Abstract
Background
Sleep problems are common in patients with chronic obstructive pulmonary disease (COPD), but the validity of patient-reported outcome measures (PROMs) that measure sleep dysfunction has not been evaluated. We have reviewed the literature to identify disease-specific and non-disease-specific sleep PROMs that have been validated for use in COPD patients. The review also examined the psychometric properties of identified sleep outcome measures and extracted point and variability estimates of sleep instruments used in COPD studies.
Methods
The online EMBASE, MEDLINE, PsycINFO, and SCOPUS databases for all years to May 2014 were used to source articles for the review. The review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Criteria from the Medical Outcomes Trust Scientific Advisory Committee guidelines were used to evaluate the psychometric properties of all sleep PROMs identified.
Results
One COPD-specific and six non-COPD-specific sleep outcome measures were identified and 44 papers met the review selection criteria. We only identified one instrument, the COPD and Asthma Sleep Impact Scale, which was developed specifically for use in COPD populations. Ninety percent of the identified studies used one of two non-disease-specific sleep scales, ie, the Pittsburgh Sleep Quality Index and/or the Epworth Sleep Scale, although neither has been tested for reliability or validity in people with COPD.
Conclusion
The results highlight a need for existing non-disease-specific instruments to be validated in COPD populations and also a need for new disease-specific measures to assess the impact of sleep problems in COPD.
Keywords: sleep, symptom assessment, chronic obstructive pulmonary disease, systematic review
Introduction
Sleep problems are a common and important, but poorly understood and under-researched, aspect of chronic obstructive pulmonary disease (COPD). After breathlessness and fatigue, sleep disturbance is considered to be the third most common symptom experienced by people with respiratory disease1 and is also predictive of exacerbations, respiratory-related emergency hospital visits, and all-cause mortality.2 Insomnia describes any reported difficulty a person has with sleep3 and has four elements: difficulties falling asleep, interrupted sleep, trouble staying asleep, and still feeling tired and worn out even after a usual amount of sleep.3–5 Around 10% of the adult population is affected by insomnia, but the occurrence is much higher in people with COPD, where estimates range between 16% and 75%.6 The benefits of sleep are well known, and long-term interruption of normal sleeping patterns has a detrimental impact on physical, emotional, and social functioning, and is also associated with anxiety, depression, bodily pain, and a wide variety of pre-existing chronic medical conditions.4 In addition to insomnia, narcolepsy (suddenly falling asleep at inappropriate times), restless legs syndrome, and obstructive sleep apnea are the most common sleep disorders found in the general population,6 and people with COPD are disproportionately affected. Restless legs syndrome involves a need to move the legs, usually at night-time, is associated with marked sleep disturbance, and affects 7%–14% of the general population and 29% of patients with COPD.7,8 Obstructive sleep apnea is the periodic interruption of airflow in the upper airway during sleep and affects 3%–7% of the general population9 and 25%–29% of people with COPD.10 A summary of the occurrence of four common sleep disorders in COPD populations is provided in Table 1.8,11–13
Table 1.
Summary of the occurrence of common sleep disorders in COPD populations
| Sleep disorder | Author | Occurrence in COPD |
|---|---|---|
| Insomnia (chronic sleep disturbance with impaired daytime functioning) | Budhiraja et al11 | 27% |
| Excessive sleepiness | Ali Zohal et al12 | 35% |
| Restless legs syndrome | Kaplan et al8 | 29% |
| Obstructive sleep apnea | McNicholas13 | 1% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Given the importance of sleep disorders in COPD, being able to accurately classify their nature and severity is important in the management of COPD. Although self-reported sleep disorders are associated with COPD symptoms and poorer health-related quality of life,14 their relationship with traditional diagnostic markers of lung function (such as forced expiratory volume in one second, forced vital capacity, and oxygen saturation) is weak.15 This emphasizes the need for clinical instruments to accurately assess the impact of the disease and its treatment on a patient’s health and well-being through patient-reported outcome measures (PROMs)16,17 as well as recording changes in physiological function.
Many of the instruments that have measured sleep disturbance in epidemiological studies were originally developed for people with a range of psychological conditions and/or pre-existing sleeping disorders.18,19 However, the validity of these measures cannot be assumed to transfer between clinical populations.19 Thus, the aim of this review was to identify and evaluate the suitability of published measures of sleep disturbance for use in people with COPD in order to make recommendations for best practice for clinical and research purposes. Our objectives were to:
Identify which patient-reported outcome sleep measures have been used in people with COPD
Identify which instruments have been developed and validated specifically for people with COPD
Summarize the evidence for reliability and validity of sleep instruments in COPD patients
Examine associations with sleep disturbance recorded by sleep instruments used in clinical studies of COPD patients.
Materials and methods
Ethical approval was not needed to undertake this review, which was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.20
Search strategy
In this study, we conducted a systematic computerized literature review designed to identify all PROMs concerned with sleep problems experienced by people with COPD. The search included all instruments that had been developed and validated in people with COPD as well as generic instruments that had been developed for use in other disease areas and then administered to adult COPD patients.
Stage 1: Identification of sleep outcome measures used in COPD
The first stage of the search was to identify sleep outcome measures that had been used in COPD. This was conducted using EMBASE, MEDLINE, and PsycINFO electronic databases for all years up to May 2014 using both key words, ie, the Medical Subject Headings (MeSH) “COPD” AND “sleep” and expanded to include all recognized subheadings. All titles, abstracts, and full texts from the identified papers were examined by the lead author (APG) for reference to specific sleep instruments or data indicating that at least one sleep outcome measure had been used. A list of sleep outcome measures was then produced. The reference lists and citations of selected articles were also searched to identify any additional sleep PROMs not found by the electronic database search.
Stage 2: Selection and evaluation of sleep instruments used in COPD
A SCOPUS database search was carried out on each of the detected sleep outcome measures to identify all publications in which the original paper had been cited. The search included the following related terms:
Construct-related terms: sleep problems
Population terms: COPD patients (in the title, abstract, text, or reference section)
Outcome-related terms: development, validation, or psychometric properties of sleep PROMs designed specifically for people with COPD. Sleep outcome measures not specifically designed for people with COPD but used in a COPD patient group whether psychometric data were reported or not
Method-related terms: instrument* OR measure* OR question* OR scale OR assess
Quality assessment terms: valid* or reliab* or evaluat* OR psychometric.
We also screened the reference lists and citations of included articles to identify additional relevant publications.
Eligibility criteria
To be included in the review, all identified articles had to meet the following inclusion criteria: the article described PROMs that either had been specifically designed and validated for use in patients with COPD or included a generic instrument that had been administered to COPD patients; information on at least one measurement property of the outcome measure was reported; the study sample consisted of adults with a clinical diagnosis of COPD; a full text of the original publication was published electronically, in English, in a peer-reviewed journal.
Articles were excluded if reference to COPD and/or sleep only appeared in the text or reference section. Similarly, we excluded all articles with mixed study samples where the results from COPD patients were not reported separately. Review articles, protocols, and case studies were also excluded. Two investigators (APG and JY) read independently all titles, abstracts, and full texts of all the retrieved articles to determine which were eligible for review. Any disagreements were resolved at a consensus meeting.
Methodological quality assessment
The COSMIN (COnsensus-based Standards for the selection of health Measurement INstruments) checklist21 is a standardized tool for evaluating the methodological quality of PROMs. COSMIN checklists are used to evaluate the measurement properties of instruments in terms of their internal consistency, reliability, measurement error, content validity, structural validity, hypothesis testing, cross-cultural validity, criterion validity, and responsiveness to change. As it was anticipated that the number of PROMs that had been developed and validated for use in COPD populations was likely to be very small, rather than using the full COSMIN checklist we used four PROM characteristics recommended by the US Food and Drug Administration22 to evaluate the measurement properties of identified sleep PROM instruments in relation to their use in COPD patients, ie, conceptual and measurement model, reliability, validity, and responsiveness to change.
