Abstract
Background
Abnormal resting arterial stiffness is present in middle‐aged and elderly persons with abnormalities of fasting glucose (diabetes or impaired fasting glucose) and is associated with exercise intolerance. We sought to determine whether these same persons exhibited stress‐related abnormalities of arterial stiffness.
Methods and Results
We analyzed dobutamine magnetic resonance stress imaging results from 373 consecutively recruited persons aged 55 to 85 years with normal fasting glucose, impaired fasting glucose, or diabetes who were at risk for but without symptomatic heart failure. Personnel blinded to participant identifiers measured arterial stiffness (brachial pulse pressure/left ventricular stroke volume indexed to body surface area, the aortic elastance index [brachial end‐systolic pressure/left ventricular stroke volume indexed to body surface area], and thoracic aortic distensibility) at 80% of the maximum predicted heart rate response for age. Participants averaged 69±8 years of age; 79% were white, 92% were hypertensive, and 66% were women. After accounting for hypertension, sex, coronary artery disease, smoking, medications, hypercholesterolemia, and visceral fat, we observed an effect of glycemic status for stress measures of arterial stiffness in those with diabetes and impaired fasting glucose relative to those with normal fasting glucose (P=0.002, P=0.02, and P=0.003, respectively).
Conclusion
Middle‐ and older‐aged individuals with diabetes or impaired fasting glucose have higher stress measures of arterial stiffness than those with normal fasting glucose. These data emphasize the need for future studies with larger sample sizes to determine whether stress‐related elevations in arterial stiffness are related to exercise intolerance and future episodes of heart failure experienced by those with abnormalities of fasting glucose.
Clinical Trial Registration
URL: http://clinicaltrials.gov/. Unique identifier: NCT00542503.
Keywords: arterial stiffness, diabetes, dobutamine, elderly
Introduction
Although symptomatic congestive heart failure (HF) occurs more frequently in people who are diabetic and contributes to their mortality and morbidity,1–4 factors and mechanisms that precipitate the onset of HF in these persons are incompletely understood. Those with diabetes often exhibit abnormal resting stiffness of their proximal thoracic aorta.5 This condition is associated with advanced age and systemic hypertension (HTN), increases left ventricular (LV) afterload,6 diminishes coronary artery perfusion,7–8 stimulates LV hypertrophy,9–11 and impairs LV diastolic filling,11–12 all of which can impair LV performance.13
Although resting measures of aortic stiffness are increased in middle‐aged and older persons with abnormalities of fasting glucose, it is uncertain whether the same persons may experience abnormalities of stress‐related measures of aortic stiffness. This question is important to address for 2 reasons. First, in those with abnormalities of fasting glucose, the aorta exhibits enhanced distensibility and reduced stiffness during stress in order to propagate blood to working skeletal muscle. Second, the mechanisms of and contributors to exercise intolerance and the onset of symptomatic HF in those with abnormalities of fasting glucose are not completely understood.13–17
We sought to measure stress‐induced metrics of thoracic aortic stiffening in middle‐aged and older persons with abnormalities of fasting glucose at high risk for but yet to experience a first episode of symptomatic HF. Our goal was to compare these metrics with those of other similarly aged persons with normal fasting glucose (NFG) who were also at risk for a first episode of symptomatic HF.13 To accomplish this objective, we performed dobutamine stress cardiovascular magnetic resonance (CMR) and measured changes in thoracic aortic stiffness in middle‐aged and older persons with diabetes mellitus (DM), impaired fasting glucose (IFG), and NFG at high risk for developing incident HF.
Methods
Study Design
The study was approved by the institutional review board of Wake Forest Health Sciences, and each participant provided witnessed, written informed consent. The study was registered with ClinicalTrials.gov (identifier NCT00542503) and was performed in accordance with National Institutes of Health grants R01HL076438 and P30AG021332. The purpose of this joint initiative was to use advanced CMR imaging techniques to identify rest and stress‐induced cardiac and vascular abnormalities in middle‐aged and older persons that may forecast the development of HF. To achieve this purpose, those at risk for but yet to develop symptomatic HF were interviewed, and baseline historical, physical examination, laboratory, and demographic data were obtained. This was followed by the administration and analysis of a dobutamine stress CMR pharmacological stress test designed to assess cardiac and vascular function.
Study Population
The study included participants from rural counties of central and western North Carolina who (1) exhibited risk factors for but had yet to develop symptomatic HF; (2) had no contraindication to intravenous dobutamine administration; (3) had no contraindication for dobutamine stress CMR, such as CMR‐incompatible biometallic implants or claustrophobia; and (4) had a resting LV ejection fraction >45%. Study participants were recruited through newspaper and television advertisements and mailings to randomly selected persons aged >55 years within Forsyth, Davie, and Davidson counties of North Carolina. Identification of those at risk for HF was substantiated through interview and review of medical records to verify the presence (according to established diagnostic criteria) of coronary artery disease (CAD),14 diabetes,15 or HTN16 of >5‐year duration. Absence of symptomatic HF was confirmed by thorough review of participants' medical records. Participants then underwent a dobutamine stress CMR examination in which each participant achieved 80% of the maximum predicted heart rate (HR) response for age without evidence of an inducible LV wall motion abnormality indicative of ischemia.
