Abstract
Background
In epidemiologic studies, obesity has been associated with reduced natriuretic peptide (NP) concentrations. Reduced NP production could impair the ability of obese individuals to respond to salt loads, increasing the risk of hypertension and other disorders. We hypothesized that weight loss enhances NP production before and after salt loading.
Methods and Results
We enrolled 15 obese individuals (mean BMI 45±5.4 kg/m2) undergoing gastric bypass surgery. Before and 6 months after surgery, subjects were admitted to the clinical research center and administered a large‐volume intravenous saline challenge. Echocardiography and serial blood sampling were performed. From the pre‐operative visit to 6 months after surgery, subjects had a mean BMI decrease of 27%. At the 6‐month visit, N‐terminal pro‐atrial NP (Nt‐proANP) levels were 40% higher before, during, and after the saline infusion, compared with levels measured at the same time points during the pre‐operative visit (P<0.001). The rise in Nt‐pro‐ANP induced by the saline infusion (≈50%) was similar both before and after surgery (saline, P<0.001; interaction, P=0.2). Similar results were obtained for BNP and Nt‐proBNP; resting concentrations increased by 50% and 31%, respectively, after gastric bypass surgery. The increase in NP concentrations after surgery was accompanied by significant decreases in mean arterial pressure (P=0.004) and heart rate (P<0.001), and an increase in mitral annular diastolic velocity (P=0.02).
Conclusion
In obese individuals, weight loss is associated with a substantial increase in the “setpoint” of circulating NP concentrations. Higher NP concentrations could contribute to an enhanced ability to handle salt loads after weight loss.
Keywords: natriuretic peptide, obesity, salt intake
Introduction
Obesity affects more than one‐third of US adults1 and is a major contributor to cardiovascular morbidity and mortality.2–3 A significant proportion of the cardiovascular risk in obese people is attributed to the development of hypertension,4–5 which predisposes them to increased risk of atrial fibrillation, coronary heart disease, and stroke.6 Abnormal salt handling is thought to be one of the mechanisms underlying obesity‐related hypertension.7–8
The natriuretic peptide system, the primary salt‐excreting system in humans, acts as an endogenous antagonist of the renin‐angiotensin‐aldosterone system.9 The primary circulating natriuretic peptides are atrial natriuretic peptide (ANP) and B‐type natriuretic peptide (BNP).10 ANP is produced primarily in the atria, while BNP is derived from both the atria and the ventricles.10–11
Obese individuals have been found to have lower natriuretic peptide levels in multiple previous studies.12–14 The finding of lower natriuretic peptides in obese subjects is unexpected because obesity promotes increased plasma volume and hypertension, which are known to lead to left ventricular stress and hypertrophy. These conditions should trigger natriuretic peptide release from the heart. Thus, it has been proposed that obese individuals may have a primary “natriuretic peptide deficiency”8,13 that could contribute to the development of hypertension.
Prior studies have been largely observational, and based on measurement of natriuretic peptide levels collected in individuals with random salt intake. Because natriuretic peptide levels are dependent on loading conditions, more controlled physiologic data are needed. Accordingly, the aim of the current investigation was to study the natriuretic peptide axis in the context of a well‐controlled physiologic stimulus (intravenous saline infusion) in obese, otherwise healthy, individuals. This study design also enabled us to compare the relative effects of weight loss and intravenous saline infusion on circulating natriuretic peptide levels.
Methods
Study Sample
A study research coordinator screened charts for eligibility from a pool of patients who were referred to the weight center of the Massachusetts General Hospital for Roux‐en‐Y gastric bypass surgery. Eligible patients were informed about the details of the research protocol, including the need for 2 saline infusion visits at the Clinical Research Center (CRC) 6 months apart. A study physician investigator verified the medical history of study participants including the use of medications at the time of the saline protocol visit. The subjects were asked to keep a detailed diary of all the food and beverages consumed for the 48 hours prior to each study visit to estimate their nutritional status.
