Abstract
Background
Randomized control trials from the developed world report that clinical decision support systems (DSS) could provide an effective means to improve the management of hypertension (HTN). However, evidence from developing countries in this regard is rather limited, and there is a need to assess the impact of a clinical DSS on managing HTN in primary health care center (PHC) settings.
Methods and Results
We performed a cluster randomized trial to test the effectiveness and cost‐effectiveness of a clinical DSS among Indian adult hypertensive patients (between 35 and 64 years of age), wherein 16 PHC clusters from a district of Telangana state, India, were randomized to receive either a DSS or a chart‐based support (CBS) system. Each intervention arm had 8 PHC clusters, with a mean of 102 hypertensive patients per cluster (n=845 in DSS and 783 in CBS groups). Mean change in systolic blood pressure (SBP) from baseline to 12 months was the primary endpoint. The mean difference in SBP change from baseline between the DSS and CBS at the 12th month of follow‐up, adjusted for age, sex, height, waist, body mass index, alcohol consumption, vegetable intake, pickle intake, and baseline differences in blood pressure, was −6.59 mm Hg (95% confidence interval: −12.18 to −1.42; P=0.021). The cost‐effective ratio for CBS and DSS groups was $96.01 and $36.57 per mm of SBP reduction, respectively.
Conclusion
Clinical DSS are effective and cost‐effective in the management of HTN in resource‐constrained PHC settings.
Clinical Trial Registration
URL: http://www.ctri.nic.in. Unique identifier: CTRI/2012/03/002476.
Keywords: cluster randomized trials, computers, cost‐effectiveness, effectiveness, hypertension
Introduction
Decision support systems (DSS) have been defined as tools that help clinicians decide on a course of action in response to an understanding of the patient's status. Recent studies from the developed world report that DSS provide an effective means to improve the physician performance.1–4 An improvement in quality of antihypertensive treatment, concurrently leading to a considerable reduction in drug costs, has also been shown for DSS.5 Randomized control trials from the developed world report that DSS provide an effective means to improve the management of hypertension (HTN).2,5–9 However, evidence from developing countries in this regard is rather limited. An increasing penetration of information technology in noncommunicable diseases health domain in India,10–11 and a shortage of primary health care workforce conversant with guideline‐based clinical management,12 are opportune reasons to find answers for a pertinent question: Are DSS effective in managing hypertension in resource constrained primary health center (PHC) settings?
Systematic reviews on DSS, as an intervention, report a paucity of studies on patient outcomes for cardiovascular disease (CVD) in the Western world.3,13–14 The potential role of DSS for the management of blood pressure (BP) among low‐ and middle‐income countries remains unclear. In a resource‐constrained setting such as a PHC, the implementation of a DSS intervention should test not only the physician performance, but also on related patient outcomes. Individual randomization dilutes the intervention effect size owing to contamination between individuals.15 Diffuse and complex interventions such as DSS (having information technology inputs) need cluster randomization.16 Moreover, coefficient of variation (CV) between PHC clusters can only be accounted for by analyzing cluster‐level data. Hence, we performed a cluster randomized trial that aimed to test the effectiveness and cost‐effectiveness of DSS for BP management among Indian hypertensive patients.
Methods
Study Design
We performed a cluster randomized trial to test the effectiveness and cost‐effectiveness of a DSS among Indian hypertensive patients, wherein PHC clusters were randomized to receive either a DSS or a chart‐based support (CBS) system in a district of Telangana state, India (baseline vs. 12‐month follow‐up; 8 PHC clusters per intervention). The processes involved in development, validation, and pilot testing of the DSS have been previously described.17
Intervention
The process of development and validation of our clinical DSS for management of HTN have been previously published.17 Physicians from the PHCs, who were randomized to receive the DSS arm, were trained and instructed to follow the algorithms and prompts that would arise out of the computer‐based software for management of BP. DSS was a software that helped the physician to (1) undertake a thorough evaluation of risk factors that hypertensive patients may have for developing a CVD; (2) classify the risk level based on data entered by the physician. These included lifestyle‐related risk factors, such as tobacco use, unhealthy diet (high salt intake), obesity (waist circumference >90 cm in men and >80 cm in women), and physical inactivity (physical activity less than 30 minutes a day for at least 5 days in a week); nonmodifiable risk factors, such as age >55 years in males and >65 years in females, family history of premature coronary artery disease (males <55 years, female >65 years), history of heart disease (heart attack, angina, heart failure [HF], or any surgical procedure on coronary vessels, ie, coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty), history of stroke or transient ischemic attack, history of diabetes, high levels low‐density lipoprotein (>100 mg/dL), low levels of high‐density lipoprotein in blood (<40 mg/dL), and hypertriglyceridemia (≥150 mg/d:); (3) follow a software‐prompted algorithmic guideline‐based drug management (which was developed based on Indian hypertension guidelines II [2007])18; and (4) give alerts on the counseling on lifestyle changes and adherence to medication. Only lifestyle‐related counseling was provided for patients suffering from comorbid conditions, such as hyperlipidemia and diabetes, in the primary care centers. However, all patients suffering from any other comorbid conditions were referred to secondary care centers (community health centers) for their disease management. The algorithm had a defined set of criteria for referral to a higher center in the HTN treatment algorithms (Table S1).
The DSS was installed in a netbook and given to the physicians in the DSS group. All the physicians allocated to the DSS group had given a written informed consent to enter all the study patients' data in the netbook (containing the DSS software) and view the DSS recommendations. However, they had the choice to agree or disagree with the DSS recommendations. The final summary output sheet containing the patient‐tailored recommendations (based on the data entered by the physician) in the DSS had a tracking variable, which specifically asked the treating physician to document their agreement or disagreement with the DSS recommendations and, if agreeable, whether they implemented or did not implement the suggested recommendations. The physicians randomized to receive the CBS had the guidelines and lifestyle advices printed as a poster format, which was fixed to the wall opposite the place where the physician usually sat and examined the patients in the outpatient department. Posters were displayed prominently and were visible even at a distance of 3 m. The risk factors that need to be specifically elicited, classification of risk among hypertensive subjects, flow chart for drug management, and advice for lifestyle interventions was included as a simple flow chart in the form of a poster.
Study Endpoints
The primary endpoint was to compare the systolic blood pressure (SBP) at 0 and 12 months among hypertensive patients randomized to receive either the DSS or the CBS group. The secondary endpoints were to compare the cost‐effectiveness of the DSS versus the CBS at the end of 12 months among hypertensive patients.
Study Participants
Adult male and female Indians in the age group of 35 to 64 years, with SBP of 140 mm Hg or greater and/or diastolic blood pressure (DBP) of 90 mm Hg or greater (irrespective of antihypertensive medications) who had given an informed written consent form were included in the study. Hypertensive subjects who have been hospitalized within the last 12 months, pregnant and lactating women, and participants with a history of cancer (physician certified) were excluded. The study protocol was approved by the (1) Human Biology Research Ethics Committee, University of Cambridge, UK, and (2) Institutional Ethics Committee, Public Health Foundation of India, New Delhi, India. Written informed consent was obtained from both the physicians and patients who volunteered to take part in the study. Figure 1 depicts the consort flow chart, which details the number of participants from each PHC cluster.
Figure 1.
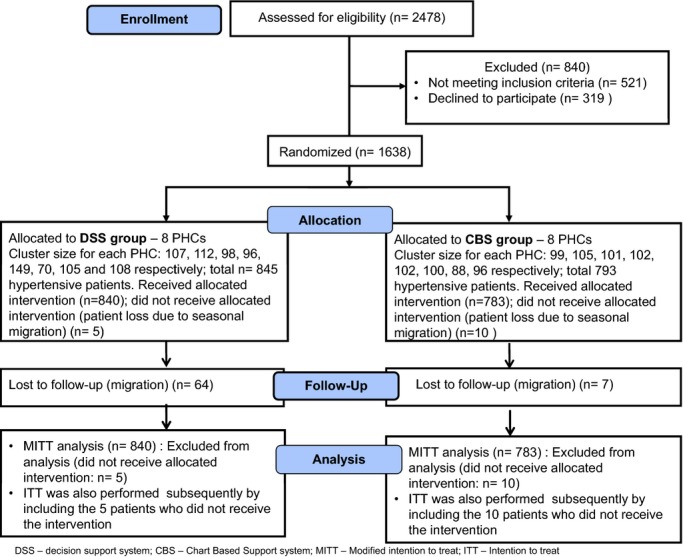
Consort flow chart. CBS indicates chart‐based support; DSS, decision support systems; MITT, modified intention to treat; PHC, primary health care center.
Study Settings
The study site, Mahabubnagar district, belongs to the state of Telangana, India (latitude between 15°55′ and 17°29′N; longitude between 77°15′ and 79°15′E) and has an area of 18 432 km2.19 We chose Mahabubnagar district because it has been identified as one of the backward districts (regional imbalances in development and health indicators) of Telangana State by the Backward Regions Grant Fund, Ministry of Panchayat Raj, Government of India.20 Moreover, Mahabubnagar had low health infrastructure and lack of qualified and trained staff for managing the PHCs. Because our intervention was aimed to improve the capacity of the existing primary health care workforce conversant with guideline‐based clinical management, we chose Mahabubnagar district.
Sample‐Size Calculations
To detect a difference of 5 mm Hg SBP, the individual randomization sample size was calculated at 239 subjects per intervention arm (SD, 19.5 mm21), with 80% power and an alpha of 0.05. Because cluster randomization was attempted in this study, the individual randomization sample size was adjusted by a design effect of 2.98 (design effect=1+[size of cluster−1]×intracluster correlation [ICC]). The ICC for the clusters among the Mahabubnagar district, AP, India (study site), was calculated to be 0.02 (based on the Indian sentinel surveillance study done on a representative sample from 10 sites in India).21 Hence, the cluster‐adjusted sample size was 713 hypertensive patients per intervention arm to detect a 5 mm Hg difference in SBP with a power of 80% and an alpha of 0.05. After adjusting for the CV among the various clusters (CV=0.25), the sample size per intervention arm was 741, that is, a total of 8 clusters (with equal cluster size of 100 each) was required per intervention arm (Table 1).
Table 1.
