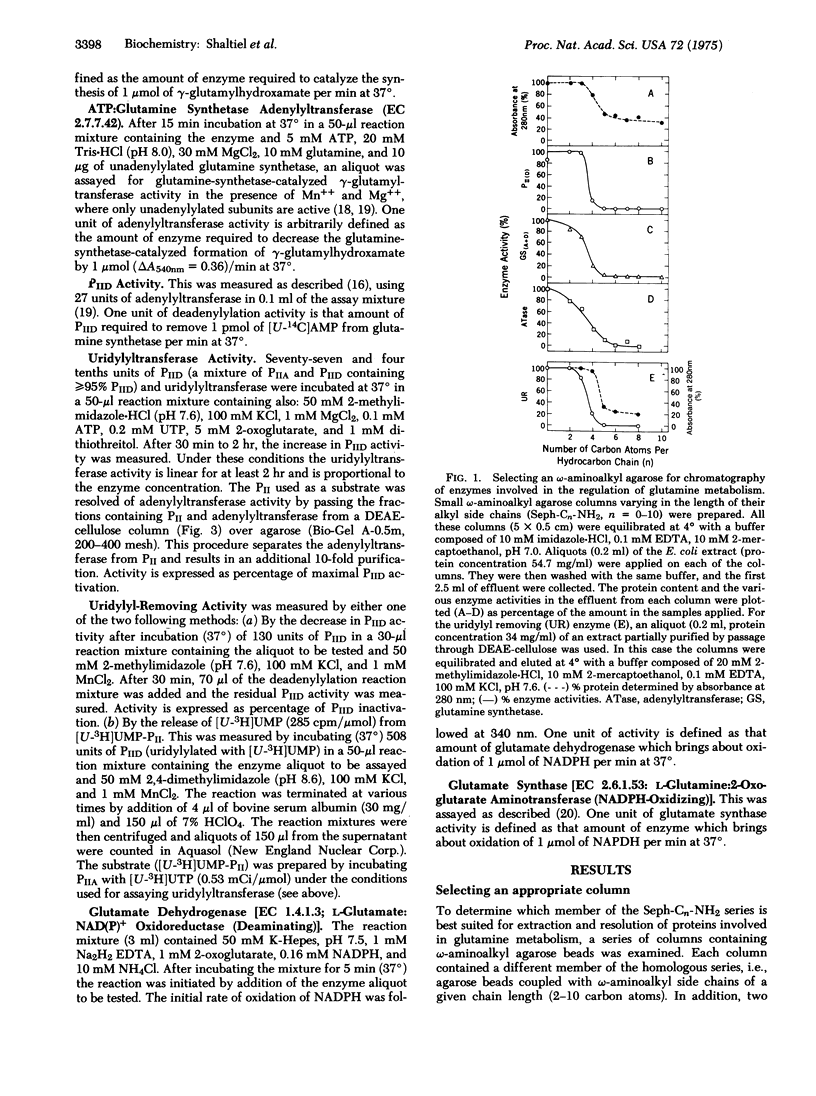
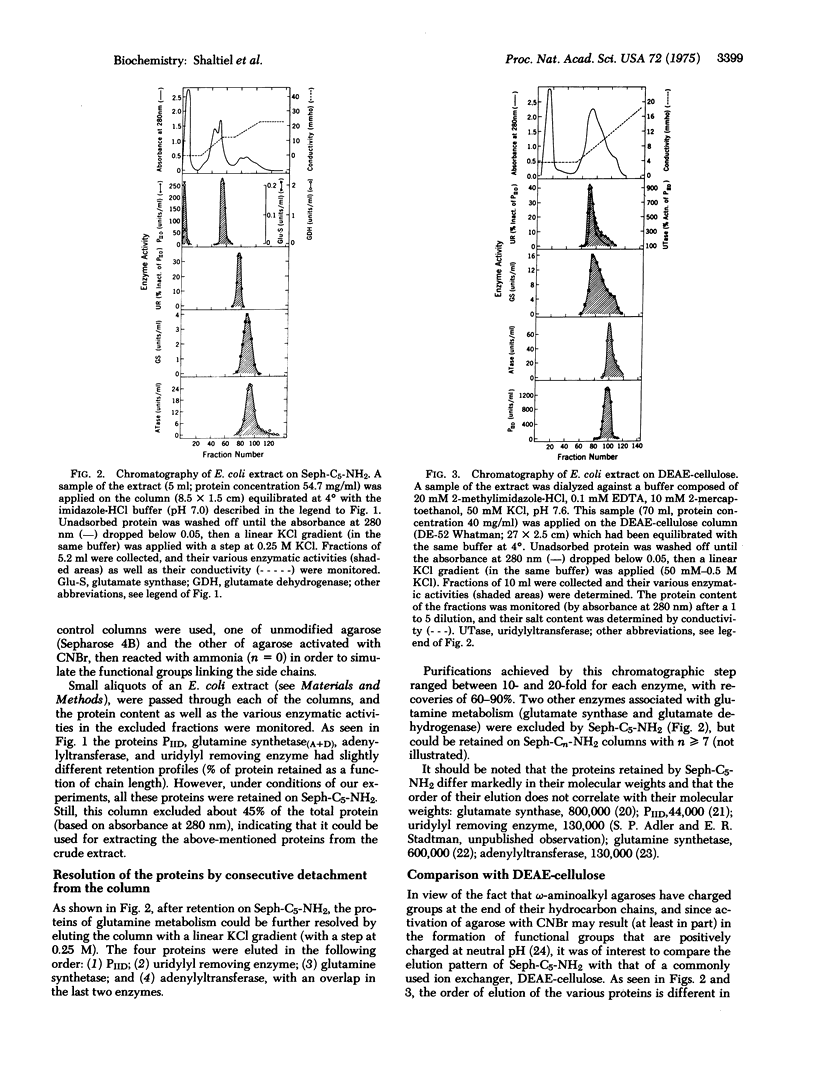
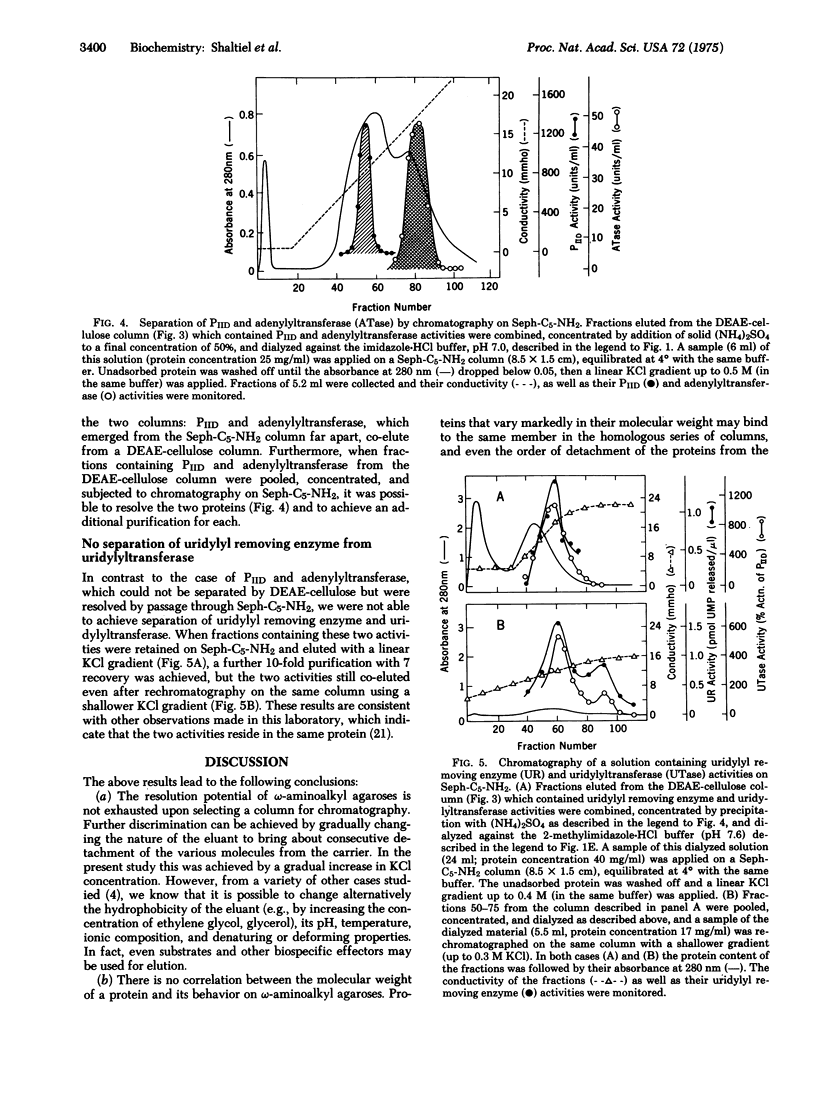
Systematic examination of a homologous series of omega-aminoalkyl agaroses showed that the pentyl derivative (Seph-C5-NH2) was best suited for the retention and subsequent separation of several proteins involved in the regulation of glutamine metabolism in Escherichia coli, including: glutamine synthetase [EC 6.3.1.2; L-glutamate:ammonia ligase (ADP-forming)], ATP:glutamine synthetase adenylyltransferase (EC 2.7.7.42), the PII regulatory protein which regulates the adenylylation and deadenylylation activities of the adenylyltransferase, the UTP:PII protein uridylyltransferase, and the uridylyl removing enzyme which catalyzes the removal of uridylyl groups from uridylylated PII protein. Resolution of these proteins was achieved by gradually increasing the concentration of KCl in the eluant, which resulted in consecutive detachment of the proteins from the column. Proteins that co-elute from a DEAE-cellulose column can be resolved and further purified on epsilon-aminopentyl agarose, probably due to the fact that with the homologous series it is possible to adjust the contribution of hydrophobic interactions for optimal resolution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Er-El Z., Shaltiel S. Hydrophobic chromatography in the resolution of the interconvertible forms of glycogen phosphorylase. FEBS Lett. 1974 Mar 15;40(1):142–145. doi: 10.1016/0014-5793(74)80913-0. [DOI] [PubMed] [Google Scholar]