Subjects were excluded if they had any of the following: history of myocardial infarction, heart failure, or left ventricular (LV) ejection fraction <50%, greater than mild valvular stenosis or regurgitation or any regional wall motion abnormalities by cardiac imaging, chronic renal failure or serum creatinine ≥3.0 mg/dL, atrial fibrillation, diabetes mellitus requiring insulin therapy, systolic blood pressure ≥170 mm Hg or diastolic blood pressure ≥100 mm Hg at the most recent weight center visit, a history of current loop or thiazide diuretic use, a history of obstructive lung disease, or thyroid dysfunction. Female subjects who were pregnant or planned to become pregnant within 6 months were also excluded. The Partners Human Research Committee approved the protocol. All subjects provided informed consent.
Saline Protocol
Eligible study subjects were admitted after overnight fasting for an outpatient visit at the MGH CRC. Upon admission, two intravenous catheters were placed for phlebotomy and 10 mL/m2 of body‐surface area (BSA)/minute normal saline (0.9 mEq/mL) was infused over 2 hours. Blood pressure, heart rate, and oxygen saturation were measured every 20 minutes during the saline infusion. BSA was calculated according to the DuBois algorithm (BSA (in m2)=0.20247×height (m)0.725×weight (kg)0.425). Venous blood was sampled beginning immediately prior to the start of the infusion and at 40, 80, 120, and 180 minutes after the start of the infusion.
The study subjects were brought back to the MGH CRC 6 months after gastric bypass surgery and underwent an identical saline infusion protocol. Subjects were excluded from completing the second saline challenge protocol if they had developed complications of gastric bypass surgery including significant peri‐operative complications (myocardial infarction, persistent atrial fibrillation, sepsis, or gastrointestinal bleeding requiring blood transfusion >2 units).
Echocardiograms
Echocardiograms were performed before and after saline infusion at both the baseline and post‐gastric bypass surgery visits. Each subject had four echocardiograms in total during the entire study. Interpretations were made by investigators blinded to clinical status (before or after saline infusion, before or after surgery). The following standard measures were made on two‐dimensional (2D) images in each echocardiogram: interventricular septal and posterior wall thickness (IVS and PWT), left ventricular internal diameter at end‐diastole and end‐systole (LVID, LVIS) and left atrial anteroposterior diameter (LA Dia) in the parasternal view, left ventricular (LV) volumes using a modified Simpson's rule (apical 4 chamber and 2 chamber views), mitral inflow E and A velocities and E deceleration time, and mitral annular early diastolic (e′) velocity at the lateral annulus. We did not calculate left atrial volumes due to limited echocardiographic windows in severely obese patients. Estimation of left atrial filling pressure was obtained every 20 minutes during the second hour of the infusion by determining the ratio of the early diastolic mitral inflow velocity to the early diastolic mitral annular velocity.15
Natriuretic Peptide Measurements
Plasma Nt‐proANP levels were measured by ELISA (proANP 1‐98; Biomedica Medizinprodukte GmbH & Co KG, Austria). Plasma Nt‐proBNP levels were measured using an electrochemiluminescence immunoassay (Elecsys proBNP; Roche, Indianapolis, IN). Mature ANP was measured using an in‐house immunoassay at the Mayo Clinic (Rochester, MN; J. Burnett). Mature BNP was measured by immunoassay (Siemens, New York, NY). Intra‐assay coefficients of variation were <10% for all assays.
Statistical Analysis
Natriuretic peptide measurements were tested for normality and were logarithmically transformed for analysis. We used paired t tests to examine the change in BMI, blood pressure, and natriuretic peptides before and after surgery. Mixed effect models using all non‐missing data from 18 study subjects were used to assess the effects of surgery and intravenous saline and their interaction on plasma Nt‐proANP, Nt‐proBNP, and mature ANP and BNP levels, as well as echocardiographic measures. To account for repeated measures for each subject, a spatial power structure for ANP. Nt‐proANP, BNP, and Nt‐proBNP, and a completely general (unstructured) covariance matrix for echocardiographic outcomes were used. None of the interactions terms were significant and P values reported are based on models without interaction. A secondary analysis after covariate adjustment for age, sex, and blood pressure was also performed. All analyses were conducted using SAS (Cary, NC). A two‐sided P<0.05 was considered statistically significant for the primary outcome.