Sample‐Size Calculations for the Cluster Randomized Trial
| Detectable Difference in SBP Between Both the Groups | 80% Power With an ICC of 0.02 | 90% Power With an ICC of 0.02 | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Size Required for Individual Rand Per Arm | Sample Size Required for Cluster Rand per Arm (DE=2.98) | Sample Size Required for Cluster Rand With CV=0.25 (DE=3.1) | Min no of Clusters With a Cluster Size of 100 | Sample Size Required for Individual Rand Per Arm | Sample Size Required for Cluster Rand Per Arm (DE=2.98) | Sample Size Required for Cluster Rand With CV=0.25 (DE=3.1) | Min No. of Clusters With a Cluster Size of 100 | |
| 4 mm Hg | 374 | 1115 | 1160 | 24 | 500 | 1490 | 1550 | 32 |
| 5 mm Hg | 239* | 713* | 741* | 16* | 320 | 954 | 992 | 20 |
| 6 mm Hg | 166 | 495 | 515 | 12 | 222 | 622 | 689 | 14 |
CV indicates coefficient of variation; DE, design effect; ICC, intracluster correlation.
Sample size that was chosen for this study.
Sampling Techniques
The average distance of the 84 PHCs in Mahabubnagar district from the nearest secondary level of care, the community health center (CHC), was 20 km. We stratified the 84 PHCs into two groups (1) less than 20 km from the nearest CHC and (2) more than or equal to 20 km away from the nearest CHC. A line listing of all PHCs was done in both the groups. A random double‐digit number table was used, by an independent statistician not belonging to the study group, to randomly choose 8 PHCs from the “less than 20 km distance” group. Matched pairs of PHCs based on population size were then created. Simple randomization by an independent statistician was then used to distribute the paired PHC into any one of the randomisation arms (ie, either DSS or CBS intervention arms). Similar steps were repeated for the “more than 20 km distance” group. Hence, 4 PHCs from the less than 20 km group and 4 PHCs from the more than 20 km group were randomized to receive DSS. Four PHCs (population matched to those PHCs that entered DSS arm) from the less than 20 km group and 4 PHCs from the more than 20 km group (population matched to those PHCs that entered the DSS arm) were randomized to receive CBS. Only those assessing outcomes were blinded. Figure2 depicts the randomization arms and the randomization process adopted for the study.
Figure 2.
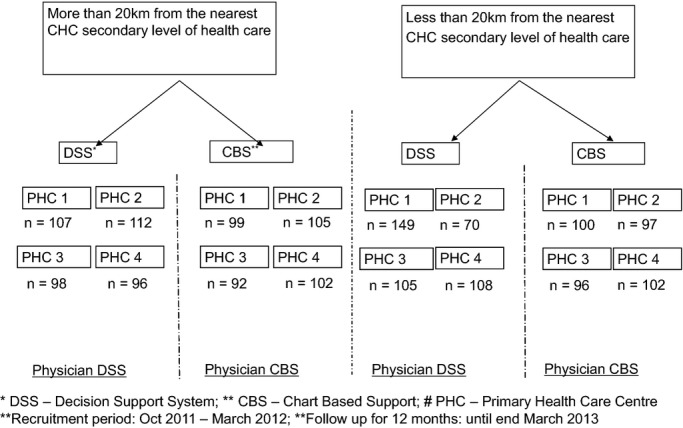
Randomization procedure followed during the study. CBS indicates chart‐based support; CHC, community health center; DSS, decision support systems; PHC, primary health care center.
Study Procedures
The full details of the study procedures have been published previously in the study protocol.14 Baseline data collection included demographic variables, such as age, gender, residence, total household members, religion, marital status, education level, employment status, occupation, and total household income per year. Data on known cardiovascular risk factors, such as type and quantity of smoking tobacco (cigarette or locally consumed products, such as beedi, chillum, and/or hukkah); type and quantity of oral tobacco and consumption of smokeless tobacco manufactured products (indigenous and locally consumed products, such as chewable tobacco, gutka, and paan with tobacco); and number of days alcohol was consumed in a typical week, whether alcohol was consumed regularly (defined as consumption of alcohol on more than or equal to 10 days in a month) were collected at 0, 6, and 12 months after study entry. The number of days in a week wherein physical activity was undertaken as a part of daily activity or leisure sport activity for 30 minutes a day was also collected. Dietary history questions were restricted to number of times of daily intake of salty food (locally high‐salt prevalent food items, such as pickles and papad), portions of fruits or vegetables consumed per day, quantity oil and fat consumed by the family members in a month, and type of oil/fat consumed by the family in a month were collected at 0, 6, and 12 months after study entry. Participants were explained that a portion could be any one of the following: 1 portion of vegetables (fresh, raw, tinned, or frozen)=3 tablespoons; salad, 1 portion=1 bowl; fresh fruit, 1 portion=1 medium apple, 1 banana; fruit juice (excluding cordials, fruit drinks, and squashes); and 1 portion=1 small glass. Duration of HTN since first diagnosed, whether on current hypertensive medications, presence or absence of any physician certified comorbid conditions, such as peripheral vascular diseases, kidney disease, diabetes mellitus, HF, cerebrovascular disease, myocardial infarction, arthritis, chronic obstructive pulmonary disease, and asthma were collected under medical history at 0, 6, and 12 months after randomization.
Measurements
Physical measurements, such as height, weight, waist circumference, pulse, and BP, were measured by a digital BP monitor at 0, 6, and 12 months after study entry. Care was taken to ensure that all the PHCs had the same equipment make, type, and validation procedures.
BP and pulse measurement
All BP measurements were taken by the physician in a standardized way using digital BP equipment (automatic digital BP monitor, Omron model HEM‐7203; Omron Corporation, Kyoto, Japan). The HEM‐7203 model was a validated one with an accuracy of ±3 mm Hg. BP was measured on the right upper arm in the sitting position, after a rest of 5 minutes. Using an appropriate‐sized cuff connected to a digital device, and the same arm at a similar time of day, two measurements were taken at 5‐minute intervals. Instructions were be given to the physicians to ensure that the lower edge of the bladder be placed 2 to 3 cm above the position of maximal pulsation of the brachial artery in the arm, just above the antecubital fossa. Care was taken to ensure that the cuff fit firmly, was comfortabe, and was well secured. The mean of the 2 readings were used for analysis.
Pulse measurements
Pulse measurements were recorded by the digital BP equipment (automatic digital blood pressure monitor, Omron model HEM‐7203; Omron Corporation), after a rest of five minutes. The digital BP equipment records the SBP, DBP, and the pulse in the same sitting, which was then recorded. The average of the two readings was used for the analysis.
Height, weight, and waist circumference measurements
Participants were weighed in light indoor clothing with a digital weighing machine (patient weighing scale, mechanical [Seca Nera Big; Seca, Hamburg, Germany] model with 150‐kg capacity) with 100‐g accuracy, which was standardized across all the centers. Height was measured in bare feet with a 2‐m wall‐mounted stadiometer. Waist circumference was measured on bare skin at the narrowest part of the abdomen between the ribs and the iliac crest, as seen from the anterior aspect, with a nonstretch metallic tape because this technique was documented to be least invasive and more culturally appropriate.
Training for Health Care Staff on DSS and HTN Management
Trainings were given to each PHC staff member during delivery of the study equipment. Centers randomized to receive CBS received trainings centered around the four key elements: staging of BP; risk stratification; drug algorithms; and follow‐up schedule and lifestyle advice (the same elements of the DSS, but in the form of a ready‐made poster). Centers randomized to receive DSS were trained on handling of netbooks, date entry, safekeeping procedures for the equipment, data archival and data entry, data export (excel and .csv formats), data synchronization with server (where Internet access was available), and data archival procedures (where no access to Internet) as well as the interpretation of the final page summary and recommendations.
Pilot Testing of the Questionnaire (Data Collection Form)
A questionnaire was developed with validated questions adapted from published recent Indian studies.22 The questionnaire was translated into the local language (Telugu) and back‐translated by 2 independent translators. A third reviewer compared the translated and back‐translated versions. The finalized questionnaire was field tested in 10% of the proposed sample size for cultural appropriateness. Questions deemed to hurt cultural sensitivity were modified based on pilot test results.
Study Recruitment
The average outpatient department (OPD) load was 50 to 100 patients per day in most centers. All consecutive patients suffering from physician‐certified HTN who were reporting to the OPD of the PHC were recruited. One to 2 hypertensive patients were recruited per day by the physician, per PHC, from the patients attending the outpatient clinic in the PHC. The average time for recruitment was approximately 4 to 8 patients per week per PHC and 16 to 32 per month per PHC. Hence, in approximately 4 to 6 months, all the required patients were recruited for the study from each center. Study recruitment lasted from October 2011 to March 2012. A 12‐month follow‐up phase for all recruited patients ended in March 2013. Patients were followed up for 1 year from the time they entered the study. Data collection using questionnaires on personal and dietary habits, risk factors, and physical measurements were done at baseline and during the 6th and 12th month after study entry. Baseline and end of 12th‐month comparisons were done for the primary endpoint. The assessments at 0‐, 6‐, and 12‐month time points were integrated with their monthly visits to the PHCs (PHCs provide medications on a monthly basis).
Ethical Consent Procedures
The study protocol was approved by the (1) Human Biology Research Ethics Committee, University of Cambridge, UK, and (2) Institutional Ethics Committee, Public Health Foundation of India, New Delhi, India. Participants were given sufficient time to understand the contents of the patient information sheet, which contained details on the study purpose, duration, study procedures, right to withdrawal from the study, details of patient confidentiality, ethical committee clearances, and the study team contact details. Participants were encouraged to ask questions regarding any aspects of the study procedures that they have not understood. The written informed consent form was read out aloud in the native language to the study participants. They were given an opportunity to seek clarifications on any issues that were not understood. Signatures were obtained by the physician who was recruiting the hypertensive patients for the study. The physician also signed and dated the informed consent form. One copy was given to the patients and 1 copy was retained at the study site. Written informed consent was obtained from both the representatives of the cluster (medical officer in charge of the PHC) and individual cluster members (hypertensive patients), and consent was sought before randomization.
Cost‐Effectiveness Analysis
Drummond's 10‐point check list for cost‐effectiveness analysis23 was used as a benchmark for our cost‐effective analysis (CEA). A societal perspective was employed because this would enable the tangible costs to be directly compared between both the intervention arms. The physician services, hospital services, drugs, costs of the DSS software development, and costs for charts (posters, etc.) involved in delivery of health care were included in the direct health care costs. Transportation to and from the site of care were included in the indirect costs. The time horizon for the costs involved was for a 1‐year duration. Costs were discounted at 3%. Quantities and unit prices for each item were calculated in Indian National Rupees and U.S. dollars. Costs for human resources were estimated from within the study. The building maintenance costs, telephone, electrical, vehicle, and other capital costs were sourced from published costing studies done among PHCs in India.24
Cost‐Effectiveness Ratio
A comparison of costs in monetary units with outcomes in quantitative nonmonetary units was done in the CEA. Resources required for intervention and values attached to those resources and effects of treatment (either benefit or harm) and values attached to those effects for both the groups were compared to determine the cost‐effectiveness ratio (CER). CERs were derived as the cost of the intervention per mm reduction in SBP for both the DSS and CBS groups.