Conceptual model
Identified articles were examined for descriptions of concepts contained within the instrument, including the rationale and process for deriving scale scores from raw scores, identifying and dealing with floor and ceiling effects, and scale variability.
Reliability
Articles were scrutinized for estimates of reliability, including inter-item correlations, test-retest repeatability, internal consistency, and/or kappa statistics.
Validity
Any reference to content, construct, and criterion-related validity were noted. When considering construct validity, we also recorded methods to differentiate between people with different levels of lung function or disease severity, such as the Global Initiative for Chronic Obstructive Lung Disease staging system that classifies people with COPD according to the results of pulmonary tests. Where available, we also collected data regarding the relationships between sleep outcome instruments and other established COPD outcome measures (such as the St George’s Respiratory Questionnaire,23 the Medical Research Council Dyspnea scale,24 and routine clinical tests). Any analyses intended to examine dimensionality using factor analysis or Rasch analysis were noted, along with any assessments of differential item functioning that evaluated group differences in PROM item responses.
Responsiveness to change
All data relating to the ability of the instrument to detect changes over time in terms of sleep disturbance were noted. Where correlations between changes in scores of two measures are reported, these had to relate to predefined hypotheses.
Results
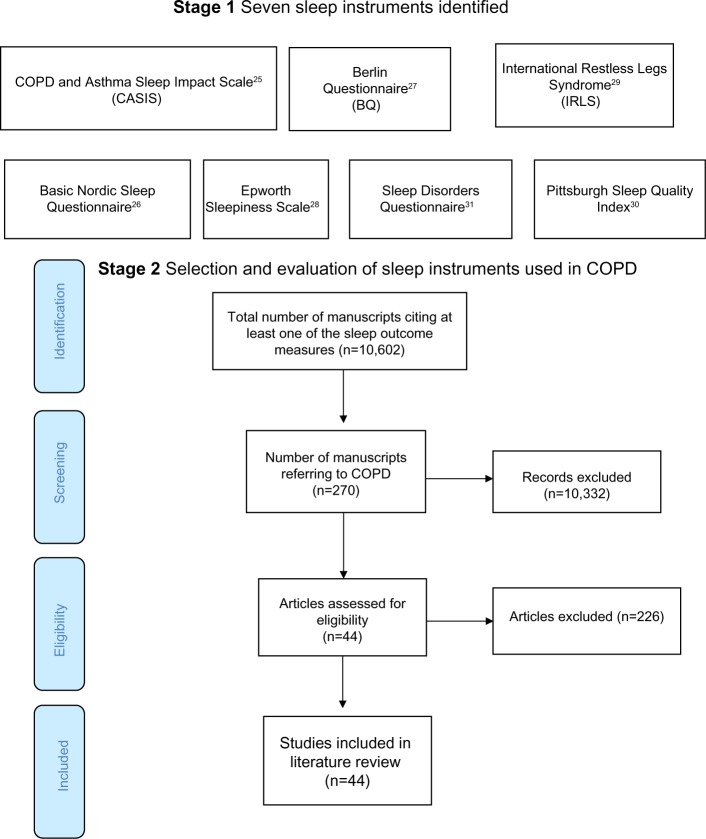
The stage 1 database search identified articles referring to COPD and sleep (Medline 804, EMBASE 2,314, and PsycINFO 59) from which one COPD-specific and six non-disease-specific sleep instruments were identified (Table 2).25–31 In stage 2, the SCOPUS search found 10,602 articles citing any of the seven sleep outcome measures, 270 of which referred to COPD. After applying the exclusion criteria, 44 manuscripts were selected for review (Figure 1). Nearly 90% of the reviewed publications either used the Pittsburgh Sleep Quality Index (PSQI;30 19/44, 43.1%) or the Epworth Sleepiness Scale (ESS; 20/44, 45.5%).28 The 19-item PSQI measures sleep quality in seven domains and the ESS assesses the likelihood of a person dozing off or falling asleep in eight common life situations. Most studies involved patients with moderate-severe COPD recruited from hospital outpatient or specialist respiratory clinics.
Table 2.
Number of papers found and excluded or included in the review
| Outcome measures | SCOPUS references (n) | References to COPD (n) | Excluded | Reviewed |
|---|---|---|---|---|
| COPD and Asthma Sleep Impact Scale25 | 8 | 6 | 5 | 1 |
| Basic Nordic Sleep Questionnaire26 | 200 | 6 | 5 | 1 |
| Berlin Questionnaire27 | 720 | 22 | 21 | 1 |
| Epworth Sleepiness Scale28 | 4,720 | 153 | 133 | 20 |
| International Restless Legs Syndrome29 | 548 | 4 | 3 | 1 |
| Pittsburgh Sleep Quality Index30 | 4,144 | 71 | 52 | 19 |
| Sleep Disorders Questionnaire31 | 262 | 8 | 7 | 1 |
| Total | 10,602 | 270 | 226 | 44 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Figure 1.
Flow diagram showing the total number of studies screened, assessed for eligibility and included in the review.
Abbreviation: COPD, chronic obstructive pulmonary disease.
COPD-specific sleep outcome measures
After assessing the methodological properties of the identified PROMs, only one instrument appeared to have been developed and validated for use in COPD patients, ie, the COPD and Asthma Sleep Impact Scale (CASIS).25
The CASIS is a seven-item measure of sleep impairment during the previous week. Five items relate to disturbance falling asleep or staying awake during the day. The remaining two items concern sleep quality. The items are scored on a five-point scale ranging from 0 if the item never applies, to 4 if the item applies very often. A total raw score is produced from the sum of the seven individual scores which is then linearly transformed to a 0–100 total scale score. A mean CASIS score of 43.3±24.7 was reported in patients with mild COPD. The results of the original psychometric testing of the CASIS (Table 3), showed that the scale had good internal consistency (Cronbach’s alpha 0.91), test-retest reproducibility (intraclass coefficient 0.84), and concurrent validity (correlated with the St George’s Respiratory Questionnaire, r=0.68).
Table 3.
Psychometric properties of COPD and Asthma Sleep Impact Scale
| Conceptual and measurement model | |
| Rationale for deriving scale scores | Items generated from focus group discussions in UK and US samples |
| Scale structure | 15 item scale scored 1= never to 5= very often – transferred onto a 0–100 scale |
| Variability | Mean score COPD patients (n=112) 47.1±24.0 |
| Reliability | |
| Inter-intra observer repeatability | Not tested |
| Item correlations | 9 items highly correlated r>0.75; 6 items indicating item redundancy |
| Internal consistency | Cronbach’s alpha 0.91 |
| Stability over time | 2-week test-retest repeatability ICC 0.84 |
| Validity | |
| Convergent validity | Correlated with SGRQ r=0.68 P=0.0001 Correlations between CASIS scores and number of bad days r=0.61, overall health status (0.5), and higher mean CASIS scores in COPD patients receiving oxygen treatment (51.4 vs 43.3) Correlates with living with COPD questionnaire 0.58 |
| Responsiveness to change | Not tested |
Abbreviations: COPD, chronic obstructive pulmonary disease; CASIS, COPD and Asthma Sleep Impact Scale; ICC, intra-class correlation coefficient; SGRQ, St George’s Respiratory Questionnaire.