Results
We identified 34 patients who met eligibility criteria based on the chart review. Two subjects were excluded because they were found to have diabetes requiring insulin therapy, 1 was excluded because of hypothyroidism, and 13 were excluded because they did not undergo gastric bypass surgery or did not wish to participate. A final study sample of 18 individuals (15 women) was enrolled into the protocol. We had 3 individuals who did not complete the 6‐month follow‐up visit; the sample that completed both visits consisted of 15 individuals (12 women). No subjects were excluded because of peri‐operative complications. Table 1 displays the characteristics of the study sample at baseline and 6 months after surgery. From baseline to 6 months after surgery, subjects had a mean decrease of 27% in body mass index (P<0.0001). There were significant reductions in mean arterial blood pressure (P=0.004) and heart rate (P<0.001) after surgery. Only 2 out of 18 subjects were on any class of anti‐hypertensive medications at the pre‐op visit before gastric bypass surgery. At the 6‐month visit, anti‐hypertensive medication was discontinued for one of these subjects, and continued at the same dose for the other subject. The mean±SD volume of saline infusion pre‐bypass was 2.6±0.4 L and post‐bypass was 2.3±0.3 L.
Table 1.
Baseline Characteristics of the Study Sample Before and After Bypass Surgery
| Characteristics | Pre‐Bypass Surgery (N=18) | Post‐Bypass Surgery (N=15) | P Values |
|---|---|---|---|
| Age, y | 43±11 | 45±10 | NA |
| Female, % | 83 | 80 | NA |
| Body mass index, kg/m2 | 45±5.4 | 33±6.5 | <0.001 |
| Systolic blood pressure, mm Hg | 117±12 | 108±9 | 0.05 |
| Diastolic blood pressure, mm Hg | 70±9 | 64±12 | 0.04 |
| Mean arterial pressure, mm Hg | 86±7 | 79±9 | 0.004 |
| Resting heart rate, beats/min | 71±10 | 65±10 | <0.001 |
Values expressed as mean±SD or %, where appropriate. NA indicates not applicable (since these are the same individuals before and after bypass surgery).
Resting plasma concentrations (mean±SEM) of mature ANP and Nt‐proANP were 14±2 pg/mL and 4±0.6 pg/mL at baseline. After gastric bypass surgery, the resting plasma concentrations rose to 24±5 pg/mL and 7±0.7 pg/mL for ANP and Nt‐proANP (increased by 23% and 43%), respectively (P=0.016 and 0.008). Absolute concentrations of ANP and Nt‐proANP were higher in individuals after bypass surgery at all acute time points during and after administration of intravenous saline (Figure 1A and 1B; P<0.001). A secondary analysis, adjusted for age, sex, and mean arterial pressure yielded similar results.
Figure 1.
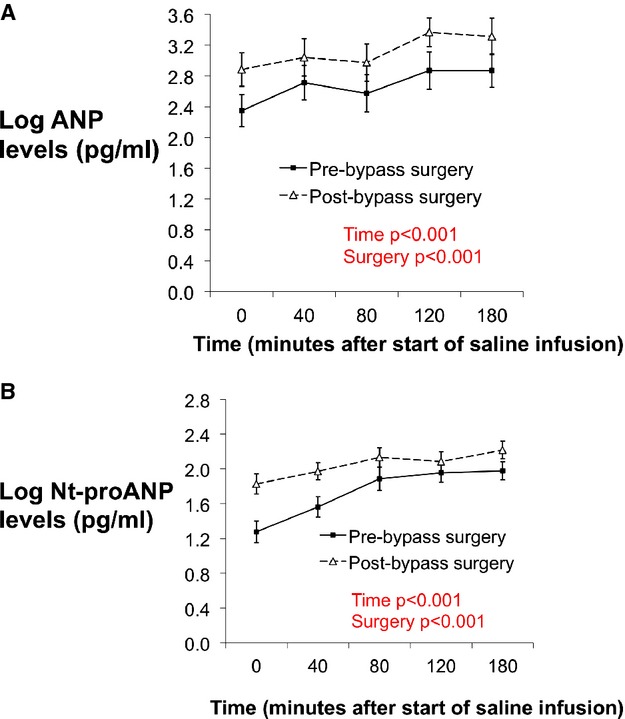
A, Concentrations of plasma mature ANP levels at baseline and at 40, 80, 120, and 180 minutes after the start of saline infusion. Solid line with squares represents pre‐bypass surgery subjects and dotted line with triangles represents post‐bypass surgery subjects. B, Concentrations of plasma Nt‐proANP levels at baseline and at 40, 80, 120, and 180 minutes after the start of saline infusion. Solid line with squares represents pre‐bypass surgery subjects and dotted line with triangles represents post‐bypass surgery subjects. ANP indicates atrial natriuretic peptide; Nt‐proANP, N‐terminal pro‐ANP.