Sensitivity Analysis
The hardware equipment costs in the DSS intervention arm are expected to fall with time and increase in usage. The software costs would also reduce given that they would entail no development, but only maintenance costs in the future. Owing to the fact that DSS intervention per se costs form a significant proportion of costs, we performed a sensitivity analysis at 50% and 80% reduction in DSS equipment costs.
Statistical Analysis Plan
Linear mixed‐regression modeling was used to test the hypothesis about difference in mean SBP levels over time by trial group (interventions arms: DSS or CBS), accounting for clustering of observations within centers and individuals over time, using a random‐effects model. Mean difference in change in SBP and DBP (95% confidence interval [CI]) between the baseline and 12th month, for both intervention arms, were reported as unadjusted values. The SBP and DBP difference in changes from baseline between DSS and CBS groups at 12th month of follow‐up were adjusted for age, gender, height, waist, body mass index (BMI), alcohol intake, pickle and papad intake, and portions of vegetable/fruit consumed per day and allowing for clustering of observations within centers and individuals. The linear mixed‐modeling approach included all data available on participants, and the between trial group comparisons correspond to modified intention to treat (mITT) analyses. We performed an mITT, wherein we excluded those patients who were randomized, but had not received the allocated intervention in our analysis (randomized, but not received the allocated intervention: 5 of the 845 patients in the DSS arm and 10 of 793 in the CBS arm). We excluded the patients who had not received the allocated intervention from our analysis since we did not collect their data at 6th and 12th month time points (only baseline data collected). We acknowledge this as a limitation in our analysis plan. Additionally, we performed ITT by including both types of missing data: (1) the patients who were lost to follow‐up after randomization and (2) patients who were randomized, but had not received the intervention (5 and 10 patients in the DSS and CBS groups, respectively). We have conducted further sensitivity analysis by assuming the worst‐case scenario for patients lost to follow‐up (no improvement in BP in the DSS group and a reduction of at least 5 mm Hg in the CBS group). A limitation of our study is that we have not adjusted the alpha for secondary endpoints (lifestyle and dietary changes and BP under control and cost comparisons) analysis.
Results
During the recruitment phase, which lasted from October 2011 to March 2012, 2478 consecutive HTN patients were observed at the OPDs of the study centers and assessed for eligibility to participate in the trial. A total of 521 did not fit our eligibility criterion (age greater than 64 years: 310; hospitalized within the last 12 months: 162; pregnant and lactating females: 24; and participants with a history of physician certified cancer: 25). Response rate was 83.6% (of 1957 eligible patients, 319 had declined to participate). Tables 2 and 3 summarize the baseline features of patients randomized to the 2 groups. There were no significant differences in age, age category (3 age categories: 35 to 44; 45 to 54; and 55 to 64 years), gender, religion, education, smoking status at baseline, and prevalence of pre‐existing HTN status between the 2 groups. The treating physicians who were randomized to receive the DSS agreed with the DSS recommendations and implemented the suggested DSS recommendations in more than 93% of patients at both 0‐ and 12‐month time points (at 0 months: agreed with and implemented DSS in 794 and 787 patients, respectively, of 845 patients at baseline; at 12th month: agreed with and implemented DSS in 747 and 742 patients, respectively, of 781 patients at 12 months).
Table 2.
Baseline Features for CBS and DSS Groups
| Variable | CBS Group N (%) or Mean (SD) | DSS Group N (%) or Mean (SD) |
|---|---|---|
| Gender | ||
| Male % (n) | 409 (51.58) | 419 (49.41) |
| Female | 384 (48.42) | 426 (50.41) |
| Age category | ||
| 35 to 44 years | 117 (14.75) | 126 (14.91) |
| 45 to 54 years | 262 (35.04) | 254 (30.08) |
| 55 to 64 years | 414 (52.21) | 465 (55.03) |
| Education | ||
| None | 390 (52.56) | 457 (54.08) |
| Primary (1 to 4 years of school education) | 190 (25.61) | 227 (26.86) |
| Secondary (5 to 12 years of school education) | 116 (15.63) | 110 (13.02) |
| Above secondary | 46 (6.20) | 51 (6.04) |
| Marital status | ||
| Married | 648 (86.98) | 740 (87.57) |
| Single | 18 (2.42) | 6 (0.71) |
| Divorce | 1 (0.13) | 2 (0.24) |
| Widow | 67 (8.99) | 96 (11.36) |
| Cohabitation (live in partner) | 11 (1.48) | 1 (0.12) |
| Employment status | ||
| Currently employed | 113 (15.48) | 150 (17.75) |
| Unemployed | 457 (62.60) | 558 (66.04) |
| Retired | 17 (2.33) | 23 (2.72) |
| Unemployment benefits | 143 (19.59) | 114 (13.49) |
| Occupation | ||
| Housewife | 217 (29.73) | 227 (32.78) |
| Skilled manual worker | 52 (7.12) | 37 (4.38) |
| Unskilled manual | 159 (21.78) | 117 (20.95) |
| Owner of business | 11 (1.51) | 27 (3.20) |
| Office worker | 18 (2.47) | 25 (2.96) |
| Self‐employed professional | 29 (3.97) | 24 (2.84) |
| Farmer | 244 (33.42) | 243 (28.76) |
| Household income per year | ||
| Less than Rs 24 000 | 311 (42.78) | 324 (38.34) |
| Between Rs 24 000 and Rs 50 000 | 232 (31.91) | 260 (30.77) |
| Between Rs 50 001 and Rs 10 000 | 142 (19.53) | 187 (22.13) |
| Between Rs 100 001 and Rs 200 000 | 32 (4.40) | 46 (5.44) |
| Between Rs 200 001 and Rs 400 000 | 10 (1.38) | 23 (2.72) |
| Height, cm | 151.13 (10.48) | 158.15 (9.97) |
| Weight, kg | 60.64 (11.80) | 61.41 (10.55) |
| Waist, inches | 33.99 (4.11) | 36.99 (5.04) |
| BMI | 26.52 (4.53) | 24.54 (3.66) |
| SBP | 148.01 (15.86) | 151.03 (16.06) |
| DBP | 88.31 (10.19) | 89.44 (9.26) |
| Age, y | 53.50 (7.78) | 53.93 (7.77) |
| HTN duration in years | 3.79 (3.69) | 3.64 (4.1) |
| Prevalence of pre‐existing HTN | 634 (79.9%) | 666 (78.82%) |
| Number of patients on more than 3 medications | 24 (3.7%) | 19 (2.8%) |
| BP | ||
| Stage 2 | 64 (8.1%) | 58 (6.8%) |
| Stage 3 | 29 (3.3%) | 24 (2.8%) |
BP stage 2: SBP in between 160 and 179 mm Hg and DBP <110 mm Hg; BP (OR) DBP in between 100 and 109 mm Hg and SBP <180 mm Hg; BP stage 3: SBP ≥180 mm Hg or DBP ≥110 mm Hg. BMI indicates body mass index; BP, blood pressure; CBS, chart‐based system; DBP, diastolic blood pressure; DSS, decision support system; HTN, hypertension; Rs, rupees (Indian currency); SBP, systolic blood pressure.
Table 3.
Baseline Comparisons: Lifestyle and Dietary Features
| CBS Group: N (%) | DSS Group: N (%) | |
|---|---|---|
| Smoking status | ||
| Yes, he/she currently smokes | 147 (18.85) | 125 (14.79) |
| Yes, but he/she no longer smokes | 100 (12.82) | 121 (14.32) |
| No | 533 (68.33) | 599 (70.89) |
| Cigarettes | ||
| 1 to 5 per day | 12 (37.50) | 20 (60.61) |
| 6 to 10 per day | 9 (28.13) | 8 (24.24) |
| >11 per day | 11 (34.38) | 5 (15.15) |
| Beedies | ||
| 1 to 5 per day | 46 (53.49) | 38 (51.35) |
| 6 to 10 per day | 8 (9.30) | 19 (25.68) |
| >11 per day | 32 (37.21) | 17 (22.97) |
| Oral tobacco | ||
| Chewable tobacco | 42 (53.16) | 45 (41.28) |
| Gutka | 19 (24.05) | 62 (56.88) |
| Paan with tobacco | 18 (22.78) | 2 (1.83) |
| Alcohol | ||
| 7 days in a week | 84 (10.81) | 49 (5.80) |
| 5 to 6 days in a week | 57 (7.34) | 15 (1.78) |
| 2 to 4 days in a week | 60 (7.72) | 56 (6.63) |
| Once per week | 105 (13.51) | 92 (10.89) |
| Less than once a week | 64 (8.24) | 98 (11.60) |
| Don't drink | 407 (52.38) | 535 (63.31) |
| Physical activity | ||
| At least 5 times a week | 264 (33.29) | 270 (31.95) |
| 3 to 4 times a week | 145 (18.28) | 187 (22.13) |
| Less than 3 times a week | 182 (22.95) | 259 (30.65) |
| Never | 152 (19.17) | 92 (10.89) |
| Don't know | 49 (6.18) | 37 (4.38) |
| Sports | ||
| At least 5 times a week | 9 (1.13) | 15 (1.78) |
| 3 to 4 times a week | 38 (4.79) | 22 (2.60) |
| Less than 3 times a week | 23 (2.90) | 60 (7.10) |
| Never | 595 (75.03) | 630 (74.56) |
| Don't know | 128 (16.14) | 118 (13.96) |
| Never | 595 (75.03) | 630 (74.56) |
| Don't know | 128 (16.14) | 118 (13.96) |
| Pickle | ||
| Daily for every meal | 14 (1.86) | 28 (3.31) |
| Once daily | 84 (11.17) | 232 (27.46) |
| At least 3 to 4 times in a week | 103 (13.70) | 213 (25.21) |
| Less than once a week | 551 (73.27) | 372 (44.02) |
| Oil/fat consumed in a month | ||
| 250 to 500 mL | 3 (0.41) | 6 (0.71) |
| 500 mL to 1 L | 2 (0.27) | 29 (3.43) |
| 1 to 1.5 L | 693 (94.80) | 720 (85.21) |
| 1.5 to 2 L | 0 (0) | 63 (7.46) |
| More than 2 L | 33 (4.51) | 27 (3.20) |
| Oil/fat often used for cooking | ||
| Desi ghee | 45 (6.07) | 25 (2.96) |
| Ghee | 83 (11.20) | 71 (8.40) |
| Oil | 379 (51.15) | 387 (45.80) |
| Butter | 72 (9.72) | 157 (18.58) |
| Dalda | 162 (21.86) | 204 (24.14) |
| Vanaspati | 0 (0) | 1 (0.12) |
| Vegetables and fruits consumed per day | ||
| Less than 1 portion in a day | 485 (66.35) | 468 (55.38) |
| 1 portion in a day | 151 (20.66) | 234 (27.69) |
| 2 portions in a day | 28 (3.83) | 77 (9.11) |
| 3 portions in a day | 61 (8.34) | 44 (5.21) |
| 4 portion in a day | 3 (0.41) | 21 (2.49) |
| More than 4 portions in a day | 3 (0.41) | 1 (0.12) |
CBS indicates chart‐based system; DSS, decision support system.