None of the non-disease-specific sleep scales reported any tests of reliability or validity to justify their use in the COPD population. Significant associations were observed in only 8/20 (40%) of studies where the ESS was compared with other COPD-related outcome measures. For example, the prevalence of daytime sleepiness (ESS >10) was significantly greater in patients diagnosed with insomnia.11 Compared with people who had obstructive sleep apnea/hypopnea syndrome, COPD patients were more likely to be affected by daytime sleepiness.32 Significant differences in mean ESS scores were observed between patients with COPD and restless legs syndrome compared with controls who had restless legs syndrome.33 However, no differences in daytime sleepiness were observed in a study that compared use of temazepam between COPD patients and controls.34 Similarly, no significant differences in ESS scores were detected in patients with and without restless legs syndrome35 (Table 4).
Table 4.
Summary of studies that used the Epworth Sleepiness Scale
| Reference | Study focus | COPD study sample | Measures of COPD severity | ESS (mean ± SD/median and range) | ESS >10 (%) | Associations with ESS score |
|---|---|---|---|---|---|---|
| Aras et al36 | RLS symptoms in COPD patients during an exacerbation period | 22 male inpatients | GOLD stage IV: FEV1 30% or 50% plus chronic respiratory failure; mean FEV1 39.4%±9.97% | Not reported | Not reported | Free thyroxine values negatively correlated with ESS (rs =−0.481 P=0.043) |
| Bednarek et al46 | Prevalence of SDB and COPD in a representative urban sample aged 41–72 years | 676 participants from the electoral register | FEV1/FEV <0.7, 10.6% | 6.4±3.9 | Not reported | Mean ESS in people with excessive sleep disorder: men 12.6±2.0 versus women 12.9±2.4 (P>0.05) |
| Budhiraja et al11 | Prevalence of insomnia in patients with COPD, and characteristics associated with insomnia in COPD patients | 183 hospital patients | GOLD stage I, 3%; stage II, 39%; stage III, 29%; stage IV, 28%; % predicted post-bronchodilator FEV 45.9±18.6. FEV1/FVC ratio 49.6±12.5 | Not reported | Not reported | Daytime sleepiness (ESS >10) greater in patients with insomnia (36.5% versus 14.6%, P=0.004) |
| Cavalcante et al35 | Occurrence and associations with RLS in a COPD population | 104 hospital outpatient attenders | mMRC 0 (4.8%); 1 and 2 (48.1%); 3 (34.6%); 4 (12.5%) | 6.9±5.1 | 20.2 | No difference in mean values between patients without RLS (6.6±4) versus with RLS (7.7±6.0). ESS positively correlated with BMI (P<0.003) |
| De Lima et al47 | Whether clinically stable COPD patients without cognitive symptoms may present with subtle cognitive impairments | 30 hospital outpatients | Mean FEV1 42.1±15.9 | 6.7±3.7 | Not reported | Not reported |
| Kapella et al39 | Feasibility and assessment of the impact of a CBT intervention for people with COPD and insomnia | 23 patients recruited from advertisements and word of mouth | FEV1/FVC ratio <70% 1 | 9.2±5.0 | Not reported | Not reported |
| Karachaliou et al32 | Association between OSAHS-related symptoms and physician-diagnosed asthma and COPD | 1,501 primary care patients (323 with COPD) | GOLD stage I, 28.8%; stage II, 53.3%; stage III, 15.2%; stage IV, 2.8% | Not reported | Not reported | Increased odds of people with COPD having an ESS score ≥10; OR 2.04, 95% CI (1.33–3.14) |
| Lewis et al48 | Variability of nocturnal desaturation in COPD over a 3-week period and impact the variability may have on clinical decision-making | 26 stable COPD hospital outpatients | Mean post-bronchodilator FEV1 28.6% | 4.1±6.2; range 0–11 | Not reported | Not reported |
| Lewis et al49 | Prevalence and clinical impact of nocturnal desaturation in a typical outpatient population with COPD | 59 COPD outpatients | Mean predicted FEV1 37.2±14.9; FVC 1.9 ±0.9; FVC predicted 62.1±17.6; TB90% 38.4±34.9 | 5.0; range 2.0–8.0 | Not reported | No significant difference between desaturators and nondesaturators (P=0.88) |
| Lo Coco et al33 | Prevalence, severity, and associations with RLS in COPD patients | 87 COPD outpatients | GOLD stage II, 42.5%; stage III, 40.2%; stage IV, 17.3% | 8.98±3.89 | Not reported | Significant difference in mean ESS score between COPD with RLS and controls with RLS 11.81±1.09 versus 8.62±3.66 (P=0.009) |
| McNicholas et al50 | Placebo-controlled, double-blind trial of severe, stable COPD patients comparing the effect of tiotropium on sleeping oxygen saturation | 56 hospital outpatients | FEV1 <65% predicted; FEV1/FVC <70%; Awake paO2 <9.98 kPa (75 mmHg) prior to entry | 5.7 in intervention group versus 6.4 in control group | Not reported | None reported |
| Nunes et al51 | Sleep quality in COPD patients at home using actigraphy and association between sleep quality and daytime somnolence | 26 hospital patients | GOLD stage II, 50%; stage III, 3 8.5%; stage IV, 11.5%; FEV1% predicted 47.62±16.04 | 8.27±4.4 | 61.5 | No difference between COPD and controls (8.27±4.4 versus 6.07±3.9, P=0.12). No difference in proportion with ESS ≥10 COPD (61% versus controls 86%; P=0.09) |
| Oliveira et al52 | Evaluate accuracy of a portable monitoring device in detection of OSA in patients with COPD | 26 hospital outpatients | FEV1/FVC 0.6±0.10; FEV1 (%) post-BD 55±0.08; FVC (%) post-BD 77±8.9 | 10.5±4.1 | Not reported | None reported |
| Scharf et al53 | Correlation between disturbed sleep and COPD | 180 pulmonary clinic patients | GOLD stage I, 10.6%; stage II, 3 0.6%; stage III, 46.1%; stage IV, 12.8%. FEV1 % predicted 47.6±15.2 | 7.0±4.8 | 24.7 | No associations with ESS and other symptoms |
| Soriano et al54 | Natural history of the most common respiratory chronic conditions, including COPD and OSA | 500 primary care patients | GOLD stage I (27%); stage II (58%); stage III (15%) | Not reported | 29.2 | None reported |
| Stege et al34 | Effects of long-term use of a benzodiazepine (temazepam) on breathing, dyspnea, and gas exchange during sleep, sleep quality, and sleepiness | 14 respiratory clinic patients | FEV1 % predicted 33.5±9.2; FEV1/FVC% 32.7±13.0; FEV1 (L) 0.99 ±0.30 | 6.0±4.0 | 50.0 | No difference between temazepam (5.0±4.0) and controls (6.0±4.0; P=0.13) |
| Toraldo et al55 | Pattern of daytime clinical variables that distinguish desaturator patients from nondesaturator COPD patients using cluster analysis | 51 consecutive hospital patients | FEV1 % predicted 53 (SE 1.5); FEV1/FVC ratio 37.6 (SE 0.5); FVC % predicted 81.5 (SE 1.2); AHI 2.8 (SE 0.1). Daytime paO2 values 60–70 mmHg |
3.9 (SE ±0.1) | None | No difference between desaturators and nondesaturators, both 3.8 (± SE 0.4) |
| Toraldo et al56 | Effect of regular use of nCPAP in patients with overlap syndrome | 12 hospital outpatients | FEV1 (%) 60.3±1.3; FEV1/FVC (%) 69.5±0.7 | 16.58±0.86 | Not reported | Reductions in ESS score between baseline and 3 months (16.6±0.86 versus 11.7±0.46; P=0.0001); 3 months and 12 months (11.7±0.46 versus 5.7±0.4; P=0.0001), and 12 and 24 months (5.67±0.4 versus 4.75±0.49; P=0.033) |
| Trauer et al57 | Relationship between 24-hour oximetry and resting partial pressure of oxygen | 35 community-living patients | GOLD stage II, 20%; stage III, 4 9%; and stage IV, 31%; FEV1 % predicted 37.5±13.2 | Median 4 (IQR 2, 8) | Not reported | Negative correlation between ESS and time below 90% SpO2 −24 hours −0.18 (0.29); waking hours −0.13 (0.46); sleeping hours −0.17 (0.24) |
| Tsolaki et al58 | Effect of non-invasive ventilation as an additional treatment for severe COPD patients | 24 hospital outpatients | FEV1 (%) 34.7±11.3; FVC (%) 50.8±15.7 | 9.2±3.7 | Not reported | Significant reductions in ESS score between baseline and 1 month in patients who received noninvasive ventilation (10.3 versus 4.9; P=0.0001). ESS was an independent predictor of the Mental Component Score of the SF-36 (P<0.001) |
Abbreviations: AHI, apnea-hypopnea index; BD, bronchodilator; BMI, body mass index; CI, confidence interval; OR, odds ratio; COPD, chronic obstructive pulmonary disease; RLS, restless legs syndrome; SDB, sleep-disordered breathing; CBT, cognitive behavioral therapy; OSAHS, obstructive sleep apnea/hypopnea syndrome; mMRC, modified Medical Research Council Dyspnoea scale; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; GOLD, Global initiative for chronic Obstructive Lung Disease; SpO2, oxygen saturation; ESS, Epworth Sleepiness Scale; SE, standard error; pO2, oxygen partial pressure; paO2, arterial oxygen tension; IQR, interquartile range; TB90%, time spent with saturation below 90%; SF-36, Short-Form 36 Health Survey; nCPAP, nasal continuous positive airway pressure; SD, standard deviation; OSA, obstructive sleep apnea.