- Er-el Z., Zaidenzaig Y., Shaltiel S. Hydrocarbon-coated sepharoses. Use in the purification of glycogen phosphorylase. Biochem Biophys Res Commun. 1972 Oct 17;49(2):383–390. doi: 10.1016/0006-291x(72)90422-6. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Shaltiel S., Snell E. E. Omega-aminoalkylagaroses in the purification of L-histidinol-phosphate aminotransferase. Biochemistry. 1974 Oct 8;13(21):4335–4338. doi: 10.1021/bi00718a015. [DOI] [PubMed] [Google Scholar]
- Hennig S. B., Anderson W. B., Ginsburg A. Adenosine triphosphate: glutamine synthetase adenylyltransferase of Escherichia coli: two active molecular forms. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1761–1768. doi: 10.1073/pnas.67.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstee B. H. Protein binding by agarose carrying hydrophobic groups in conjunction with charges. Biochem Biophys Res Commun. 1973 Feb 5;50(3):751–757. doi: 10.1016/0006-291x(73)91308-9. [DOI] [PubMed] [Google Scholar]
- Holzer H., Duntze W. Metabolic regulation by chemical modification of enzymes. Annu Rev Biochem. 1971;40:345–374. doi: 10.1146/annurev.bi.40.070171.002021. [DOI] [PubMed] [Google Scholar]
- Jakubowski H., Pawelkiewicz J. Chromatography of plant aminoacyl-tRNA synthetases on omega-aminoalkyl sepharose columns. FEBS Lett. 1973 Aug 15;34(2):150–154. doi: 10.1016/0014-5793(73)80780-x. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Stadtman E. R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972 Nov 25;247(22):7407–7419. [PubMed] [Google Scholar]