Comparison of Unadjusted SBP at 0 and 12 Months (Within Group)
Statistically significant differences were found in the DSS arm when unadjusted mean SBP (139.9; 95% CI: 135.1 to 144.8) at the 12th month was compared to the unadjusted mean SBP (151.1; 95% CI: 146.9 to 155.3) at 0 months (P<0.001). Significant differences were not found in the CBS arm when unadjusted mean SBP (144.7; 95% CI: 140.7 to 148.9) at the 12th month was compared to the unadjusted mean SBP (148.2; 95% CI: 143.3 to 153) at 0 months (P=0.0670).
Comparison of Unadjusted DBP at 0 and 12 Months (Within Group)
Statistically significant differences were found in DSS arm when unadjusted mean DBP (84.3; 95% CI: 82.0 to 86.5) at the 12th month was compared to the unadjusted mean DBP (89.7; 95% CI: 87.7 to 91.7) at 0 months (P<0.001). Significant differences were not found in the CBS arm when unadjusted mean DBP (86.3; 95% CI: 84.6 to 87.9) at the 12th month was compared to the unadjusted mean DBP (88.4; 95% CI: 85.9 to 90.8) at 0 months (P=0.06).
Comparisons Between the DSS and CBS Arms
The unadjusted (without covariate adjustments, but allowing for clustering of observations within centers and individuals) mean difference in SBP change from baseline between DSS and CBS groups at 12 months was −7.11 mm Hg (95% CI: −13.11 to −2.23 mm Hg; P=0.008). The unadjusted mean difference in DBP change from baseline between DSS and CBS groups at 12 months was −3.29 mm Hg (95% CI: −6.32 to −0.87 mm Hg; P=0.041). The mean difference in SBP change from baseline between the DSS and CBS groups at the 12th month, adjusted for age, gender, height, waist, BMI, alcohol intake, pickle and papad intake, and portions of vegetable or fruit consumed per day was −6.59 mm Hg (95% CI: −12.18 to −1.42 mm Hg; P=0.021). The mean difference in DBP change from baseline between the DSS and CBS at the 12th month, adjusted for age, gender, height, waist, BMI, alcohol intake, pickle and papad intake, and portions of vegetable/fruit consumed per day between both the groups was −2.83 mm Hg (95% CI: −5.78 to 0.13 mm Hg; P=0.083). The chosen variables to adjust for differences between the 2 groups were based on the baseline data differences between CBS and DSS groups, systematic literature review performed by our group, and other articles (from India) that reported on the associations between the covariate(s) and HTN.25–36 Table 4 and Figure 3 show the unadjusted and adjusted mean difference in SBP and DBP change from baseline between DSS and CBS groups at 6 and 12 months.
Table 4.
SBP and DBP Difference in Changes From Baseline Between DSS and CBS Groups at 6th and 12th Month of Follow‐up
| Variable | Trial Arm | Month | Difference (95% CI) vs. Baseline for Each Randomized Group | P Value | Difference (95% CI) in Changes From Baseline Between Trial Arms | P Diff Value |
|---|---|---|---|---|---|---|
| (A) Without covariate adjustments | ||||||
| Difference in SBP | CBS | 6 | −2.57 (−7.98 to 3.58) | 0.39 | Ref | — |
| Difference in SBP | DSS | 6 | −7.5 (−12.23 to −2.31) | 0.003 | −5.01 (−11.74 to 1.69) | 0.131 |
| Difference in SBP | CBS | 12 | −4.11 (−6.79 to 0.87) | 0.06 | Ref | — |
| Difference in SBP | DSS | 12 | −11.42 (−14.93 to −7.12) | <0.001 | −7.11 (−13.11 to −2.23) | 0.008 |
| Difference in DBP | CBS | 6 | −0.92 (−3.84 to 2.1) | 0.71 | Ref | — |
| Difference in DBP | DSS | 6 | −6.78 (−8.12 to −3.21) | <0.001 | −5.71 (−9.79 to −2.34) | 0.005 |
| Difference in DBP | CBS | 12 | −2.79 (−4.91 to −0.22) | 0.048 | Ref | — |
| Difference in DBP | DSS | 12 | −5.02 (−7.94 to −3.39) | <0.001 | −3.29 (−6.32 to −0.87) | 0.041 |
| (B) Adjusted for covariates | ||||||
| Difference in SBP | CBS | 6 | −2.11 (−7.24 to 3.02) | 0.40 | Ref | — |
| Difference in SBP | DSS | 6 | −6.42 (−11.12 to −1.29) | 0.027 | −4.19 (−11.21 to 2.88) | 0.30 |
| Difference in SBP | CBS | 12 | −3.59 (−7.71 to 0.12) | 0.061 | Ref | — |
| Difference in SBP | DSS | 12 | −10.13 (−14.24 to −6.45) | <0.001 | −6.59 (−12.18 to −1.42) | 0.021 |
| Difference in DBP | CBS | 6 | −0.71 (−3.0 to 2.07) | 0.72 | Ref | — |
| Difference in DBP | DSS | 6 | −5.97 (−8.57 to −3.92) | <0.001 | −5.23 (−9.11 to −1.47) | 0.003 |
| Difference in DBP | CBS | 12 | −2.82 (−4.72 to 0.97) | 0.06 | Ref | — |
| Difference in DBP | DSS | 12 | −5.09 (−7.64 to −2.97) | <0.001 | −2.83 (−5.78 to 0.13) | 0.083 |
Without covariate adjustments allows for clustering of observations within centers and individuals; covariates included: age, gender, height, waist, BMI, alcohol, pickle intake, vegetable/fruit intake; P value=P value for difference (95% CI) vs. baseline for each randomized group; P diff value=P value for difference (95% CI) in changes from baseline between trial arms. CBS indicates chart‐based system; CI, confidence interval; DBP, diastolic blood pressure; DSS, Decision support system; SBP, systolic blood pressure.
Figure 3.
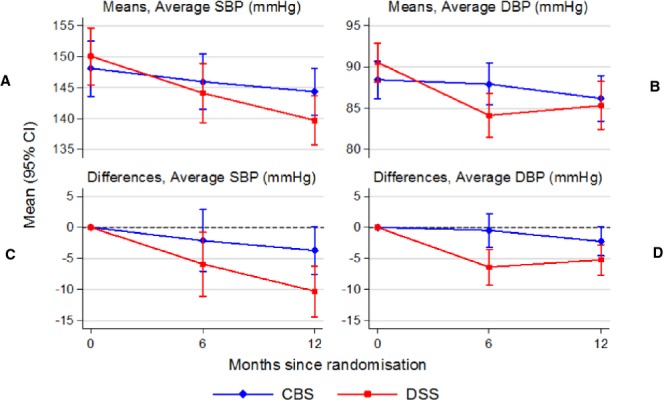
Mean blood pressure in randomized groups by month and differences versus baseline. CBS indicates chart‐based support; CI, confidence interval; DBP, diastolic blood pressure; DSS, decision support systems; SBP, systolic blood pressure.
Comparison of BP Under Control
Figure4 depicts the number of individuals who had their BP under control in both the groups at 0 and 12 months. The CBS group did not show an improvement in BP control, whereas the DSS group significantly improved BP control from 12.6% at baseline to 40.8% at the end of the study (P<0.001).
Figure 4.

Comparison of CBS and DSS groups: BP under control (SBP <140 and DBP <90 mm Hg). BP indicates blood pressure; CBS, chart‐based support; DBP, diastolic blood pressure; DSS, decision support systems; SBP, systolic blood pressure.
Comparison of Lifestyle and Dietary Factors Between CBS and DSS Arms
Table 5 shows the comparison of lifestyle and dietary factors between CBS and DSS at the 12th month. Significant differences were noted between the CBS and DSS groups at the 12th month for current smokers (13.2% vs. 9.9%), nonsmokers (67.6% vs. 75.3%), consumption of alcohol more than or equal to less than once a week (26.5% vs. 20.9%), taking part in physical activity at least 5 or more times in a week for at least 30 minutes a day (87.4% vs. 94%), intake of pickle less than once a week (77.3% vs. 47.2%), usage of ghee (13.1% vs. 7.3%), and consumption of 2 or more portions of vegetables/fruits per day (12.1% vs. 42.5%).
Table 5.