For the PSQI, significant associations were noted in 11/19 (57.9%) of the relevant studies. PSQI scores were found to be significantly higher in patients with restless legs syndrome.36 PSQI total scores also correlated with total scores from the St George’s Respiratory Questionnaire37,14 and the Fatigue Severity Scale.35 In contrast, no correlation was observed between PSQI and St George’s Respiratory Questionnaire scores in an investigation of factors affecting health status in COPD patients with comorbid anxiety or depression.38 Further, although significant PSQI score reductions were observed in patients receiving a course of cognitive behavioral therapy (where the primary outcome was insomnia),39 no reductions in pre- and post-sleep quality were observed in a randomized controlled trial that compared cognitive behavioral therapy with usual care, where sleep was a secondary outcome measure to anxiety and depression40 (Table 5).
Table 5.
Summary of papers that used the Pittsburgh Sleep Quality Index
| Reference | Study focus | COPD study sample | Measures of COPD severity | PSQI (mean ± SD) | PSQI >5 (%) | Associations with PSQI score |
|---|---|---|---|---|---|---|
| Akinci and Yildirim37 | Associations between quality of life and breathlessness, fatigue, sleep quality, and FEV1 % predicted in patients with COPD | 79 stable hospital outpatients | FEV1 (%) 51.5±16.1 (range 18–80); FEV1/FVC (%) 63.4±9.3 (range 34.6–70.2) | 7.1±3.9 | Not reported | Correlations between SGRQ and PSQI total scores (0.428, P<0.001); daytime dysfunction (0.400, P<0.001); sleep disturbance (0.481, P<0.001), habitual sleep efficiency (0.271, P<0.05); sleep latency (0.309, P<0.01); subjective sleep quality (0.421, P<0.001) but not sleep duration or use of sleep medication |
| Aras et al36 | RLS symptoms in COPD patients during an exacerbation | 22 male inpatients | GOLD stage IV: FEV1 30% or 50%, plus chronic respiratory failure; mean FEV1 39.4%±9.97% | 6.0±3.81 | Not reported | PSQI score was higher in patients with RLS symptoms (7.76±3.74) compared with patients without RLS symptoms (3.44±2.18; P<0.05) |
| Cavalcante et al35 | Occurrence and associations with RLS in a COPD population | 104 hospital outpatients | mMRC 0 (4.8%); 1 and 2 (48.1%); 3 (34.6%); 4 (12.5%) | 7.6 ±4.3 | 59.6% | PSQI correlated with Fatigue Severity Scale (P<0.005). Patients with RLS had poor quality sleep (P<0.002). PSQI score correlated with mMRC (P<0.005); higher BMI (P=0.01); serum ferritin (P=0.005). mMRC and creatinine influenced PSQI sleep quality |
| Hynninen et al38 | Factors affecting health status in COPD patients with comorbid anxiety or depression | 58 hospital outpatients/responders to newspaper advertisements | 29 (50%) had mild-moderate COPD and 29 (50%) had severe/very severe COPD. Mean FEV1 53.79±23.96; 50% had FEV1 ≥50% |
Men 8.1±3.6; women 9.2±3.8 | Not reported | PSQI total scores not correlated with SGRQ. PSQI daytime functioning correlated with SGRQ total (0.57, P≤0.001); symptoms (0.374, P≤0.01); activity (0.364, P<0.01); impact (0.56, P≤0.001). PSQI sleep disturbance correlated with SGRQ total (0.404 P≤0.01); symptoms (0.378, P<0.01), and impact (0.409, P≤0.01) |
| Hynninen et al40 | Effect of CBT on anxiety and depression compared with usual care and associations with age and sex | 25 hospital patients/respondents to newspaper advertisements | FEV1 (%) 59.8±21.1 | 9.8±4.4 | Not reported | No significant difference between pre- and post-treatment sleep quality or at 6 months follow-up as a result of the CBT intervention |
| Ito et al59 | Prevalence and associations between depression and sleep disorders in COPD patients and whether depression and sleep disorders are risk factors for exacerbations, hospitalization, and mortality due to COPD | 85 hospital patients | GOLD stage I, 21.2%; stage II, 38.8%; stage III, 28.2%; stage IV, 11.8%. Mean post-BD FEV1 1.6±0.7 L; FVC 3.3±0.9 L; FEV1/FVC % 47.1±13.9 | 5.5±3.3 | 43.5% | PSQI scores higher in COPD versus non-COPD patients (5.5±3.3 versus 4.1±2.6; P=0.0076). An increase in RR in patients with COPD versus non-COPD controls (RR 1.82, 95% CI 1.03–3.22; P=0.042). A weak correlation between PSQI scores and CESD scores (r=0.22; P=0.044). Annual number of exacerbations was higher in COPD patients with depression (3.3±3.5) compared with patients having sleep problems alone |
| Kapella et al39 | Feasibility and impact of a CBT intervention for people with COPD and insomnia | 23 patients recruited from advertisements and word of mouth | FEV1/FVC ratio <70% predicted. Mean FEV1 % predicted 62±18; mean FEV1/FVC 50±10 |
11.0±3.6 | Not reported | PSQI scores reduced following COPD treatment (11.0±3.6 versus 6.5±3.4; P=0.0002). Within group effect size 1.02 |
| Lewis et al48 | Variability of nocturnal desaturation in COPD as measured by OPO and the impact the variability may have on clinical decision-making | 26 stable hospital outpatients | Mean FEV1 % 28.6±10.6 | Not reported | Not reported | No significant association between PSQI and resting pO2 (P=0.89) |
| Lewis et al49 | Prevalence and clinical impact of nocturnal desaturation in COPD outpatients | 59 consecutive outpatient and pulmonary rehabilitation patients | FEV1 <60% predicted and FEV1/FVC <70% predicted; mean FEV1 % predicted 37.2±14.9; mean FVC % predicted 37.2±14.9; mean FEV1 0.9±0.4 L | Median 7 (IQR 4, 11) | 61% | No significant difference in PSQI total score between desaturators and nondesaturators (8 [IQR 4, 11] versus 7 [IQR 4, 11]; P=0.63) |