- Peters T., Jr, Taniuchi H., Anfinsen C. B., Jr Affinity chromatography of serum albumin with fatty acids immobilized on agarose. J Biol Chem. 1973 Apr 10;248(7):2447–2451. [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel S., Ferro-Luzzi Ames G., Noel K. D. Hydrophobic chromatography in the purification of the histidine-binding protein J from Salmonella typhimurium. Arch Biochem Biophys. 1973 Nov;159(1):174–179. doi: 10.1016/0003-9861(73)90442-6. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Ginsburg A. Effects of specific divalent cations on some physical and chemical properties of glutamine synthetase from Escherichia coli. Taut and relaxed enzyme forms. Biochemistry. 1968 Jun;7(6):2153–2167. doi: 10.1021/bi00846a018. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]
- Yon R. J. Chromatography of lipophilic proteins on adsorbents containing mixed hydrophobic and ionic groups. Biochem J. 1972 Feb;126(3):765–767. doi: 10.1042/bj1260765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon R. J. Enzyme purification by hydrophobic chromatography: an alternative approach illustrated in the purification of aspartate transcarbamoylase from wheat germ. Biochem J. 1974 Jan;137(1):127–130. doi: 10.1042/bj1370127. [DOI] [PMC free article] [PubMed] [Google Scholar]