Comparison of Lifestyle and Dietary Factors Between CBS and DSS at 12th Month
| Variable | CBS at 12th Month | DSS at 12th Month | P Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Do you smoke, or have you ever smoked | |||||
| Yes, he/she currently smokes | 102 | 13.16 | 75 | 9.96 | <0.001 |
| Yes, but he/she longer smokes | 149 | 19.23 | 111 | 14.74 | |
| No | 524 | 67.61 | 567 | 75.29 | |
| Cigarettes | |||||
| 1 to 5 per day | 21 | 70.00 | 29 | 63.04 | 0.027 |
| 6 to 10 per day | 8 | 26.67 | 11 | 23.91 | |
| >11 per day | 1 | 3.33 | 6 | 13.04 | |
| Beedies | |||||
| 1 to 5 per day | 57 | 58.76 | 50 | 62.50 | 0.001 |
| 6 to 10 per day | 20 | 20.62 | 21 | 26.25 | |
| >11 per day | 20 | 20.62 | 9 | 11.25 | |
| Alcohol | |||||
| 7 days in a week | 51 | 6.76 | 27 | 3.59 | <0.001 |
| 5 to 6 days in a week | 37 | 4.91 | 20 | 2.66 | |
| 2 to 4 days in a week | 42 | 5.57 | 42 | 5.58 | |
| Once per week | 70 | 9.28 | 68 | 9.03 | |
| Less than once per week | 32 | 4.24 | 91 | 12.08 | |
| Don't drink | 522 | 69.23 | 505 | 67.07 | |
| Physical activity | |||||
| At least 5 times in a week | 339 | 43.63 | 282 | 37.45 | <0.001 |
| 3 to 4 times in a week | 170 | 21.88 | 169 | 22.44 | |
| Less than 3 times in a week | 170 | 21.88 | 257 | 34.13 | |
| Never | 94 | 12.10 | 32 | 4.25 | |
| Don't know | 4 | 0.51 | 13 | 1.73 | |
| Sport | |||||
| At least 5 times in a week | 9 | 1.16 | 60 | 7.97 | <0.001 |
| 3 to 4 times in a week | 53 | 6.82 | 72 | 9.56 | |
| Less than 3 times in a week | 52 | 6.69 | 46 | 6.11 | |
| Never | 519 | 66.80 | 518 | 68.79 | |
| Don't know | 144 | 18.53 | 57 | 7.57 | |
| Pickle | |||||
| Daily for every meal | 17 | 2.22 | 15 | 1.99 | <0.001 |
| Once daily | 98 | 12.81 | 139 | 18.46 | |
| At least 3 to 4 times in a week | 59 | 7.71 | 242 | 32.14 | |
| Less than once a week | 591 | 77.25 | 357 | 47.41 | |
| Oil/fat consumed | |||||
| 250 to 500 mL | — | — | 2 | 0.27 | <0.001 |
| 500 mL to 1 L | 4 | 0.52 | 60 | 7.97 | |
| 1 to 1.5 L | 761 | 99.09 | 684 | 90.84 | |
| 1.5 to 2 L | — | — | 4 | 0.53 | |
| More than 2 L | 3 | 0.39 | 3 | 0.40 | |
| Oil/fat often in cooking | |||||
| Desi ghee | 36 | 4.64 | 3 | 0.40 | <0.001 |
| Ghee | 102 | 13.14 | 55 | 7.30 | |
| Oil | 458 | 59.02 | 437 | 58.03 | |
| Butter | 68 | 8.76 | 148 | 19.65 | |
| Dalda | 112 | 14.43 | 110 | 14.61 | |
| Fruits and vegetables | |||||
| Less than 1 portion | 472 | 61.22 | 297 | 39.44 | <0.001 |
| 1 portion | 205 | 26.59 | 136 | 18.06 | |
| 2 portion | 49 | 6.36 | 195 | 25.90 | |
| 3 portion | 40 | 5.19 | 82 | 10.89 | |
| 4 portion | 3 | 0.39 | 31 | 4.12 | |
| More than 4 portions | 2 | 0.26 | 12 | 1.59 | |
CBS indicates chart‐based system; DSS, decision support system.
Loss to Follow‐up
A difference in loss to follow‐up was noticed between DSS (845 patients at baseline, 64 patients lost to follow‐up at 12th month; loss=7.5%) and CBS (793 patients at baseline, 7 patients lost to follow‐up at 12th month; loss ≤1%) arms at the end of 12 months of follow‐up. To address the discrepancy in the loss to follow‐up rates between both arms, we conducted further sensitivity analysis by imputing the missing data assuming a worst‐case scenario (no improvement in BP in the DSS group and a reduction of at least 5 mm Hg in the CBS group). The trend for the results remained unchanged in the worst‐case scenario (unadjusted SBP change [95% CI] from baseline to 12 months was −8.09 [−11.17, −4.92] mm Hg in DSS vs. −4.10 [−7.33, −1.56] mm Hg in CBS).
ITT Analysis
Upon inclusion of the patients who were randomized but had not received the intervention (5 and 10 patients in the DSS and CBS arms, respectively), and by imputing the missing values assuming the worst‐case scenario for patients lost to follow‐up, the mean difference in SBP change from baseline between the DSS and CBS at 12th month was –4.54 mm Hg (95% CI: −5.89 to −3.18 mm Hg; P=0.013) and the mean difference in DBP change from baseline between the DSS and CBS at the 12th month was −1.89 mm Hg (95% CI: −2.74 to −1.03 mm Hg; P=0.01).
Stratified Analysis of Primary Outcome Based on Stage of BP
In the DSS group, at the study beginning, the proportion of patients having BP under control (SBP <140 mm Hg and DBP <90 mm Hg), stage 1 BP (SBP 140 to 159 mm Hg or DBP 90 to 99 mm Hg), stage 2 BP (SBP 160 to 179 mm Hg or DBP 100 to 109 mm Hg), and stage 3 BP (SBP >180 mm Hg or DBP >110 mm Hg) was 12.6%, 60.02%, 21.2%, and 6.06% (106, 503, 178, and 51 patients). At the study end, the proportion of patients having normal, stage 1, stage 2, and stage 3 BP had changed to 40.7%, 52.19%, 5.4%, and 0.7%, respectively (307, 393, 47, and 6 patients). In the CBS group, at the study beginning, the proportion of patients having normal BP, stage 1, stage 2, and stage 3 BP was 23.7%, 47.6%, 21.8%, and 6.7%, respectively (186, 373, 171, and 53 patients). At the study end, the proportion of patients having normal, stage 1, stage 2, and stage 3 BP had changed to 22.9%, 64.03%, 10.7%, and 2.3%, respectively (178, 495, 83, and 18 patients).
Given that the proportion of patients having BP under control varied between both the groups (12.6% in DSS vs. 23.7% in CBS), we performed a matched analysis. Upon comparing the patients with BP under control at baseline, the mean difference in SBP between both the CBS (N, mean [SD]: 186, 130.7 [7.3]) and DSS (N, mean [SD]: 106, 128.1 [1.0]) groups was −2.6 mm Hg (95% CI: −4.6 to −0.5; P=0.01), which was significantly higher in the CBS group. Upon comparing the patients with BP under control at baseline, the mean difference in DBP between both the CBS (N, mean [SD]: 186, 80.8 [5.3]) and DSS (N, mean [SD]: 106, 78 [8.9]) was 2.8 mm Hg (95% CI: 1.1 to 4.4; P=0.01), which was significantly higher in the CBS group. The baseline SBP mean (SD) values in the CBS group for stage 1, 2, and 3 (n=373, 168, and 53 patients, respectively) was 146.2 (7.2), 163.1 (11), and 173.8 (17.9) mm Hg, respectively. Baseline SBP mean (SD) values in the DSS group for stage 1, 2, and 3 (n=503, 178, and 51 patients, respectively) was 147.5 (6.8), 164.2 (9.2), and 186.7 (14.1) mm Hg, respectively. Baseline DBP mean (SD) values in the CBS group for stage 1, 2, and 3 were 86.5 (7.2), 94.8 (8.3), and 107.4 (10.1) mm Hg, respectively. Baseline DBP mean (SD) values in the DSS group for stage 1, 2, and 3 were 89.2 (5.6), 92.2 (8.1), and 105.3 mm Hg, respectively. There was no statistically significant difference in the baseline SBP values for stage 2 hypertensive patients between the groups (−1.1 mm Hg, 95% CI: −3.2 to 1.03; P=0.31); however, statistically significant difference in baseline SBP (between CBS and DSS groups) was noted for patients who had their BP under control, and for patients who were in stage 1 and 3 BP (−1.3 mm Hg, 95% CI: −2.2 to −0.3, P=0.006; −12.9 mm Hg, 95% CI: −19.2 to −6.5, P<0.001, respectively).
Within‐Group Comparison Between 0 and 12th Month Based on Stages of BP
A 0‐ and 12th‐month comparison was done to find out the mean change in SBP and DBP (within‐group difference at 0‐ and 12th‐month readings) among all patients in the stage 1, 2, and 3 BP categories. All patients suffering from BP stages 1 to 3 in the DSS group (n at 0 month=503, 178, and 51; n at 12th month=460, 151, and 38, respectively) had a statistically significant change in mean SBP (stage 1: 147.5 [6.8] vs. 140 [8.5], P<0.001; stage 2: 164.1 [9.2] vs. 147.8 [12.2], P<0.001; and stage 3: 186.7 [14.1] vs. 153.3 [16.8], P<0.001) and DBP levels (stage 1: 89.1 [5.5] vs. 85.1 [6.3], P<0.001; stage 2: 92.1 [8.1], 85.7 [6.7], P<0.001; and stage 3: 105.3 [12.9] vs. 87.69 [9.9], P<0.001) at the study end, when compared with the baseline.
Patients suffering from BP stage 2 (n=167) in the CBS group had a statistically significant change in SBP (mean [SD] at 0 and 12 months: 163.1 [11], 149.5 [12.6], P<0.001) and DBP (mean [SD] at 0 and 12 months: 94.8 [8.2], 88.2 [9.1] P<0.001). Patients suffering from stage 3 BP in the CBS group (n=52) had a statistically significant change in SBP (mean [SD] at 0 and 12 months: 173.8 [17.9], 153.3 [13.9], P<0.001) and DBP (107.4 [10.1], 90.1 [10.4], P<0.001) at the study end, when compared with the baseline. However, stage 1 BP patients in the CBS (n=372) did not a show a statistically significant mean change in either SBP or DBP (mean SBP and DBP [SD] at 0 and 12 month: SBP, 146.2 [7.1], 145.2 [11.3], P=0.13; DBP, 86.52 [7.2], 86.03 [6.7], P=0.33).
Between‐Group Comparison Between 0 and 12th Month Based on Stages of BP
Among stage 1 BP patients, difference in mean SBP change between the CBS and DSS group at the 12th month was −5.3 mm Hg (95% CI: −7.3 to −4.5; P<0.001). Among stage 2 BP patients, difference in mean SBP change between the CBS and DSS group at the 12th month was −1.9 mm Hg (95% CI: −5.1 to 1.2; P=0.22). Among stage 3 BP patients, difference in mean SBP change between the CBS and DSS group at the 12th month was –14.1 mm Hg (95% CI: −22.5 to −5.6; P<0.001).
Among stage 1 BP patients, difference in mean DBP change between the CBS and DSS group at the 12th month was −1.1 mm Hg (95% CI: −2.5 to −0.31 P=0.01). Among stage 2 BP patients, difference in mean DBP change between the CBS and DSS group at the 12th month was −4.6 mm Hg (95% CI: −6.5 to −2.6; P<0.001). Among stage 3 BP patients, difference in mean DBP change between the CBS and DSS group at the 12th month was −16.1 mm Hg (95% CI: −21.1 to −10.93; P<0.001).
Cost Comparisons
In a usual care scenario, the direct and indirect costs put together worked out to be $30 330 per year per PHC (Table 6). Average unit cost estimates per outpatient visit without drug or diagnostics (adjusted for inflation for the years 2008–2012) were calculated to be $22.34. The total costs for DSS intervention were $19 513 and the total costs for CBS were $7510. Tables 7 and 8 provide the calculations for the cost estimates for both the interventions. Briefly, in the DSS arm, the equipment cost was $2852.06 and development and validation of DSS costs were $10 622.64, whereas in the CBS arm equipment cost was $515.46 and poster and stationary cost (data collection forms) was $956.6. Similar costs were incurred for doctor compensation charges ($3018) and patient travel compensation costs ($3018) in both the groups. The cost per patient in the DSS arm was $370.48, whereas the cost in the CBS arm per patient was $344.69 (Table 9).