| Nisbet et al60 | Occurrence of overnight desaturation; if resting oxygen saturation predicts overnight desaturation and whether desaturation correlates with HRQoL and sleep quality | 38 consecutive outpatient and pulmonary rehabilitation patients | FEV1 <60% predicted and FEV1/FVC ratio <70% predicted. Mean FEV1 (L) 0.8±0.37; mean FVC (L) 2.1±0.65 |
7.1±3.99 | Not reported | No significant difference in PSQI total score between desaturators and nondesaturators (6.7±3.78 versus 7.1±3.99; P=0.82) |
| Nunes et al14 | Impact of sleep quality on HRQoL in COPD | 30 hospital COPD patients | GOLD stage II, 50.0%; stage III, 33.3%; stage IV, 16.7%; mean FEV1% predicted 48.55±17.27 FEV1/FVC % 52.11±9.85 | 7.37±3.6 | 70.0% | Significant positive correlation between PSQI total score and SGRQ total score (r=0.42; P=0.02) and impact domain score (r=0.47; P=0.01); global PSQI score was a predictor of SGRQ total score (adjusted r2 0.373, P=0.001) and SGRQ impact score (adjusted r2 0.329, P=0.001) |
| Nunes et al51 | Sleep quality in COPD patients at home using actigraphy and association between sleep quality and daytime somnolence | 26 stable respiratory outpatients | GOLD stage II, 50%; stage III, 38.5%; stage IV, 11.5%; mean FEV1 % predicted 47.62±16.04 | 6.96±3.5 | 57.7% | Mean PSQI total score significantly worse in COPD than in controls (6.96±3.5 versus 4.8±2.4; P=0.043); no significant correlation between PSQI and actigraphy variables |
| Oh et al61 | Characteristics of fatigue in patients with chronic lung disease | 128 hospital patients, 80% of whom had COPD, 13% had bronchiectasis, and 4% had interstitial lung disease | Mean FEV1 64.5±28.8 | Mean score 1.9±0.7 (range 0–3) | Not reported | In the regression analysis, sleep quality was not independently associated with fatigue; standardized β coefficient 0.02: t=0.25 P=0.8 |
| Reishtein62 | Impact of dyspnea, fatigue, and sleep difficulty on functional performance | 30 home and 47 clinic patients | FEV1 <60% predicted; mean FEV1 41.2±11.79 | 8.69±4.33 | Not reported | Weak non-significant correlation between sleep difficulty and functional performance (−0.17, P>0.05) |
| Scharf et al53 | Extent of sleep problems in COPD; predictors of HRQoL and the contribution of sleep disturbance to HRQoL | 180 pulmonary clinic patients | GOLD stage I, 10.6%; stage II, 30.6%; stage III, 46.1%; stage IV, 12.8%; mean FVC% predicted 64.7±16.3; FEV1/FVC % 57.5 ±12.3; FEV1 (L) 1.24±0.50 FVC (L) 2.17±0.70 | 11.0±5.4 | 77.7 | HRQoL and SGRQ scores significantly associated with PSQI (adjusted r2 HRQoL 0.24 and SGRQ 0.23 both P<0.0001). HRQoL and SGRQ were independently associated with PSQI score (r2 0.06, P=0.0002 and r2 0.05, P=0.0005, respectively) |
| Suh et al63 | Effect of anxiety on heart rate variability, depression, and sleep in COPD | 30 COPD pulmonary rehabilitation patients and 30 non-COPD controls | COPD patients with anxiety PSQI 12.0 (4.06) versus healthy patients with anxiety 7.8 (4.02) GOLD criteria: stage I, 13.3%; stage 2, 43.3%; stage 3, 30%; stage 4, 13.3% | 12.0±4.02 | Not reported | COPD patients with anxiety had poorer sleep quality than non-COPD controls with anxiety (12.0 versus 7.8; t=2.74, P=0.01). The COPD only group had significantly lower PSQI scores than COPD anxiety group (6.9 versus 12.0; t=−3.49, P=0.002) |
| Soler et al64 | PR improves sleep quality in chronic lung disease | 46 obstructive 13 restrictive 5 mixed |
FEV1 % predictive Obstructive 44.2 (12.3) Restrictive 82.2 (6.8) Mixed 62.7 (11.5) |
6.6 (3.9) obstructive 8.2 (3.7) restrictive 6.6 (4.7) mixed |
58 | Poor sleep quality was reported by 58% of patients before PR and 47% after PR (P<0.001) |
| Halvani et al65 | Evaluation of exogenous melatonin administration in improvement of sleep quality in patients with COPD | 48 stable hospital patients | Confirmed diagnosis of GOLD stage II, GOLD stage IV | Intervention 11.63±3.96 Controls 10.66±2.48 |
Not reported | Melatonin group Baseline 11.63±3.96; follow-up 8.7±4.15 (P=0.002). Placebo group Baseline 10.66±2.48; follow-up 10.11±2.66 (P=0.065) |
| Bhatt et al66 | NPPV in subjects with stable COPD | 15 stable hospital patients who received NPPV versus 12 controls | FEV1/FVC ratio <0.70 PaCO2 <52 mmHg | 3.7 (3.0) NPPV 6.1 (3.2) Controls |
Not reported | Results after 6 months NPPV 3.7 (3.0) versus 3.4 (2.0; P=0.2) Controls 6.1 (3.3) vs 5.7 (3.2) P=0.77 |
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; RLS, restless legs syndrome; mMRC, modified Medical Research Council Dyspnea scale; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; GOLD, Global initiative for chronic Obstructive Lung Disease; IQR, interquartile range; nCPAP, nasal continuous positive airway pressure; PR, pulmonary rehabilitation; RR, relative risk; BD, bronchodilator; CBT, cognitive behavioral therapy; SD, standard deviation; NPPV, noninvasive positive pressure ventilation; PaCO2, partial pressure of carbon dioxide; HRQoL, health-related quality of life; PSQI, Pittsburgh Sleep Quality Index; OPO, overnight pulse oximetry; SGRQ, St George’s Respiratory Questionnaire; CESD, Center for Epidemiologic Studies-Depression.
Table 6 shows the papers that used the four remaining generic outcome measures in studies of COPD patients.33,35,41,42 With so few studies, there are currently insufficient data to evaluate the utility of these instruments; however, in one study,33 International Restless Leg Study Group scores correlated significantly with ESS scores.
Table 6.