Resting plasma concentrations of mature BNP and Nt‐proBNP were 14±3 pg/mL and 42±9 pg/mL before gastric bypass surgery and increased to 32±5 pg/mL and 107±20 pg/mL (increased by 50% and 31%), respectively (P=0.0009 and 0.0001) after the surgery. Circulating BNP and Nt‐proBNP concentrations during saline infusion were also higher after surgery compared with before surgery (Figures 2A and 2B; P<0.0001). The saline infusion itself was not associated with an increase in BNP or Nt‐proBNP levels at either visit (P=0.65 and 0.60, respectively).
Figure 2.
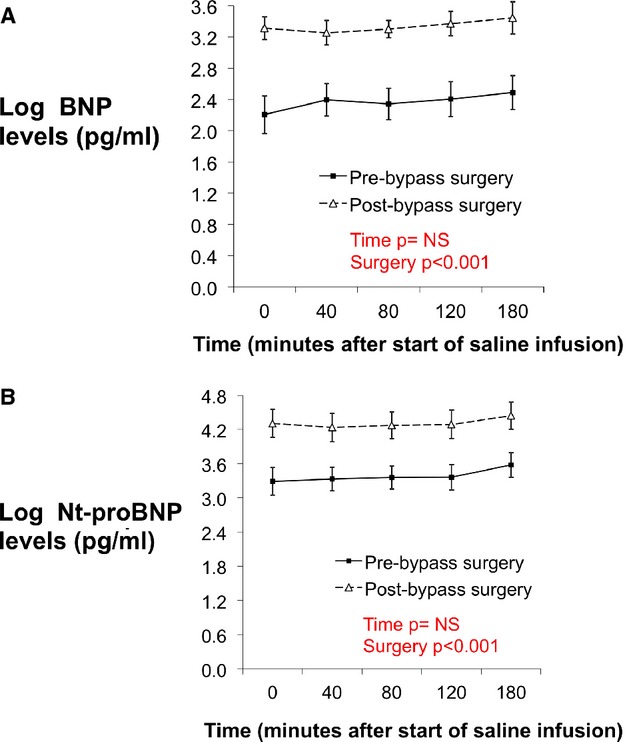
A, Concentrations of plasma mature BNP levels at baseline and at 40, 80, 120, and 180 minutes after the start of saline infusion. Solid line with squares represents pre‐bypass surgery subjects and dotted line with triangles represents post‐bypass surgery subjects. B, Concentrations of plasma Nt‐proBNP levels at baseline and at 40, 80, 120, and 180 minutes after the start of saline infusion. Solid line with squares represents pre‐bypass surgery subjects and dotted line with triangles represents post‐bypass surgery subjects. BNP indicates B‐type natriuretic peptide; Nt‐proBNP, N‐terminal pro‐BNP.
Echocardiographic measurements obtained at pre‐ and post‐bypass visits are outlined in Table 2. Due to limitations in scanning windows and poor image quality, interpretable echocardiograms were obtained in 12 of 15 patients. Transmitral E increased from 76±19 cm/s at pre‐bypass to 83±19 cm/s at the post‐bypass surgery visit while no significant increase was noted in transmitral A. The mean intra‐individual change in transmitral E was 15 cm/s, with 95% confidence interval 3 to 26 cm/s. The increases in transmitral E were significant for the effects of saline (P=0.005) and surgery (P=0.002). There was also a significant increase in the early diastolic mitral annular velocity e′ (P=0.02 for effect of surgery). However, the E/e′ ratio did not change after surgery (Table 2). Left atrial diameter showed a trend towards decrease at the post‐bypass surgery visit (P=0.3).