Table 6.
Health System Costs for a 1‐Year Time Period per PHC
| Source | INR Per Month | No. of Personnel Per PHC/Units | Costs Per Year (INR/Year) | US Dollars Per ($/Year) | |
|---|---|---|---|---|---|
| Personnel costs | |||||
| (A) Recurrent costs | |||||
| Physician salary/month | Estimated within study | 30 000.00 | 1.00 | 360 000.00 | 6792.45 |
| Staff nurses | Estimated within study | 12 190.00 | 2.00 | 292 560.00 | 5520.00 |
| Second ANMs | Estimated within study | 10 200.00 | 1.00 | 122 400.00 | 2309.43 |
| Pharmacist | Estimated within study | 12 190.00 | 1.00 | 146 280.00 | 2760.00 |
| Lab technician | Estimated within study | 9000.00 | 1.00 | 108 000.00 | 2037.74 |
| Data entry operator | Estimated within study | 9500.00 | 1.00 | 114 000.00 | 2150.94 |
| Health worker (female) | Estimated within study | 10 020.00 | 1.00 | 120 240.00 | 2268.68 |
| Health assistant (male) | Estimated within study | 10 020.00 | 1.00 | 120 240.00 | 2268.68 |
| Health assistant (female)/lady health visitor | Estimated within study | 9000.00 | 1.00 | 108 000.00 | 2037.74 |
| Multiskilled Group D worker | Estimated within study | 6700.00 | 2.00 | 160 800.00 | 3033.96 |
| Sanitary worker cum watchman | Estimated within study | 6700.00 | 1.00 | 80 400.00 | 1516.98 |
| Accredited Social Health Activists (ASHA) | Estimated within study | 800.00 | 6.00 | 57 600.00 | 1086.79 |
| Cost of consumables, electrical charges, telephone charges, building maintenance, vehicle charges | Published literature | 6250.00 | 12.00 | 75 000.00 | 1415.09 |
| Drugs—provided by the government | 0.00 | 0.00 | 0.00 | 0.00 | |
| Laboratory—blood and urine—free | 0.00 | 0.00 | 0.00 | 0.00 | |
| Total recurrent cost (Rs) | 132 570.00 | 1 590 840 | 30 015.85 | ||
| (B) Capital cost | |||||
| PHC building depreciation | Published literature | 709.67 | 8516.00 | 160.68 | |
| Subcenter building depreciation (6) | Published literature | 368.89 | 4426.66 | 83.52 | |
| Furniture depreciation | Published literature | 282.31 | 3387.73 | 63.92 | |
| Electrical fitting depreciation | Published literature | 24.79 | 297.51 | 5.61 | |
| Vehicle depreciation | Published literature | 0.00 | 0.00 | 0.00 | |
| Total capital cost | 1385.66 | 16 627.90 | 313.73 | ||
| Total cost (A+B) | 133 955.66 | 1 607 467 | 30 329.58 | ||
ANM indicates auxillary nurse midwife; INR, Indian National Rupees; PHC, primary health care center; US$, United States dollars.
Table 7.
Intervention Costs for DSS Group
| Items | Cost/Unit (INR) | No. of PHCs | Amount (INR) | Amount (US$) |
|---|---|---|---|---|
| Equipment cost | ||||
| Girth measuring tape | 15 | 8 | 120 | 2.26 |
| Height measuring scale, 2 m, wall mounted | 450 | 8 | 3600 | 67.92 |
| Patient weighing scale, mechanical, (Seca Nera Big, 150‐kg capacity) | 850 | 8 | 6800 | 128.30 |
| netbook/notebook/laptop costs | 15480 | 8 | 123 840 | 2336.60 |
| Automatic digital blood pressure monitor (Omron HEM‐7203) | 2100 | 8 | 16 800 | 316.98 |
| DSS, development cost | 563 000 | 10 622.64 | ||
| Patient travel compensation | 160 000 | 3018.87 | ||
| Doctor compensation and training costs | 160 000 | 3018.87 | ||
| Grand total | 1 034 160 | 19 512.45 | ||
DSS indicates decision support system; INR, Indian National Rupees; PHC, primary health care center; US$, United States dollars.
Table 8.
Intervention Costs for CBS Group
| Items | Cost/Unit (INR) | No. of PHCs | Amount (INR) | Amount (US$) |
|---|---|---|---|---|
| Equipment cost | ||||
| Girth measuring tape | 15 | 8 | 120 | 2.26 |
| Height measuring scale, 2 m, wall mounted | 450 | 8 | 3600 | 67.92 |
| Patient weighing scale, mechanical (Seca Nera Big, 150‐kg capacity) | 850 | 8 | 6800 | 128.30 |
| Automatic digital blood pressure monitor (Omron HEM‐7203) | 2100 | 8 | 16 800 | 316.98 |
| Patient travel compensation | 160 000 | 3018.87 | ||
| Poster charges | 18 380 | 346.79 | ||
| Doctor compensation and training costs | 160 000 | 3018.87 | ||
| Stationary (data collection forms) | 30 070 | 567.36 | ||
| Translation charges | 2250 | 42.45 | ||
| Grand total | 398 020 | 7509.81 | ||
CBS indicates chart‐based support; INR, Indian National Rupees; PHC, primary health care center; US$, United States dollars.
Table 9.
Comparison of Total Costs Involved for Both Groups for 1 Year
| Items | DSS | CBS |
|---|---|---|
| Health system cost in USD | $242 637 ($30 330 per PHC; total for 8 PHCs) | $242 637 ($30 330 per PHC; total for 8 PHCs) |
| Patient care costs, OPD health care costs in USD | $16 822.02 ($22.34 for each patient; total for 753 patients) | $17 335.84 ($22.34 for each patient; for 776 patients) |
| Intervention costs in USD | $19 512.45 (for 8 PHCs) | $7509.81 (for 8 PHCs) |
| Total costs | $278 971.79 | $267 482.65 |
| Cost per patient | $370.48 | $344.69 |
CBS indicates chart‐based support; DSS, decision support systems; OPD, outpatient department; PHC, primary health care center.
Sensitivity Analysis for Costings
Equipment costs contributed a significant proportion of DSS costs. If equipment prices fall by 50%, estimated mean costs per year for the DSS group would decrease from $19 513 to $12 776. If equipment prices fall by 80%, the DSS mean costs per year would be $8733 (less than 50% of total costs for DSS intervention). However, total costs of the DSS group would remain slightly higher than those of the CBS group (difference of $1223). SDs for the equipment costs could not be calculated owing to the fact that the procurement was centralized and each center had the same model and make of equipment.
Cost‐Effectiveness Ratios
The average costs per patient for the CBS group patient ($344.69) divided by the average reduction in SBP over a 12‐month period (3.59 mm Hg) yielded a CER of $96.01 per mm reduction in SBP for the CBS group; the corresponding CER for DSS intervention arm was $36.57 ($370.48 divided by 10.13) per mm reduction in SBP.
Discussion
Our cluster randomized trial has shown that DSS help physicians to undertake guideline‐based risk staging, prescribe appropriate drug therapy, provide follow‐up advice, and offer tailor‐made counseling suggestions to patients based on their lifestyle and dietary factors. The adjusted mean difference in systolic BP, at 12th month of follow‐up, between the DSS and CBS in our study was −6.59 mm Hg (95% CI: −12.18 to −1.42 mm Hg; P=0.021). The adjusted mean difference in DBP, at 12th month of follow up, between the DSS and CBS intervention arms was statistically not significant (−2.83 mm Hg, 95% CI: −5.78 to 0.13 mm Hg, P=0.083). Statistically significant differences between baseline and 12th month of follow‐up have been found for both SBP and DBP in the DSS arm. A trend toward statistical significance for both differences between baseline and 12th month of follow‐up in SBP and DBP was noted even in the CBS arm (P values of 0.061 and 0.06, respectively). The CERs for CBS and DSS groups ($96.01 and $36.57 per mm of SBP reduction) are very cost‐effective because they are less than the per‐capita gross domestic product of India ($1509; most recent estimate from World Bank in 2011).
The magnitude of difference for SBP between the intervention arms at study endpoint that has been found in our study (−6.59 mm Hg; 95% CI: −12.18 to −1.42 mm Hg) has not been shown in any of the other DSS studies done thus far in the developed world. This could have arisen owing to 3 reasons. First, our cluster randomized study has been undertaken in a resource‐limited PHC setting, wherein the experience of the treating doctors and the health care staff for management of BP on clinical guideline based management is limited owing to the fact that the usual practice is to refer all cases to second‐tier health care (to the nearest CHC for initial screening and management of all hypertensive patients). Second, rigorous adherence of all sequential steps in the DSS algorithm by all the participating physicians made sure that risk, staging, history, and measurements were followed up with tailor‐made recommendations on drug management and counseling for lifestyle support. Finally, repeated counseling on lifestyle modifications at 0, 6, and 12 months by treating physicians and regular governmental supply of quality antihypertensives may have yielded the desired results.
Our study does not show a statistical significance for the adjusted mean difference in DBP, at 12th month of follow‐up, between the DSS and CBS in our study. This could have arisen owing to various reasons. First, it has been documented, in clinical trials, that restriction of salt intake results in greater decrease in SBP than in DBP37–38 (restricting salt intake to 80 mmol daily reduces SBP by 4.3 mm Hg and DBP by 2 mm Hg39). Consumption of salty food reduced in both groups at study end, when compared with baseline intake (owing to the lifestyle counseling given at 0‐, 6‐, and 12‐month time points), and thus might explain the greater drop in SBP, as compared to DBP, in both the study groups. Second, changes in lifestyle and dietary habits, such as increased physical activity, reduced alcohol consumption, and increased consumption of fruits and vegetables, all of which are known to lower SBP more than DBP,40–43 may have led to more SBP reduction, as compared to DBP, in our study. Third, the magnitude of within‐group reduction in SBP at study end was more than the magnitude of within‐group reduction in DBP (SBP: 10.13 mm Hg in the DSS group and 3.59 mm Hg in the CBS group; DBP: 5.09 mm Hg in the DSS group and 2.82 mm Hg in the CBS group), which could have caused a statistical significant difference in SBP and an insignificant difference in DBP between DSS and CBS groups at the 12th month.
Strengths and Limitations
This study has several important strengths.
Blending within existing clinical work flow: We followed the SAGE (Standards‐Based Sharable Active Guideline Environment) consortium project recommendations by developing a DSS that has “a complex clinical guideline as a series of recommendation sets.”44 Furthermore, we followed the SAGE guidelines model, which suggests that DSS must seamlessly blend within the clinical work flow nonobtrusively and allow for easy inspection of the underlying clinical logic, by having “info” buttons displaying the logic behind the recommendations.44
Stakeholder involvement in DSS development process: A validated and pilot‐tested DSS was developed in resource‐constrained settings after extensive consultations and focus group discussions with all stakeholders involved.