Study participants were characterized into 1 of 3 fasting glucose groups according to criteria established by the American Diabetes Association15: (1) NFG, defined as those without a history of diabetes and a fasting serum glucose level <100 mg/dL; (2) IFG, defined as those without a history of diabetes and a fasting serum glucose level between 100 and 125 mg/dL; or (3) DM, defined as those with a previous clinical diagnosis of DM substantiated by a fasting glucose level >125 mg/dL or receipt of insulin or hypoglycemic drugs regardless of fasting glucose level.
Dobutamine/Atropine Stress Cardiac Magnetic Resonance Protocol
The dobutamine stress CMR protocol was accomplished according to previously published techniques.17–18 Dobutamine CMR images were acquired on a 1.5‐T (Siemens Avanto) whole‐body imaging system with a phased‐array cardiothoracic surface coil applied around the chest. Dobutamine was infused incrementally from low dose (7.5 μg/kg per minute) to peak dose (20 to 40 μg/kg per minute), with atropine administered (up to 1.5 mg) to achieve 80% of the maximum predicted HR response for age. Cine imaging of the LV short‐ and long‐axis views (2‐, 3‐, and 4‐chamber) were accomplished at rest, at low‐ and peak‐dose dobutamine, and then after 10 minutes of recovery. Brachial systolic and diastolic blood pressures were monitored and measured using a CMR‐compatible sphygmomanometer, and HR was monitored and recorded with a CMR‐compatible monitor throughout each stage of stress.
CMR Measurements of LV Volumes
According to previously published techniques,19 LV volumes were measured from the short‐axis series of cine white blood imaging sequences. In each slice, the epicardial and endocardial contours at end‐systole and end‐diastole at rest and at peak stress were drawn, and the LV myocardial volumes and mass were determined using a modified Simpson's rule method.20 Image acquisition parameters included a 45‐ms repetition time, a 1‐ms echo time, a 78° flip angle, a 400×324‐mm field of view, a 192×109 matrix, an 8‐mm‐thick slice with a 2‐mm gap, and an acceleration factor of 2.
Measures of Arterial Stiffness
One primary and 2 secondary measures of arterial stiffness were used in this study. The primary measure of arterial stiffness was the aortic stiffness index (mm Hg/mL per m2), which was calculated as the ratio of the brachial artery pulse pressure (PP; measured by the CMR‐compatible sphygmomanometer) relative to the LV stroke volume indexed to body surface area (SVi).21–23 The PP/SVi has been shown to correlate with other measures of arterial stiffness (r=0.98, P<0.001).24
The secondary measures of arterial stiffness included the aortic elastance index (EaI) and proximal thoracic aortic distensibility (AoD). The EaI was measured as the ratio of end‐systolic pressure to LV stroke volume index (mm Hg/mL per m2), in which end‐systolic pressure was estimated by multiplying the brachial arterial systolic blood pressure by 0.85. Previously, this metric to assess end‐systolic pressure has been shown to correlate well with that obtained invasively (r=0.98, P<0.0001).25 Redfield et al studied EaI in correlation with the development of diastolic dysfunction and adjusted the EaI for changes in HR and peripheral vascular resistance. Similarly, we studied changes in HR and systemic vascular resistance to assess whether changes in EaI reflected that of arterial stiffness. The HR was recorded, and the systemic vascular resistance index was estimated from the measured cardiac output and the mean arterial pressure calculated from the measured systolic and diastolic blood pressures.26 Chandler et al extensively validated the use of EaI in assessing sex‐ and age‐related differences in arterial stiffness with rest and stress by measuring the ventricular volumes using noninvasive nuclear isotope imaging.27
Although PP/SVi represents a measure of the pulsatile load of the entire vascular system, EaI represents a net measure of the resistive and pulsatile load.28 EaI is related directly to the HR and peripheral vascular resistance and inversely related to aortic compliance.28 In addition to the measures of aortic stiffness, we measured the HR and calculated the systemic vascular resistance index to assess the influence of these parameters on the EaI. The HR was recorded, and the systemic vascular resistance index was estimated from the measured cardiac output and the mean arterial pressure calculated from the measured systolic and diastolic blood pressures.26
According to previously published techniques,5,29 proximal thoracic AoD (10−3 mm Hg−1) was measured in the ascending thoracic aorta 4 cm distal to the aortic annulus.5 Proximal thoracic AoD was defined as the ratio of the cardiac cycle‐dependent (systole–diastole) changes in the area of the ascending aorta divided by the PP corrected for the resting end‐diastolic vessel area. Imaging parameters included a phase contrast gradient‐echo sequence with a 34‐cm field of view, a 10‐ms repetition time, a 1‐ms echo time, a 20° flip angle, an 8 mm‐thick slice, a 256×224 matrix, a 32‐kHz bandwidth, and velocity encoding of 150 cm/s.