Summary of articles using generic sleep measures
| Outcome measure and authors | Study focus | COPD study sample | Measures of COPD severity | Outcome reported (mean ± SD) | Associations |
|---|---|---|---|---|---|
| Berlin Questionnaire Cavalcante et al35 | Occurrence and associations with RLS in a COPD population | 104 hospital outpatient attenders | mMRC 0 (4.8%); 1 and 2 (48.1%); 3 (34.6%); 4 (12.5%) | 30 (29%) of patients had a high probability of OSA | Risk of OSA not associated with RLS (P=0.25) |
| BNSQ Saaresranta et al41 | Sleep quality and excessive daytime sleepiness in ambulatory patients with moderate to severe COPD | 15 consecutive female clinic outpatients | FEV1 predicted <65% of daytime hypoxemia (PaO2 <10.0 kPa) and/or hypercapnia (PaCO2 >6.0 kPa); FEV1 % 36±12; FVC % 63±14; FEV1 (L) 0.73±0.45 (range 0.25–1.8); FVC (L) 1.3±0.45 | BNSQ 9.9±3.0 | BNSQ score higher than controls (9.9±3.0 versus 7.6±3.2; P=0.025) and correlated with insulin levels (r=0.59, P=0.027) and body movements (r=0.52, P=0.047) |
| IRLSG Lo Coco et al33 | Prevalence, severity, and associations with RLS in COPD patients | 87 COPD outpatients | GOLD stage II, 42.5%; stage III, 40.2%; stage IV, 17.3% | IRLSG score 32 (36.8%) | IRLSG score in COPD patients 20.5±2.8 versus 18.0±3.5 in controls (P=0.016); moderate correlation between ESS and IRLSG score (Spearman correlation 0.489, P=0.01) |
| SDQ Valipour et al42 | Differences in symptoms and polysomnographic parameters in COPD patients | 52 consecutive hospital outpatients | FEV1% predicted 60±10; FVC % predicted 93±12; FEV1/FVC 60±8 | SA 36.0±6.9 PLM 25.2±7.1 PSY 18.0±6.0 Narcolepsy 22.1±5.5 |
COPD patients had higher scores in PLM (25.2±7.1 versus 21.1±6.2, P=0.0003) and PSY (18.0±6.0 versus 15.3±5.0, P=0.035) Minimum SaO2 had an independent effect on SDQ subscale scores: SA (P=0.045), PLM (P=0.051), PSY (P=0.037), and narcolepsy (P=0.053) Mean overnight SaO2 was a significant predictor of PSY score (P=0.015) |
Abbreviations: BNSQ, Basic Nordic Sleep Questionnaire; COPD, chronic obstructive pulmonary disease; PLM, periodic limb movement; SDQ, Sleep Disorders Questionnaire; PSY, psychiatric sleep disorder; RLS, restless leg syndrome; IRLSG, International Restless Leg Study Group; SA, sleep apnea; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; OSA, obstructive sleep apnea; mMRC, modified Medical Research Council Dyspnea scale; GOLD, Global initiative for chronic Obstructive Lung Disease; SaO2, arterial oxygen saturation; ESS, Epworth Sleepiness Scale; PaCO2, partial pressure of carbon dioxide; PaO2; hypercapnia PaCO.
Although the results provide some evidence of the validity of measures of sleep disturbance in people with COPD, none of the above sleep measures were specifically evaluated for people with COPD. Similarly, we did not find any articles that provided data on test-retest, intrarater, or inter-rater reliability or responsiveness to change among COPD patient groups.
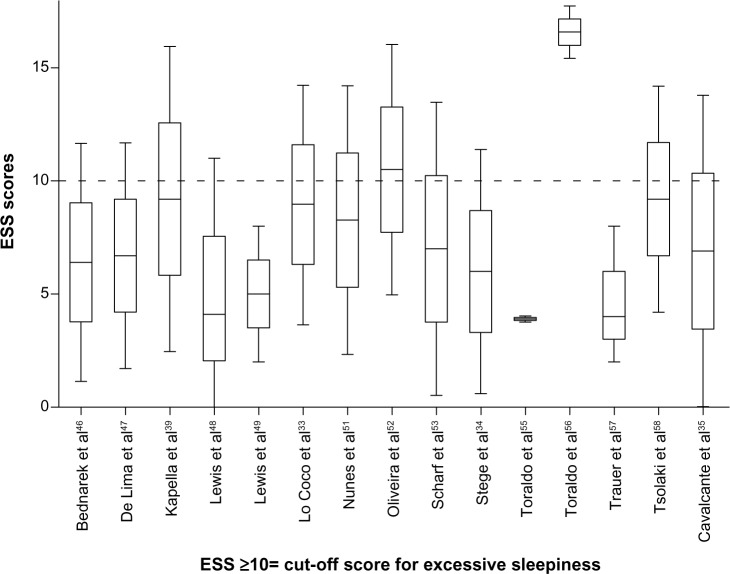
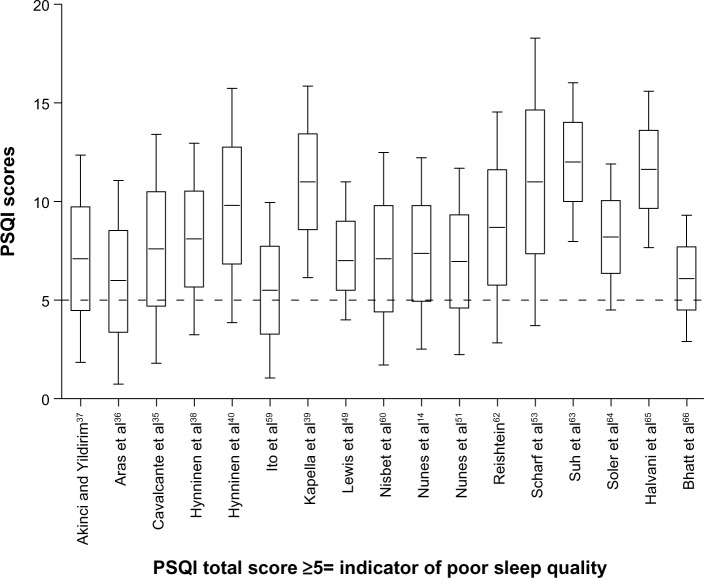
The point estimates of sleep disturbance from clinical studies of COPD patients using the ESS and the PSQI are shown on Tables 4 and 5. In 13/15 of the studies using the ESS, the mean/median scores were less than 10, ie, below the accepted cutoff value for excessive daytime sleepiness.28 Most of the observed PSQI scores were above 5, ie, above the cutoff value representing poor quality sleep.30 Point and upper and lower quartile estimates for the ESS and PSQI are displayed on Figures 2 and 3.
Figure 2.
Point estimates and variability in studies that used the ESS.
Abbreviation: ESS, Epworth Sleepiness Scale.
Figure 3.
Point estimates and variability in studies that used the PSQI.
Abbreviation: PSQI, Pittsburgh Sleep Quality Index.
Discussion
Sleep disturbances are an important problem that can seriously impact on physical and mental well-being as well as quality of life for people with COPD. This review identified seven outcome measures that have been used in COPD populations but none has been sufficiently validated to satisfy US Food and Drug Administration requirements to support labeling claims in medical product development. Only one measure, the CASIS, included item response theory modeling when evaluating the psychometric evaluation of the instrument. Incorporating item response theory is now considered to be an essential component in the design and validation of all PROMs.19
The majority of sleep studies in COPD have relied on two general measures of sleep dysfunction, the ESS and the PSQI, and although both of these instruments have been extensively used in a variety of clinical populations, neither has been validated for use in COPD patients.
As far as we are aware, this is the first systematic review of sleep measures in COPD. A strength of this study was the comprehensiveness of our literature search. We believe that we have identified all of the main PROMs of sleep disorders that have been used in COPD populations. Nevertheless, as we did not search all electronic databases or carry out a hand search, there is the possibility that we may have missed some relevant articles, particularly those that appeared in non-English language journals. However, by cross-checking the reference lists of all included papers and that of a recent systematic review of instruments designed to measure sleep dysfunction in adults,15 we believe we have minimized the loss of any important papers.
The review identified only one PROM, ie, the CASIS, which has been specifically designed and validated for use in COPD patients. In each item of the CASIS, patients are advised to “[…] think about the impact of breathing problems/COPD/asthma on your sleep during the past week […]” Most items, however, are general in nature and relate to the frequency of symptoms such as falling asleep, staying awake, and waking up feeling rested. Only one item relates specifically to breathing problems, ie, shortness of breath, coughing, and chest tightness. Further, as these symptoms are all contained within the same item, it is not possible to differentiate patients who may have different severity of symptoms; for example, between patients who wake up at night only with shortness of breath or wake up with both shortness of breath and coughing. Since the publication of the original paper, the CASIS has not been used in any intervention studies, so further evidence is needed to confirm the utility of this instrument in guiding the clinical management of COPD patients and in research.