Reporting on patient outcomes: Computerised clinical DSS (CCDSS) have reported an improvement in physician performance and in quality of care given for patients, when CCDSS were deployed in developed countries.3,13 Ours is the first study that has documented an improvement in patient‐related outcomes for managing HTN at a primary health care level in low‐ and middle‐income country settings (LMICs).
Multicomponent intervention with concurrent advice to physicians and patients in institutional settings: A recent review reported that system‐initiated CCDSS in institutional settings performed better than ambulatory settings.45 Our DSS‐HTN has been set up in PHCs, which are the first gateway for institutional health care provider services in India. Adding a multicomponent intervention (clinical guidelines for the physician and lifestyle counseling for the patients) has yielded a comprehensive risk management strategy, thereby making our DSS‐HTN an effective tool for management of CVDs among hypertensive patients. In a recent decision maker/research partnership systematic review on CCDSS, transparent documentation of steps to clearly mention the design of the system, context in which CCDSS are deployed, implementation process, costing involved, user satisfaction, and impact on workflow patterns have been stated as key factors that ensure success in development and implementation of DSS.46 Our study has taken care to carefully delineate the above‐mentioned key factors.
Inclusion of cost‐effective results: Very few DSS studies from the developed world have reported on cost‐effectiveness parameters. We studied the costs and the effect‐size gains involved among both the interventions and have arrived at reasonable estimates of accuracy (all our costs have been based on WHO‐CHOICE 200847 information, adjusted to inflation for the years 2009–2012).
Our DSS‐HTN study meets 4 of the top 5 key DSS services and capabilities that have been listed in the consensus assessment of the health level 7 clinical decision support work group report.48 “Clinical data query service, user communication service, use of appropriate and standard information models and the ability to leverage the DSS,” which were ranked on an absolute important scale,48 and are the key services and capabilities needed for a service‐oriented architecture, are present in our DSS‐HTN. Finally, we have strived to provide recommendations, rather than mere assessments, for the physician in his daily workflow pattern at the time and location of his or her decision making, which have been identified as independent predictors for improving clinical practice when using CDSS.49
Our study has some potential limitations. First, our study did not have a usual care (UC) group. The comparison of DSS was done with the CBS group in our study. Given that the quality of usual care is very low in PHC settings in India, the ethical committee suggested having a chart‐based support system in place of UC, wherein the chart‐based system mimics all that is in a DSS, except that it is optional for the physician to refer to the pocket guidelines in CBS, whereas the physicians in the DSS group necessarily had to go step by step using the software processes in the netbooks. Our original intention to perform a cluster randomized trial with 3 arms—DSS, CBS, and UC—was not feasible in our settings owing to limitations in budget, higher sample‐size estimates, and lack of ethical committee's approval to undertake a 3‐arm study. Second, there was a considerable difference between the 2 treatment groups with respect to the number of patients with BP under control at baseline (186 participants in CBS and 106 in DSS). Having fewer patients with BP under control could have allowed participants in the DSS group more opportunity to improve BP. We attempted to overcome this limitation by doing a subgroup analysis based on the stages of BP. In the between‐group comparison at baseline, a significant difference in SBP was noted for patients in stage 1 and 3 BP. However, the between‐group comparison at the 12th month showed a significant reduction in BP even after accounting for between‐group baseline differences. The mean difference in SBP change from baseline between the DSS and CBS at the 12th month for patients in all stages of BP was significantly higher than the mean difference (between group) in SBP values at baseline (stage, 12th‐month difference versus 0‐month difference in SBP, stage 1: −5.3 vs. −1.3 mm Hg; stage 2: −1.9 vs. −1.1 mm Hg; stage 3: −14.5 vs. −12.9 mm Hg, respectively).
Third, a difference in loss to follow‐up was noticed between DSS (7.5%) and CBS (<1%) arms at the end of 12 months of follow‐up. To address the discrepancy in the loss to follow‐up rates between both the arms, we conducted further sensitivity analysis by imputing the missing data assuming a worst‐case scenario, although we acknowledge that multiple imputation may be a better approach for dealing with missing data on the covariates as well. We used a linear mixed model for inferences, which allowed the inclusion of all available data from the participants at baseline and during follow‐up in the modeling. Furthermore, because the modeling allowed for clustering at the center level and modeled the within‐subject correlations over time, the likelihood‐based inferences would have been unbiased under the missing‐at‐random assumption.
Fourth, a majority (90%) of the study‐site population is rural and this may have affected the generalizability of the findings to urban areas in India. However, this may have a limited impact given that 68.84% of the Indian population resides in rural areas (Indian Census 2011 data).50 Moreover, because our target was resource‐constrained PHCs, we chose rural settings. However, our study findings would be applicable to primary care settings in other LMICs given that they, too, suffer from similar problems, including lack of trained manpower and deficient health system infrastructure.
Finally, our study does not integrate the data from patient electronic records and hospital clinical information systems owing to the fact that the infrastructure for health management information system is still at a very primitive stage in India. The knowledge‐based engine in our system, built mostly on “if and then” scenarios, limits itself to management of HTN only at primary care settings. Similarly, potential interactions with other drugs that would have had an effect on BP have not been built in the system. Referral scenarios are suggested when the reasoning engine is confronted with complex data that can be managed only at secondary and tertiary care settings.
Conclusion
DSS embedded with clinical practice guidelines aid implementation of evidence‐based clinical practice guidelines for management of HTN even in resource‐limited settings. DSS result in better management of HTN, provided patients adhere to the suggested dietary and lifestyle modifications, and medications and providers adhere to the suggested DSS recommendations. Key features of the DSS include (1) patient‐specific, tailor‐made, and guideline‐based recommendations on risk factors and disease management of HTN and (2) counseling on lifestyle modification, both of which aid the end user (clinicians) to decide on the appropriate line of management for the patient. The DSS was not only effective, but also cost‐effective in management of HTN. Future studies on DSS should be of a longer duration and look at assessing (1) the effectiveness of the intervention in reducing the number of MI and stroke cases and (2) cost utility of the DSS, which, when answered, would aid in deciding the scalability and replicability of DSS in similar LMIC settings.
Author Contributions
Conceived and designed the experiments: Anchala, Prabhakaran and Franco. Performed the experiments: Anchala. Database management: Pant. Analyzed the data: Anchala, Kaptoge, and Pant. Contributed materials/analysis tools: Anchala, Pant, and Kaptoge. Wrote the initial manuscript: Anchala. Critical comments on the manuscript: Kaptoge, Pant, Angelantonio, Prabhakaran, Franco.
Supplementary Material
Table S1 Referral criteria to a higher secondary or tertiary health care center.
Sources of Funding
This work was supported by a Wellcome Trust Capacity Strengthening Strategic Award to the Public Health Foundation of India and a consortium of UK universities. The funders had no role in the study design and analysis.
Disclosures
Prabhakaran has received honoraria from Torrent Pharmaceuticals for being a member of the data safety and monitoring board and has received funds from Medtronic for a similar kind of study in 2011–2013.
Acknowledgments
The authors thank the 16 PHC doctors, health care workers, and the patients who took part in the DSS‐HTN study. The 16 PHC centers of Mahabubnagar who took part in the study were: Chinchode (Dr Sameena Nasreen); Kothur (Dr G. Pradeep Kumar); Siddapur (Dr B. Shireesh kumar); Ambatpally (Dr Subba Reddy Naru); Veepangandla (Dr Lal Sheikh); Madanapuram (Dr Najma Farheen); Peddamandadi (Dr M. Sakru); Hanwada (Dr M. Swapna); Bhootpur (Dr Asghar Ali); Amangal (Dr Parvez Hyder Baquary); Badepally (Dr Sridevi); Palem (Dr P. Srinivasa Rao); Deverkadra (Dr B. Srinivas Reddy); Nawabpet (Dr C. Arvind Kumar); Gattiippenpally (Dr Vishnu Vardhan); and Nandigam (Dr Gurram Sudha Rani). The authors also thank the District Medical and Health Officer, Dr P. Chandrashekhar, who was instrumental in the key success of the project. Dr Vamadevan S. Ajay, Senior Research Fellow at the Center for Chronic Disease Control (CCDC), India, was instrumental in developing and reviewing the built algorithms and knowledge base. Data Template, Bangalore, India, were the DSS software developers. Sreejesh K, Anil Parakkad, and K. Krishna Kumar from Data Template organization were the software development team members. The authors also thank the Wellcome Trust Programme Public Health Foundation of India (WTP‐PHFI) secretariat for their logistical and administrative help.