Body Composition
According to previously published techniques, assessments of body composition were determined from dark blood T1‐weighted single‐inversion recovery images of the abdomen.30 Visceral and subcutaneous fat were determined from the planimetered areas of high signal intensity on a T1‐weighted image in an axial slice at the level of the L4 to L5 vertebra.30
Statistical Analysis
Descriptive statistics were initially examined among the 3 groups (NFG, IFG, and DM). Next, comparisons were obtained of rest and stress measures of PP/SVi, EaI, and AoD between those with NFG and IFG and those with NFG and DM using an ANCOVA approach with PROC GLM in SAS version 9 (SAS Institute). Because the mean values of stiffness differed among the 3 fasting glucose groups, we sought to ensure that these differences were not related to differences in age within these cohorts. To address this potential issue, we used ANOVA to assess the effect of glycemic status after accounting for age. Two post hoc, pair‐wise comparisons were made between the IFG and DM groups versus the NFG group. Two pair‐wise comparisons were proposed a priori, NFG versus IFG and DM. To control the overall false‐positive rate, these pair‐wise comparisons were made only if the overall glycemic main effect was significant. We repeated the analysis after accounting for HTN, smoking, hypercholesterolemia, sex, CAD, visceral fat, and cardiovascular medication use that included angiotensin‐converting enzyme inhibitors, β‐blockers, diuretics, and vasodilators. We included visceral fat in our adjustment because abnormalities of fasting glucose often occur in the setting of obesity as the result of increases in abdominal or visceral fat.
We initially performed an analysis of effect of glycemic status, effect of stress, and glycemic status–stress interaction in the overall population. A large study involving 3499 persons in the Multi‐Ethnic Study of Atherosclerosis (MESA) cohort demonstrated an age threshold beyond which abnormalities of fasting glucose had no influence on resting arterial stiffness. The age threshold identified in this study was 65 years. Consequently, we performed effect of glycemic status, effect of stress, and glycemic status–stress interaction only in the group aged 55 to 64 years.
For all analyses, a P value <0.05 was considered significant. Results were expressed as mean±SE of the estimate unless stated otherwise.
Results
There were 373 consecutively enrolled persons who met the entry criteria, and their demographic, cardiac, and hemodynamic measures are shown in Table 1. Those with DM were more frequently men and exhibited higher body mass index (P=0.0008), more visceral fat (P<0.0001), higher incidence of hypercholesterolemia (P<0.05) and smoking (P<0.05), and lower incidence of HTN relative to those with NFG (P=0.007). Statin use was significantly higher among those with DM compared with those with NFG (P=0.01).
Table 1.
Demographic Characteristics, Hemodynamics, and CMR Volumetric Assessments
| NFG (n=81) | IFG (n=128) | DM (n=164) | |
|---|---|---|---|
| Demographics | |||
| Age, y | 70±8 | 70±8 | 68±7 |
| Men | 29 (36) | 60 (47) | 86 (52)* |
| Race/ethnicity | |||
| White | 64 (79) | 107 (84) | 124 (76) |
| Black | 14 (17) | 18 (14) | 35 (21) |
| Hispanic | 3 (4) | 1 (0.8) | 2 (1.2) |
| Asian | 0 (0) | 2 (1.6) | 3 (1.8) |
| Body mass index, m/kg2 | 28.5±6.6 | 29.5±4.8 | 31.5±6.5* |
| Height, cm | 172±39 | 169±10 | 169±11 |
| Weight, kg | 81±20 | 84±17 | 89±18* |
| Subcutaneous fat, m2 | 210±118 | 206±96 | 229±124 |
| Visceral fat, m2 | 150±75 | 195±95* | 213±102* |
| Hypertension | 77 (95) | 123 (96) | 142 (87)* |
| Coronary artery disease | 26 (32) | 38 (30) | 41 (25) |
| Hypercholesterolemia | 54 (67) | 93 (73) | 131 (80)* |