This review has highlighted the current reliance of sleep research on generic sleep measures and the paucity of disease-specific instruments currently available to assess the patient’s experience of sleep in relation to COPD. By definition, generic measures tend to cover broad aspects such as functional status and perceptions and are more likely to identify aspects that are not disease-related. Because instruments validated in one population may not perform well in specific populations under investigation, separate validation of generic measures in each population is recommended.43 Similarly, given that disease-specific measures are generally more responsive to change, outcomes based solely on generic measures are unlikely to detect treatment-related improvements.44 These deficiencies could call into question findings from previous research on the impact of sleep problems in COPD. The need for validated COPD-specific sleep outcome measures was emphasized in an expert panel meeting held in 2011.45 While appreciating the multifactorial nature of sleep disturbance in COPD, the panel highlighted the need for an instrument to classify patients according to their night or daytime symptoms, which is not possible using existing PROMs for sleep. Development work on new COPD sleep PROMs to address these limitations is currently being carried out by the authors of this review.
Conclusion
This review highlights the complexity of sleep assessment, the inadequacy of non-disease-specific measures to capture problems experienced by people with COPD, and the absence of robust and validated methods of assessing and classifying symptoms associated with disrupted sleep in COPD. In studies using non-disease-specific sleep measures, there is a pressing need for these to be validated with COPD populations and/or for new disease-specific PROMs to be developed.
Acknowledgments
This partnership received financial support from the Knowledge Transfer Partnerships (KTP) program. The project was also supported by the UK Medical Research Council. KTP aims to help businesses to improve their competitiveness and productivity through the better use of knowledge, technology, and skills that reside within the UK knowledge base. KTP is funded by the Technology Strategy Board along with other government funding organizations.
Author contributions
All authors contributed to the design of the review. APG carried out the literature searches, produced draft manuscripts for review, and edited the manuscript prior to submission. JY read and verified the suitability of the articles for review and also participated in consensus meetings. ST provided guidance and editorial support during preparation of the review. All authors contributed and approved the final version of the manuscript. ST and JY are guarantors of the paper, taking responsibility for the integrity of the work as a whole from inception to the published article.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kinsman RA, Yaroush RA, Fernandez E, Dirks JF, Schocket M, Fukuhara J. Symptoms and experiences in chronic bronchitis and emphysema. Chest. 1983;83:755–761. doi: 10.1378/chest.83.5.755. [DOI] [PubMed] [Google Scholar]
- 2.Omachi TA, Blanc PD, Claman DM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13:476–483. doi: 10.1016/j.sleep.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health Information about sleep. 2013. [Accessed July 9, 2013]. Available from: http://science.education.nih.gov/supplements/nih3/sleep/guide/info-sleep.htm.
- 4.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–280. [PubMed] [Google Scholar]
- 5.Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res. 2000;9:35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 6.Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest. 1987;91:540–546. doi: 10.1378/chest.91.4.540. [DOI] [PubMed] [Google Scholar]
- 7.Allen RP, Earley CJ. Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol. 2001;18:128–147. doi: 10.1097/00004691-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan Y, Inonu H, Yilmaz A, Ocal S. Restless legs syndrome in patients with chronic obstructive pulmonary disease. Can J Neurol Sci. 2008;35:352–357. doi: 10.1017/s0317167100008957. [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelic S. Diagnostic and therapeutic approach to coexistent chronic obstructive pulmonary disease and obstructive sleep apnea. Int J Chron Obstruct Pulmon Dis. 2008;3:269–275. doi: 10.2147/copd.s2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budhiraja R, Parthasarathy S, Budhiraja P, Habib MP, Wendel C, Quan SF. Insomnia in patients with COPD. Sleep. 2012;35:369–375. doi: 10.5665/sleep.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali Zohal M, Yazdi Z, Kazemifar AM. Daytime sleepiness and quality of sleep in patients with COPD compared to control group. Glob J Health Sci. 2013;5:150–155. doi: 10.5539/gjhs.v5n3p150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med. 2009;180:692–700. doi: 10.1164/rccm.200903-0347PP. [DOI] [PubMed] [Google Scholar]
- 14.Nunes DM, Mota RM, de Pontes Neto OL, Pereira ED, de Bruin VM, de Bruin PF. Impaired sleep reduces quality of life in chronic obstructive pulmonary disease. Lung. 2009;187:159–163. doi: 10.1007/s00408-009-9147-5. [DOI] [PubMed] [Google Scholar]
- 15.Jones P, Miravitlles M, van der Molen T, Kulich K. Beyond FEV(1) in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis. 2012;7:697–709. doi: 10.2147/COPD.S32675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Service Patient reported outcome measures: their role in measuring and improving patient experience. 2012. [Accessed April 3, 2014]. Available from: http://patientexperienceportal.org/article/patient-reported-outcome-measures-their-role-in-measuring-and-improving-patient-experience.
- 17.Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2010;63:1195–1204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Academy of Sleep Medicine . International classification of sleep disorders, revised: diagnostic and coding manual. Chicago, IL, USA: American Academy of Sleep Medicine; 2001. [Accessed April 4, 2014]. Available from: http://www.esst.org/adds/ICSD.pdf. [Google Scholar]
- 19.Devine EB, Hakim Z, Green J. A systematic review of patient-reported outcome instruments measuring sleep dysfunction in adults. Pharmacoeconomics. 2005;23:889–912. doi: 10.2165/00019053-200523090-00003. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokkink LB, Terwee CB, Patrick DL, et al. International consensus on taxonomy, terminology, and definitions of measurement properties: results of the COSMIN study. J Clin Epidemiol. 2010;63:737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services Food and Drug Administration Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. 2009. [Accessed April 30, 2014]. Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf.