References
- 1.Anderson JA, Willson P, Peterson NJ, Murphy C, Kent TA. Prototype to practice: developing and testing a clinical decision support system for secondary stroke prevention in a veterans healthcare facility. Comput Inform Nurs. 2010; 28:353-363. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ. 2000; 320:686-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg AX, Adhikari NK, McDonald H, Rosas‐Arellano MP, Devereaux PJ, Beyene J, Sam J, Haynes RB. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005; 293:1223-1238. [DOI] [PubMed] [Google Scholar]
- 4.Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on‐screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009; 3:CD001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosworth HB, Olsen MK, McCant F, Harrelson M, Gentry P, Rose C, Goldstein MK, Hoffman BB, Powers B, Oddone EZ. Hypertension Intervention Nurse Telemedicine Study (HINTS): testing a multifactorial tailored behavioral/educational and a medication management intervention for blood pressure control. Am Heart J. 2007; 153:918-924. [DOI] [PubMed] [Google Scholar]
- 6.Persson M, Mjorndal T, Carlberg B, Bohlin J, Lindholm LH. Evaluation of a computer‐based decision support system for treatment of hypertension with drugs: retrospective, nonintervention testing of cost and guideline adherence. J Intern Med. 2000; 247:87-93. [DOI] [PubMed] [Google Scholar]
- 7.Roumie CL, Elasy TA, Greevy R, Griffin MR, Liu X, Stone WJ, Wallston KA, Dittus RS, Alvarez V, Cobb J, Speroff T. Improving blood pressure control through provider education, provider alerts, and patient education. Ann Intern Med. 2006; 145:165-175. [DOI] [PubMed] [Google Scholar]
- 8.Hicks LS, Sequist TD, Ayanian JZ, Shaykevich S, Fairchild DG, Orav EJ, Bates DW. Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med. 2008; 23:429-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinfret S, Lussier MT, Peirce A, Duhamel F, Cossette S, Lalonde L, Tremblay C, Guertin MC, LeLorier J, Turgeon J, Hamet PLOYAL Study Investigators. The impact of a multidsicplinary information technology supported program on blood pressure control in primary care. Circ Cardiovasc Qual Outcomes. 2009; 2:170-177. [DOI] [PubMed] [Google Scholar]
- 10.Nair M, Ali MK, Ajay VS, Shivashankar R, Mohan V, Pradeepa R, Deepa M, Khan HM, Kadir MM, Fatmi ZA, Reddy KS, Tandon N, Narayan KM, Prabhakaran D. CARRS surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health. 2012; 12:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah S, Singh K, Ali MK, Mohan V, Kadir MM, Unnikrishnan AG, Sahay RK, Varthakavi P, Dharmalingam M, Viswanathan V, Masood Q, Bantwal G, Khadgawat R, Desai A, Sethi BK, Shivashankar R, Ajay VS, Reddy KS, Narayan KM, Prabhakaran D, Tandon N. Improving diabetes care: multi‐component cardiovascular disease risk reduction strategies for people with diabetes in South Asia—the CARRS multi‐center translation trial. Diabetes Res Clin Pract. 2012; 98:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy SK, Roy SK, Bagchi S, Bajpayee A, Pal R, Biswas R. Study of KAP of the private medical practitioners about national disease control programmes. Indian J Public Health. 2005; 49:256-257. [PubMed] [Google Scholar]
- 13.Roshanov PS, Misra S, Gerstein HC, Garg AX, Sebaldt RJ, Mackay JA, Weise‐Kelly L, Navarro T, Wilczynski NL, Haynes RB. Computerized clinical decision support systems for chronic disease management: a decision‐maker‐researcher partnership systematic review. Implement Sci. 2011; 6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anchala R, Pant H, Prabhakaran D, Franco OH. ‘Decision support system (DSS) for prevention of cardiovascular disease (CVD) among hypertensive (HTN) patients in Andhra Pradesh, India'—a cluster randomised community intervention trial. BMC Public Health. 2012; 12:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weijer C, Grimshaw JM, Eccles MP, McRae AD, White A, Brehaut JC, Taljaard M. The Ottawa statement on the ethical design and conduct of cluster randomized trials. PLoS Med. 2012; 9:e1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards SJ, Braunholtz DA, Lilford RJ, Stevens AJ. Ethical issues in the design and conduct of cluster randomised controlled trials. BMJ. 1999; 318:1407-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anchala R, Di Angelantonio E, Prabhakaran D, Franco OH. Development and validation of a clinical and computerised decision support system for management of hypertension (DSS‐HTN) at a primary health care (PHC) setting. PLoS One. 2013; 8:e79638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Association of Physicians of India. Indian Hypertension Guidelines; 2007. Available at: http://www.apiindia.org/pdf/pg_med_2007/Chapter-36.pdf. Accessed June 05, 2013.
- 19.Official website of Mahabubnagar district. National Informatics center (NIC), Mahabubnagar Available at: http://mahabubnagar.nic.in/. Accessed June 05, 2013.
- 20.Ministry of Panchayat Raj India. Backward Regions Grant Fund, Programme Guidelines Available at:http://www.nird.org.in/brgf/doc/BRGFFINALGUIDELINES.pdf. Accessed June 05, 2013.
- 21.Prabhakaran D, Jeemon P, Goenka S, Lakshmy R, Thankappan KR, Ahmed F, Joshi PP, Mohan BV, Meera R, Das MS, Ahuja RC, Saran RK, Chaturvedi V, Reddy KS. Impact of a worksite intervention program on cardiovascular risk factors: a demonstration project in an Indian industrial population. J Am Coll Cardiol. 2009; 53:1718-1728. [DOI] [PubMed] [Google Scholar]
- 22.Kinra S, Bowen LJ, Lyngdoh T, Prabhakaran D, Reddy KS, Ramakrishnan L, Gupta R, Bharathi AV, Vaz M, Kurpad AV, Smith GD, Ben‐Shlomo Y, Ebrahim S. Sociodemographic patterning of non‐communicable disease risk factors in rural India: a cross sectional study. BMJ. 2010; 341:c4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996; 313:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur N, Kedia G, Trivedi A. A comparative study to analyze the cost of curative care at primary health center in Ahmedabad. Indian J Community Med. 2010; 35:153-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borah PK, Hazarika NC, Biswas D, Kalita HC, Mahanta J. Population‐specific left ventricular hypertrophy in three groups from the northeastern region of India. Natl Med J India. 2010; 23:336-339. [PubMed] [Google Scholar]
- 26.Mohan V, Deepa M, Farooq S, Datta M, Deepa R. Prevalence, awareness and control of hypertension in Chennai—the Chennai Urban Rural Epidemiology Study (CURES‐52). J Assoc Physicians India. 2007; 55:326-332. [PubMed] [Google Scholar]
- 27.Manimunda SP, Sugunan AP, Benegal V, Balakrishna N, Rao MV, Pesala KS. Association of hypertension with risk factors & hypertension related behaviour among the aboriginal Nicobarese tribe living in Car Nicobar Island, India. Indian J Med Res. 2011; 133:287-293. [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta A, Ray MR. Prevalence of hypertension and pre‐hypertension in rural women: a report from the villages of West Bengal, a state in the eastern part of India. Aust J Rural Health. 2012; 20:219-225. [DOI] [PubMed] [Google Scholar]
- 29.Hazarika NC, Biswas D, Narain K, Kalita HC, Mahanta J. Hypertension and its risk factors in tea garden workers of Assam. Natl Med J India. 2002; 15:63-68. [PubMed] [Google Scholar]
- 30.Sathish T, Kannan S, Sarma PS, Razum O, Thankappan KR. Incidence of hypertension and its risk factors in rural Kerala, India: a community‐based cohort study. Public Health. 2012; 126:25-32. [DOI] [PubMed] [Google Scholar]
- 31.Meshram II, Arlappa N, Balkrishna N, Rao KM, Laxmaiah A, Brahmam GN. Prevalence of hypertension, its correlates and awareness among adult tribal population of Kerala state, India. J Postgrad Med. 2012; 58:255-261. [DOI] [PubMed] [Google Scholar]
- 32.Ganguli D, Das N, Saha I, Chaudhuri D, Ghosh S, Dey S. Risk factors for hypertension in a population‐based sample of postmenopausal women in Kolkata, West Bengal, India. Asia Pac J Public Health. 2013; 25:388-397. [DOI] [PubMed] [Google Scholar]
- 33.Thankappan KR, Sivasankaran S, Sarma PS, Mini G, Khader SA, Padmanabhan P, Vasan R. Prevalence‐correlates‐awareness‐treatment and control of hypertension in Kumarakom, Kerala: baseline results of a community‐based intervention program. Indian Heart J. 2006; 58:28-33. [PubMed] [Google Scholar]
- 34.Shanthirani CS, Pradeepa R, Deepa R, Premalatha G, Saroja R, Mohan V. Prevalence and risk factors of hypertension in a selected South Indian population—the Chennai Urban Population Study. J Assoc Physicians India. 2003; 51:20-27. [PubMed] [Google Scholar]
- 35.Gupta R, Deedwania PC, Achari V, Bhansali A, Gupta BK, Gupta A, Mahanta TG, Asirvatham AJ, Gupta S, Maheshwari A, Saboo B, Jali MV, Singh J, Guptha S, Sharma KK. Normotension, prehypertension, and hypertension in urban middle‐class subjects in India: prevalence, awareness, treatment, and control. Am J Hypertens. 2013; 26:83-94. [DOI] [PubMed] [Google Scholar]
- 36.Anchala R, Kannuri NK, Pant H, Khan H, Franco OH, Di Angelantonio E, Prabhakaran D. Hypertension in india: a systematic review and meta‐analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014; 32:1170-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta‐analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002; 16:761-770. [DOI] [PubMed] [Google Scholar]
- 38.Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH, Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). Tone collaborative research group. JAMA. 1998; 279:839-846. [DOI] [PubMed] [Google Scholar]
- 39.Basile JN. Systolic blood pressure. BMJ. 2002; 325:917-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller ER, III, Erlinger TP, Young DR, Jehn M, Charleston J, Rhodes D, Wasan SK, Appel LJ. Results of the Diet, Exercise, and Weight loss Intervention Trial (DEW‐IT). Hypertension. 2002; 40:612-618. [DOI] [PubMed] [Google Scholar]
- 41.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons‐Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. N Engl J Med. 2001; 344:3-10. [DOI] [PubMed] [Google Scholar]
- 42.Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension. 2001; 38:1112-1117. [DOI] [PubMed] [Google Scholar]
- 43.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006; 47:296-308. [DOI] [PubMed] [Google Scholar]
- 44.Tu SW, Campbell JR, Glasgow J, Nyman MA, McClure R, McClay J, Parker C, Hrabak KM, Berg D, Weida T, Mansfield JG, Musen MA, Abarbanel RM. The SAGE guideline model: achievements and overview. J Am Med Inform Assoc. 2007; 14:589-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearson SA, Moxey A, Robertson J, Hains I, Williamson M, Reeve J, Newby D. Do computerised clinical decision support systems for prescribing change practice? A systematic review of the literature (1990–2007). BMC Health Serv Res. 2009; 9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roshanov PS, Fernandes N, Wilczynski JM, Hemens BJ, You JJ, Handler SM, Nieuwlaat R, Souza NM, Beyene J, Spall HG, Garg AX, Haynes RB. Features of effective computerised clinical decision support systems: meta‐regression of 162 randomised trials. BMJ. 2013; 346:f657. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organisation. Choosing Interventions that are Cost Effective (WHO‐CHOICE) Available at: http://www.who.int/choice/country/country_specific/en/index.html. Accessed April 24, 2013.
- 48.Kawamoto K, Jacobs J, Welch BM, Huser V, Paterno MD, Del Fiol G, Shields D, Strasberg HR, Haug PJ, Liu Z, Jenders RA, Rowed DW, Chertcoff D, Fehre K, Adlassnig KP, Curtis AC. Clinical information system services and capabilities desired for scalable, standards‐based, service‐oriented decision support: consensus assessment of the Health Level 7 clinical decision support Work Group. AMIA Annu Symp Proc. 2012; 2012:446-455. [PMC free article] [PubMed] [Google Scholar]
- 49.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005; 330:76510.1136/bmj.38398.500764.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Union Primary Census Data Highlights ‐ India, 2011. Available at: http://www.censusindia.gov.in/2011census/hlo/PCA_Highlights/pca_highlights_file/India/4Executive_Summary.pdf. Accessed April 24, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Referral criteria to a higher secondary or tertiary health care center.