| Smoking | 31 (38) | 54 (42) | 86 (53)* |
| Medications | |||
| Angiotensin‐converting enzyme inhibitor | 33 (41) | 53 (41) | 85 (52) |
| Angiotensin receptor blocker | 6 (7) | 8 (6) | 7 (4) |
| Statin | 46 (57) | 75 (59) | 119 (73)* |
| β‐Blocker | 35 (43) | 58 (45) | 60 (37) |
| Calcium channel antagonist | 16 (20) | 38 (30) | 42 (26) |
| CMR measures | |||
| LVEDV, mL | 122 (48) | 117 (30) | 121 (30) |
| LVESV, mL | 44 (17) | 42 (17) | 45 (17) |
| SV, mL | 74 (17) | 75 (18) | 76 (20) |
| LVEF | 64 (7) | 64 (8) | 63 (8) |
| LV mass, g | 123 (32) | 129 (33) | 134 (36)* |
| LVEDVI, mL/m2 | 63 (20) | 60 (14) | 60 (13) |
| LVESVI, mL/m2 | 23 (8) | 22 (9) | 23 (8) |
| SVI, mL/m2 | 39 (8) | 38 (8) | 38 (9) |
| LVMI, g/m2 | 64 (13) | 66 (13) | 67 (15) |
| Rest cardiac index, L/min per m2 | 2.4 (0.5) | 2.4 (0.5) | 2.5 (0.6) |
| Stress cardiac index, L/min per m2 | 4.6 (1.1) | 4.5 (1.2) | 4.7 (1.2) |
| Hemodynamics | |||
| Resting heart rate, beats/min | 63 (10) | 64 (10.0) | 66 (12)* |
| Resting SBP, mm Hg | 139 (15) | 140 (17) | 139 (16) |
| Resting DBP, mm Hg | 79 (12) | 78 (12) | 77 (12) |
| Resting pulse pressure, mm Hg | 60 (13) | 62 (15) | 62 (17) |
| Stress heart rate, beats/min | 123 (15) | 124 (16) | 126 (13) |
| MPHR | 82 (9) | 82 (10) | 83 (8) |
| Stress SBP, mm Hg | 119 (22) | 128 (25)* | 133 (24)* |
| Stress DBP, mm Hg | 65 (15) | 70 (20) | 71 (18)* |
| Stress pulse pressure, mm Hg | 54 (19) | 58 (18) | 62 (18)* |
| Stress/resting pulse pressure | 0.9 (0.4) | 0.98 (0.4) | 1.1 (0.6)* |
Values expressed as mean±SD or number (percentage). CMR indicates cardiovascular magnetic resonance; DBP, diastolic blood pressure; DM, diabetes mellitus; IFG, impaired fasting glucose; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVESVI, left ventricular end‐systolic volume index; LVMI, left ventricular mass index; MPHR, maximum predicted heart rate; NFG, normal fasting glucose; SBP, systolic blood pressure; SV, stroke volume; SVI, stroke volume index.
P<0.05 for NFG vs DM.
P<0.05 for NFG vs IFG.
Resting measures of arterial stiffness after accounting for sex, HTN, CAD, hypercholesterolemia, smoking, visceral fat, and cardiovascular medication use are shown in Table 2. There were no statistically significant differences in resting measures of arterial stiffness (PP/SVi, EaI, and AoD) among the 3 groups.
Table 2.
Resting Measures of Aortic Stiffness After Accounting for Sex, Hypertension, Coronary Artery Disease, Dyslipidemia, Smoking, Visceral Fat, and Cardiovascular Medication Use
| NFG | IFG | DM | P Value, Glycemic Effect | P Value, (NFG vs IFG) | P Value, (NFG vs DM) | |
|---|---|---|---|---|---|---|
| Rest PP/SVI, mm Hg/mL per m2 | 1.55 (0.06) | 1.68 (0.05) | 1.71 (0.04) | 0.10 | 0.06 | 0.05 |
| Rest EaI, mm Hg/mL per m2 | 3.28 (0.09) | 3.48 (0.08) | 3.50 (0.07) | 0.41 | 0.28 | 0.19 |
| Rest AoD1, 10−3 mm Hg−1 | 1.34 (0.11) | 1.45 (0.09) | 1.38 (0.08) | 0.49 | 0.33 | 0.96 |
Values are expressed as mean (SE). AoD indicates aortic distensibility; DM, diabetes mellitus; EaI, aortic elastance index; IFG, impaired fasting glucose; NFG, normal fasting glucose; PP/SVI, pulse pressure/stroke volume index.
Similar to resting measures of arterial stiffness, stress measures were also adjusted for sex, HTN, CAD, hypercholesterolemia, smoking, visceral fat, and cardiovascular medication use. After intravenous dobutamine, overall differences in stress PP/SVi and EaI were noted between those with NFG and IFG (P=0.002 and P=0.009, respectively) (Figures 1 and 2), with a trend toward significance for AoD, (P=0.34) (Figure 3). Similarly, there were differences between those with NFG and DM for stress PP/SVi and stress AoD (P=0.001 and P=0.004, respectively) (Figure 1), and a trend toward significance was noted for EaI (P=0.15) (Figure 1).
Figure 1.