- 23.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. BMJ. 1959;2:257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pokrzywinski RF, Meads DM, McKenna SP, Glendenning GA, Revicki DA. Development and psychometric assessment of the COPD and Asthma Sleep Impact Scale (CASIS) Health Qual Life Outcomes. 2009;7:98. doi: 10.1186/1477-7525-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4:150–155. doi: 10.1111/j.1365-2869.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 27.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Walters AS, LeBrocq C, Dhar A, et al. International Restless Legs Syndrome Study Group Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 32.Karachaliou F, Kostikas K, Pastaka C, Bagiatis V, Gourgoulianis KI. Prevalence of sleep-related symptoms in a primary care population – their relation to asthma and COPD. Prim Care Respir J. 2007;16:222–228. doi: 10.3132/pcrj.2007.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo Coco D, Mattaliano A, Coco AL, Randisi B. Increased frequency of restless legs syndrome in chronic obstructive pulmonary disease patients. Sleep Med. 2009;10:572–576. doi: 10.1016/j.sleep.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Stege G, Heijdra YF, van den Elshout FJ, et al. Temazepam 10 mg does not affect breathing and gas exchange in patients with severe normocapnic COPD. Respir Med. 2010;104:518–524. doi: 10.1016/j.rmed.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Cavalcante AG, de Bruin PF, de Bruin VM, et al. Restless legs syndrome, sleep impairment, and fatigue in chronic obstructive pulmonary disease. Sleep Med. 2012;13:842–847. doi: 10.1016/j.sleep.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Aras G, Kadakal F, Purisa S, Kanmaz D, Aynaci A, Isik E. Are we aware of restless legs syndrome in COPD patients who are in an exacerbation period? Frequency and probable factors related to underlying mechanism. COPD. 2011;8:437–443. doi: 10.3109/15412555.2011.623737. [DOI] [PubMed] [Google Scholar]
- 37.Akinci AC, Yildirim E. Factors affecting health status in patients with chronic obstructive pulmonary disease. Int J Nurs Pract. 2013;19:31–38. doi: 10.1111/ijn.12034. [DOI] [PubMed] [Google Scholar]
- 38.Hynninen MJ, Pallesen S, Nordhus IH. Factors affecting health status in COPD patients with co-morbid anxiety or depression. Int J Chron Obstruct Pulmon Dis. 2007;2:323–328. [PMC free article] [PubMed] [Google Scholar]
- 39.Kapella MC, Herdegen JJ, Perlis ML, et al. Cognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. Int J Chron Obstruct Pulmon Dis. 2011;6:625–635. doi: 10.2147/COPD.S24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hynninen MJ, Bjerke N, Pallesen S, Bakke PS, Nordhus IH. A randomized controlled trial of cognitive behavioral therapy for anxiety and depression in COPD. Respir Med. 2010;104:986–994. doi: 10.1016/j.rmed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 41.Saaresranta T, Irjala K, Aittokallio T, Polo O. Sleep quality, daytime sleepiness and fasting insulin levels in women with chronic obstructive pulmonary disease. Resp Med. 2005;99:856–863. doi: 10.1016/j.rmed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Valipour A, Lavie P, Lothaller H, Mikulic I, Burghuber OC. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med. 2011;12:367–372. doi: 10.1016/j.sleep.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Matza LS, Boye KS, Yurgin N. Validation of two generic patient-reported outcome measures in patients with type 2 diabetes. Health Qual Life Outcomes. 2007;5:47. doi: 10.1186/1477-7525-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiebe S, Guyatt G, Weaver B, Matijevic S, Sidwell C. Comparative responsiveness of generic and specific quality-of-life instruments. J Clin Epidemiol. 2003;56:52–60. doi: 10.1016/s0895-4356(02)00537-1. [DOI] [PubMed] [Google Scholar]
- 45.Agusti A, Hedner J, Marin JM, et al. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20:183–194. doi: 10.1183/09059180.00004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration. 2005;72:142–149. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 47.De Lima OM, Oliveira-Souza Rd, Santos Oda R, Moraes PA, Sá LF, Nascimento OJ. Subclinical encephalopathy in chronic obstructive pulmonary disease. Arq Neuropsiquiatr. 2007;65:1154–1157. doi: 10.1590/s0004-282x2007000700012. [DOI] [PubMed] [Google Scholar]
- 48.Lewis CA, Eaton TE, Fergusson W, Whyte KF, Garrett JE, Kolbe J. Home overnight pulse oximetry in patients with COPD: more than one recording may be needed. Chest. 2003;123:1127–1133. doi: 10.1378/chest.123.4.1127. [DOI] [PubMed] [Google Scholar]
- 49.Lewis CA, Fergusson W, Eaton T, Zeng I, Kolbe J. Isolated nocturnal desaturation in COPD: prevalence and impact on quality of life and sleep. Thorax. 2009;64:133–138. doi: 10.1136/thx.2007.088930. [DOI] [PubMed] [Google Scholar]
- 50.McNicholas WT, Calverley PM, Lee A, Edwards JC, Tiotropium Sleep, Study in COPD Investigators Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J. 2004;23:825–831. doi: 10.1183/09031936.04.00085804. [DOI] [PubMed] [Google Scholar]
- 51.Nunes DM, De Bruin VM, Louzada FM, et al. Actigraphic assessment of sleep in chronic obstructive pulmonary disease. Sleep Breath. 2013;17:125–132. doi: 10.1007/s11325-012-0660-z. [DOI] [PubMed] [Google Scholar]
- 52.Oliveira MG, Nery LE, Santos-Silva R, et al. Is portable monitoring accurate in the diagnosis of obstructive sleep apnea syndrome in chronic pulmonary obstructive disease? Sleep Med. 2012;13:1033–1038. doi: 10.1016/j.sleep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Scharf SM, Maimon N, Simon-Tuval T, Bernhard-Scharf BJ, Reuveni H, Tarasiuk A. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:1–12. doi: 10.2147/COPD.S15666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soriano JB, Yanez A, Renom F, et al. Set-up and pilot of a population cohort for the study of the natural history of COPD and OSA: the PULSAIB study. Prim Care Respir J. 2010;19:140–147. doi: 10.4104/pcrj.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toraldo DM, Nicolardi G, De Nuccio F, Lorenzo R, Ambrosino N. Pattern of variables describing desaturator COPD patients, as revealed by cluster analysis. Chest. 2005;128:3828–3837. doi: 10.1378/chest.128.6.3828. [DOI] [PubMed] [Google Scholar]
- 56.Toraldo DM, De Nuccio F, Nicolardi G. Fixed-pressure nCPAP in patients with obstructive sleep apnea (OSA) syndrome and chronic obstructive pulmonary disease (COPD): a 24-month follow-up study. Sleep Breath. 2010;14:115–123. doi: 10.1007/s11325-009-0291-1. [DOI] [PubMed] [Google Scholar]
- 57.Trauer JM, Gielen C, Trauer T, Steinfort CL. Inability of single resting arterial blood gas to predict significant hypoxaemia in chronic obstructive pulmonary disease. Intern Med J. 2012;42:387–394. doi: 10.1111/j.1445-5994.2010.02405.x. [DOI] [PubMed] [Google Scholar]
- 58.Tsolaki V, Pastaka C, Karetsi E, et al. One-year non-invasive ventilation in chronic hypercapnic COPD: effect on quality of life. Respir Med. 2008;102:904–911. doi: 10.1016/j.rmed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Ito K, Kawayama T, Shoji Y, et al. Depression, but not sleep disorder, is an independent factor affecting exacerbations and hospitalization in patients with chronic obstructive pulmonary disease. Respirology. 2012;17:940–949. doi: 10.1111/j.1440-1843.2012.02190.x. [DOI] [PubMed] [Google Scholar]
- 60.Nisbet M, Eaton T, Lewis C, Fergusson W, Kolbe J. Overnight prescription of oxygen in long term oxygen therapy: time to reconsider the guidelines? Thorax. 2006;61:779–782. doi: 10.1136/thx.2005.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh EG, Kim CJ, Lee WH, Kim SS. Correlates of fatigue in Koreans with chronic lung disease. Heart Lung. 2004;33:13–20. doi: 10.1016/j.hrtlng.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Reishtein JL. Relationship between symptoms and functional performance in COPD. Res Nurs Health. 2005;28:39–47. doi: 10.1002/nur.20054. [DOI] [PubMed] [Google Scholar]
- 63.Suh S, Ellis RJ, Sollers JJ, 3rd, Thayer JF, Yang HC, Emery CF. The effect of anxiety on heart rate variability, depression, and sleep in chronic obstructive pulmonary disease. J Psychosom Res. 2013;74:407–413. doi: 10.1016/j.jpsychores.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 64.Soler X, Diaz-Piedra C, Ries AL. Pulmonary rehabilitation improves sleep quality in chronic lung disease. COPD. 2013;10:156–163. doi: 10.3109/15412555.2012.729622. [DOI] [PubMed] [Google Scholar]
- 65.Halvani A, Mohsenpour F, Nasiriani K. Evaluation of exogenous melatonin administration in improvement of sleep quality in patients with chronic obstructive pulmonary disease. Tanaffos. 2013;12:9–15. [PMC free article] [PubMed] [Google Scholar]
- 66.Bhatt SP, Peterson MW, Wilson JS, Durairaj L. Noninvasive positive pressure ventilation in subjects with stable COPD: a randomized trial. Int J Chron Obstruct Pulmon Dis. 2013;8:581–589. doi: 10.2147/COPD.S53619. [DOI] [PMC free article] [PubMed] [Google Scholar]