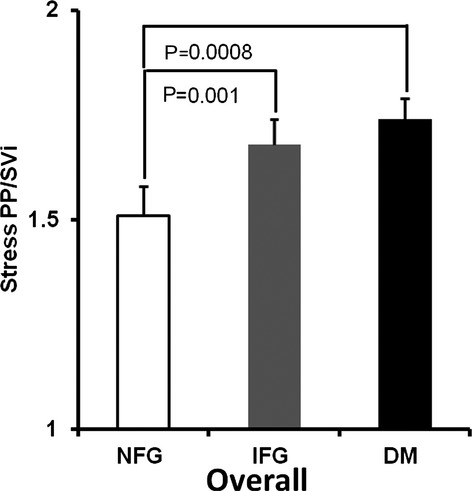
Adjusted arterial stiffness as assessed by pulse pressure/stroke volume index (PP/SVi) in the overall population. There is a difference in stress PP/SVi between those with NFG and IFG in addition to the differences between NFG and DM. DM indicates diabetes mellitus; IFG, impaired fasting glucose; NFG, normal fasting glucose.
Figure 2.
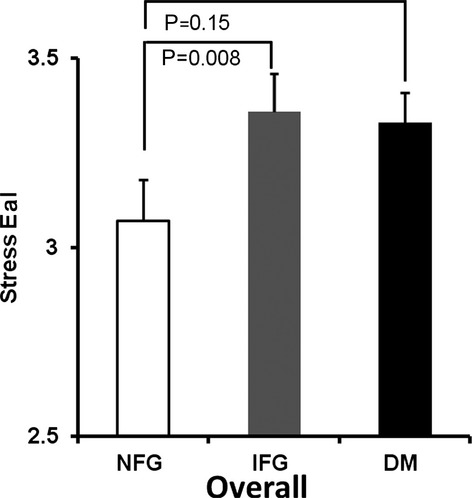
Adjusted arterial stiffness as assessed by aortic elastance index (EaI) in the overall population. Similar to pulse pressure/stroke volume index, with stress, those with NFG have lower EaI compared with those with IFG or DM, with a trend toward significance between those with NFG and DM. DM indicates diabetes mellitus; IFG, impaired fasting glucose; NFG, normal fasting glucose.
Figure 3.
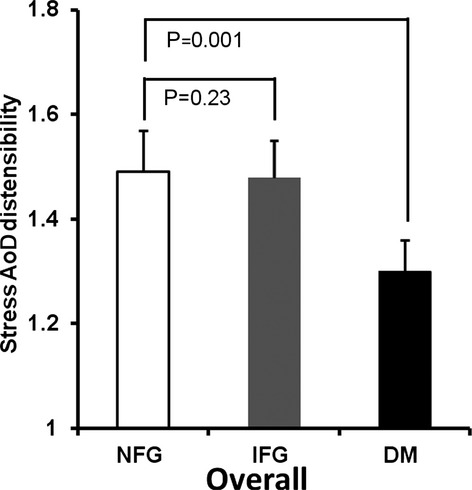
Adjusted aortic distensibility (AoD) of the ascending aorta at stress in the overall group. There are differences in stress AoD between those with NFG and those with DM, with a trend toward significance between those with NFG and IFG. DM indicates diabetes mellitus; IFG, impaired fasting glucose; NFG, normal fasting glucose.
To examine more fundamental components of the EaI, we measured both HR and systemic vascular resistance index. The NFG, IFG, and DM groups all had similar increases in HR (65±3 versus 67±2 versus 64±2 beats per minute, respectively; P=0.59) and decreases in systemic vascular resistance index (−1.89±0.14 versus −1.73±0.10 versus 1.71±0.08 dynes/s per cm−5, respectively; P=0.36). This suggests that the differences in EaI among the 3 groups were most likely the result of differences in arterial stiffness.
In addition to the pair‐wise comparisons already noted, we measured the effect of glycemic status on stress arterial stiffness in the overall population, regardless of age. An effect of glycemic status was noted for stress PP/SVi, EaI, and the AoD (P=0.002, P=0.02, and P=0.003, respectively), as shown in Table 3. However, the effect of stress was statistically significant only for EaI (P=0.001), with a trend toward significance for AoD (P=0.2) and no statistical significance for PP/SVi. No evidence of glycemic status–stress interaction was found (P=0.56, P=0.69, and P=0.10, respectively) for PP/SVi, EaI, and AoD in the overall population.
Table 3.
Effect of Glycemic Status on Stress Arterial Stiffness
| Stress Measures | Glycemic Effect | Stress Effect | Glycemic–Stress Interaction |
|---|---|---|---|
| Overall group | |||
| PP/SVi | 0.002 | 0.89 | 0.56 |
| EaI | 0.02 | 0.001 | 0.69 |
| AoD | 0.003 | 0.20 | 0.10 |
| Group aged 55 to 64 years | |||
| PP/SVi | 0.09 | 0.08 | 0.39 |
| EaI | 0.05 | 0.06 | 0.09 |
| AoD | 0.39 | 0.12 | 0.07 |
AoD indicates aortic distensibility; EaI, aortic elastance index; PP/SVI, pulse pressure/stroke volume index.
As noted, we performed effect of glycemic status, effect of stress, and glycemic status–stress interaction subsequently in the group aged 55 to 64 years. Table 3 shows a trend toward significance for the effect of glycemic status (P=0.09, P=0.05, and P=0.39 for PP/SVi, EaI, and AoD, respectively), effect of stress (P=0.08, P=0.06, and P=0.12, respectively) and a glycemic status–stress interaction (P=0.39, P=0.09, and P=0.07,respectively) in the group aged 55 to 64 years. Although we cannot state with statistical confidence that there is differential response of arterial stiffness to dobutamine in those with IFG or DM relative to those with NFG in those aged <65 years, these results should generate other hypotheses and could help explain the exercise intolerance of those with DM and IFG.
Discussion
This study has 2 important findings. First, those with diabetes and IFG at high risk for a first episode of symptomatic HF exhibit higher stress measures of arterial stiffness compared with those with NFG, even after accounting for other cardiovascular comorbidities such as HTN or preexisting CAD (Figure 1). Second, in addition to total cardiovascular stiffness, the distensibility of the proximal ascending aorta with dobutamine is impaired in those with IFG or DM relative to those with NFG. The latter conclusion extends prior observations showing impaired ascending AoD at rest in those with DM or IFG in a MESA cohort.24 Our results showing possible glycemic status–stress interactions in those aged <65 years can elicit other hypotheses and necessitate validation in a cohort adequately powered to test those hypotheses. The results of this study provide new information regarding the impact of both IFG and DM on stress‐related measures of aortic stiffening in persons at risk for but yet to experience symptomatic HF.
The administration of intravenous dobutamine in younger persons has been shown previously to reduce aortic impedance and improve aortic compliance.31 Binkley et al demonstrated a downward shift in the input impedance spectrum consistent with an increase in aortic compliance after administration of intravenous dobutamine.31 Commensurate with the results from Binkley et al, we observed a glycemic status–stress interaction with a trend toward significance only in the group aged 55 to 64 years.
Among 3499 persons aged 45 to 80 years without known CAD from the MESA cohort, those with abnormal fasting glucose (IFG and DM) exhibited increased resting measures of arterial stiffness, carotid arterial wall thickness, and LV hypertrophy relative to their counterparts with NFG.24 In the current study, we examined stress‐related measures of aortic stiffness in participants at risk for a first episode of symptomatic HF. Importantly, we discovered that those with IFG or DM have higher stress measures of arterial stiffness than those with NFG; this included both total cardiovascular stiffness and the ascending aortic stiffness. These results remained unchanged after accounting for other risk factors for HF. To further delineate the role of glycemic status in modifying the stress response of arterial stiffness, we assessed the glycemic status–stress interaction only in the group aged 55 to 64 years. The rationale for this approach was the finding of a threshold age of 65 years for the effect of fasting glucose on resting arterial stiffness in a large MESA cohort., These results raised the possibility that middle‐aged and older persons with IFG or DM at risk for a first episode of symptomatic HF may in fact experience a differential response compared with those with NFG when exposed to an external stress. This paradoxical increase could inadvertently raise LV end‐diastolic pressure, inhibit left atrial ejection, and raise pulmonary capillary wedge pressure leading to pulmonary edema.32 Our results demonstrate the need to validate this possibility in a cohort that is adequately powered to detect a differential response of arterial stiffness in persons with differing glycemic status.
One of several etiologies may be responsible for the increase in stress‐related aortic stiffness in the setting of abnormal fasting glucose. Glycation of the extracellular matrix, including both collagen33–34 and elastin,35 is noted with aging and is accelerated in hyperglycemic states. This glycation results in decreased flexibility and elasticity, increased fragmentation, and increased resistance to proteolysis.36 In addition, atherosclerosis, inflammation, and decreased endothelial function37 also decrease vascular function in the setting of hyperglycemia.
Using magnetic resonance, we were able to examine several of the components of rest‐ and stress‐related measures of cardiovascular stiffness to gain insight into potential mechanisms by which abnormal glucose states could influence stress‐related changes in arterial stiffening. Our measures of arterial elastance reflect net measures of the pulsatile and resistive load on the left ventricle. Regardless of whether participants were aged <65 or ≥65 years, dobutamine‐related changes in HR and peripheral vascular resistance were relatively similar across the 3 groups. This suggests that the abnormalities in EaI observed in this study were related to differences in pulsatile load as opposed to the resistive load of the vascular system.
Stress measures of AoD in the ascending thoracic aorta were higher in those with DM relative to those with NFG. This suggests that the mechanism by which abnormalities of fasting glucose (IFG or DM) increase aortic stiffening are related to adverse effects on the pulsatile load of the left ventricle that involve the ascending thoracic aorta. Interestingly, the proximal thoracic aorta has been the site in which the aorta stiffened at rest in persons with DM or IFG enrolled in MESA.24
Because abnormalities of fasting glucose often occur in the setting of obesity related to increases in abdominal or visceral fat, we examined whether the relationship between dobutamine‐induced change in aortic stiffness and fasting glucose was mediated by the presence of visceral fat. Previous studies have shown an independent relationship between increases in thoracic aortic wall thickness and visceral fat presumed secondary to the release of adipokines and increases in both systemic inflammation and insulin resistance.30,36,38 According to previously published techniques, we measured visceral fat within the abdomen30 and found that for those aged 55 to 85 years, abnormalities of fasting glucose remained associated with paradoxical increases in arterial stiffening after pharmacological stress, even after adjusting for the presence of visceral fat. These findings suggest that the relationship between abnormalities of fasting glucose and abnormal stress‐related increased in aortic stiffening occurs regardless of visceral fat content.
Our study exhibits some limitations. First, the majority of our subjects were white. Insufficient numbers of participants were present to examine the effects of impaired abnormalities of fasting glucose on stress‐related measures of arterial stiffening after accounting for those of differing ethnicity and race. Second, we were unable to unequivocally demonstrate a glycemic status–stress interaction and a glycemic status–stress–age interaction due to the limited sample size of our cohort. Consequently, we cautiously approach our findings as hypothesis generating, and they will need confirmation in a cohort with a larger sample size. Third, we used brachial assessments of PP in our calculations. Although brachial PP can serve as a surrogate for central aortic pressure and has been extensively published in community studies and large cohort studies,26 it is likely that greater accuracy could be achieved with invasive measures of central aortic pressure. Fourth, our cross‐sectional sampling and study design do not allow us to prove causality. Importantly, studies such as this provide important information about associations from which further studies of causation can be planned.
In conclusion, pharmacological stress‐related measures of aortic stiffness are elevated in middle‐aged and older persons with DM or IFG. Because abnormal increases in stress measures of aortic stiffness impair coronary artery perfusion and stimulate of LV hypertrophy, these results highlight the need for further investigation into whether abnormal fasting glucose–related changes in stress‐induced arterial stiffness may precipitate symptomatic HF or serve as a therapeutic target and for risk factor modification to mitigate acute HF exacerbations in older persons with diabetes or prediabetic states.
Sources of Funding
Research supported in part by National Institutes of Health grants R01HL076438, P30AG21332, N01HC95165, R01CA167821, and R33CA121296.
Disclosures
None.
References
- 1.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993; 16:434-444. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham Study. JAMA. 1979; 241:2035-2038. [DOI] [PubMed] [Google Scholar]
- 3.Pazin‐Filho A, Kottgen A, Bertoni AG, Russell SD, Selvin E, Rosamond WD, Coresh J. HbA 1C as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetologia. 2008; 51:2197-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005; 294:334-341. [DOI] [PubMed] [Google Scholar]
- 5.Stacey RB, Bertoni AG, Eng J, Bluemke DA, Hundley WG, Herrington D. Modification of the effect of glycemic status on aortic distensibility by age in the Multi‐Ethnic Study of Atherosclerosis. Hypertension. 2010; 55:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saba PS, Ganau A, Devereux RB, Pini R, Pickering TG, Roman MJ. Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens. 1999; 17:1007-1015. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe H, Ohtsuka S, Kakihana M, Sugishita Y. Coronary circulation in dogs with an experimental decrease in aortic compliance. J Am Coll Cardiol. 1993; 21:1497-1506. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsuka S, Kakihana M, Watanabe H, Enomoto T, Ajisaka R, Sugishita Y. Alterations in left ventricular wall stress and coronary circulation in patients with isolated systolic hypertension. J Hypertens. 1996; 14:1349-1355. [DOI] [PubMed] [Google Scholar]
- 9.Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000; 36:489-494. [DOI] [PubMed] [Google Scholar]
- 10.Pini R, Cavallini MC, Bencini F, Silvestrini G, Tonon E, De Alfieri W, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ. Cardiovascular remodeling is greater in isolated systolic hypertension than in diastolic hypertension in older adults: the Insufficienza Cardiaca negli Anziani Residenti (ICARE) a Dicomano Study. J Am Coll Cardiol. 2002; 40:1283-1289. [DOI] [PubMed] [Google Scholar]
- 11.Palmieri V, Bella JN, Roman MJ, Gerdts E, Papademetriou V, Wachtell K, Nieminen MS, Dahlöf B, Devereux RB. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens. 2003; 21:781-787. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O'Leary DH, Bluemke DA, Lima JA. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi‐Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008; 28:194-201. [DOI] [PubMed] [Google Scholar]
- 13.Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O'Connor F, Becker LC, Lakatta EG. Age and gender affect ventricular‐vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004; 44:611-617. [DOI] [PubMed] [Google Scholar]
- 14.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Valgimigli M, Claeys MJ, Donner‐Banzhoff N, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Husted S, James SK, Kervinen K, Kristensen SD, Maggioni AP, Pries AR, Romeo F, Rydén L, Simoons ML, Steg PG, Timmis A, Yildirir AMembers TF, (CPG) ECfPG, Reviewers D. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013; 34:2949-3003. [DOI] [PubMed] [Google Scholar]
- 15.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33suppl 1:S62-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJNational Heart L, and Blood Institute Joint National Committee on Prevention, Detection, E.aluation, and Treatment of High Blood Pressure, Committee NHBPEPC. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560-2572. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton CA, Link KM, Salido TB, Epstein FH, Hundley WG. Is imaging at intermediate doses necessary during dobutamine stress magnetic resonance imaging? J Cardiovasc Magn Reson. 2001; 3:297-302. [DOI] [PubMed] [Google Scholar]
- 18.Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002; 106:2328-2333. [DOI] [PubMed] [Google Scholar]
- 19.Charoenpanichkit C, Little WC, Mandapaka S, Dall'Armellina E, Morgan TM, Hamilton CA, Hundley WG. Impaired left ventricular stroke volume reserve during clinical dobutamine stress predicts future episodes of pulmonary edema. J Am Coll Cardiol. 2011; 57:839-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch‐Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in Multi‐Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006; 186:S357-S365. [DOI] [PubMed] [Google Scholar]
- 21.Alfie J, Waisman GD, Galarza CR, Camera MI. Contribution of stroke volume to the change in pulse pressure pattern with age. Hypertension. 1999; 34:808-812. [DOI] [PubMed] [Google Scholar]
- 22.Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume‐to‐aortic pulse pressure ratio in humans. Am J Physiol. 1998; 274:H500-H505. [DOI] [PubMed] [Google Scholar]
- 23.Fagard RH, Pardaens K, Staessen JA, Thijs L. The pulse pressure‐to‐stroke index ratio predicts cardiovascular events and death in uncomplicated hypertension. J Am Coll Cardiol. 2001; 38:227-231. [DOI] [PubMed] [Google Scholar]
- 24.Rerkpattanapipat P, D'Agostino RB, Link KM, Shahar E, Lima JA, Bluemke DA, Sinha S, Herrington DM, Hundley WG. Location of arterial stiffening differs in those with impaired fasting glucose versus diabetes: implications for left ventricular hypertrophy from the Multi‐Ethnic Study of Atherosclerosis. Diabetes. 2009; 58:946-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992; 86:513-521. [DOI] [PubMed] [Google Scholar]
- 26.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age‐ and gender‐related ventricular‐vascular stiffening: a community‐based study. Circulation. 2005; 112:2254-2262. [DOI] [PubMed] [Google Scholar]
- 27.Chantler PD, Melenovsky V, Schulman SP, Gerstenblith G, Becker LC, Ferrucci L, Fleg JL, Lakatta EG, Naijar SS. The sex‐specific impact of systolic hypertension and systolic blood pressure on arterial‐ventricular coupling at rest and during exercise. Am J Physiol Heart Circ Physiol. 2008; 295:H145-H153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chantler PD, Lakatta EG. Arterial‐ventricular coupling with aging and disease. Front Physiol. 2012; 3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rerkpattanapipat P, Hundley WG, Link KM, Brubaker PH, Hamilton CA, Darty SN, Morgan TM, Kitzman DW. Relation of aortic distensibility determined by magnetic resonance imaging in patients > or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol. 2002; 90:1221-1225. [DOI] [PubMed] [Google Scholar]
- 30.Chughtai HL, Morgan TM, Hamilton CA, Charoenpanichkit C, Ding J, Brinkley TE, Hundley WG. Intraperitoneal fat is associated with thickening of the thoracic aorta in individuals at high risk for cardiovascular events. Obesity (Silver Spring). 2011; 19:1784-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binkley PF, Van Fossen DB, Haas GJ, Leier CV. Increased ventricular contractility is not sufficient for effective positive inotropic intervention. Am J Physiol. 1996; 271:H1635-H1642. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003; 107:714-720. [DOI] [PubMed] [Google Scholar]
- 33.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988; 318:1315-1321. [DOI] [PubMed] [Google Scholar]
- 34.Vlassara H, Brownlee M, Cerami A. Advanced non‐enzymatic tissue glycosylation: significance in late diabetic complications. Diabetes Res Clin Pract. 1989; 7suppl 1:S103-S108. [DOI] [PubMed] [Google Scholar]
- 35.Tanno T, Yoshinaga K, Sato T. Alteration of elastin in aorta from diabetics. Atherosclerosis. 1993; 101:129-134. [DOI] [PubMed] [Google Scholar]
- 36.Nordstrand N, Gjevestad E, Dinh KN, Hofsø D, Røislien J, Saltvedt E, Os I, Hjelmesæth J. The relationship between various measures of obesity and arterial stiffness in morbidly obese patients. BMC Cardiovasc Disord. 2011; 11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994; 70:138-151. [PubMed] [Google Scholar]
- 38.Kim TN, Park MS, Lim KI, Yang SJ, Yoo HJ, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Res Clin Pract. 2011; 93:285-291. [DOI] [PubMed] [Google Scholar]