Table 2.
Echocardiographic Measures in Obese Subjects Before and After Saline Administration at Pre‐Bypass and Post‐Bypass Surgery Visits
| Pre‐Bypass | Post‐Bypass | P Values | ||||
|---|---|---|---|---|---|---|
| Pre Saline | Post Saline | Pre Saline | Post Saline | Saline Effect | Surgery Effect | |
| LVEDV, mL | 79±13 | 84±14 | 80±13 | 87±14 | 0.001 | 0.3 |
| LVESV, mL | 31±7 | 30±9 | 31±6 | 30±7 | 0.5 | 0.7 |
| LVEF, % | 60±5.2 | 65±5.9 | 61±4.7 | 66±4.0 | 0.002 | 0.4 |
| SV, mL | 47±8 | 54±9 | 49±1 | 57±9 | <0.001 | 0.1 |
| CO, L/min | 3.3±0.7 | 4.2±0.7 | 3.1±0.7 | 3.7±0.7 | <0.001 | 0.01 |
| LA dia (mm) | 37.3±2 | 38.3±2 | 36.7±4 | 37.3±2 | 0.9 | 0.3 |
| E, cm/s | 76±19.0 | 89±21 | 83±19.2 | 90±17 | 0.005 | 0.002 |
| A, cm/s | 64±19 | 72± 28 | 64±31 | 60±13 | 0.6 | 0.2 |
| e′ lat, cm/s | −8.7±2.8 | −9.6±2.7 | −9.1±2.7 | −9.6±2.6 | 0.09 | 0.02 |
| E/e′ | −9.9±6 | −10.9±7 | −10.2±5 | −9.5±2.1 | 0.8 | 0.4 |
CO indicates cardiac output; LA dia, left atrial anteroposterior diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; SV, stroke volume.
At both visits, saline infusion was associated with a significant increase in left ventricular (LV) end‐diastolic volume (P=0.001 for saline effect), whereas LV end‐systolic volume was unchanged. Stroke volume and cardiac output increased in response to saline administration at both pre‐ and post‐bypass visits. The effect of saline infusion on cardiac function did not differ before and after surgery (saline×surgery interaction P values non‐significant).
Discussion
In summary, we found that weight loss in obese individuals undergoing gastric bypass surgery is associated with higher natriuretic peptide concentrations across a range of loading conditions. This observation is consistent with a higher “set point” of natriuretic peptide levels after weight loss. That these findings are accompanied by improvements in blood pressure, heart rate and echocardiographic diastolic function provides persuasive evidence that the increase in natriuretic peptides with weight loss is “primary” and not secondary to alterations in cardiac structure or function.
It is also notable that the rise in absolute concentrations of ANP and Nt‐proANP after weight loss surgery was comparable to that observed with a 2‐L saline infusion. This observation suggests that the magnitude of obesity‐induced alteration in natriuretic peptide production is physiologically relevant. The slope of the natriuretic peptide response to saline challenge was similar before and after weight loss, suggesting that obesity does not blunt the responsiveness of the natriuretic peptide axis to salt challenge, but rather alters the “set point.”
We found that BNP and Nt‐proBNP concentrations were also substantially higher after weight loss surgery, both before and after saline infusion. We did not observe an acute rise in BNP or Nt‐proBNP in the first 3 hours after the saline infusion. The longer half‐lives of BNP and Nt‐proBNP may be one explanation, as these peptides may take longer to peak.16 However, we have noted a similar lack of increase after up to 8 hours of observation.17 Thus, we expect that the changes in BNP associated with surgery are likely to be substantially larger than any change induced by saline, even over longer periods of observation.
Over the same period, we noted an approximately 2‐fold increase in Nt‐proANP levels. Although, BNP co‐localizes with ANP in secretory granules,18 its release may be regulated differently, thereby making the salt loading response more variable.19–20
Several epidemiologic studies have reported lower circulating natriuretic peptide concentrations in obese individuals.12,14 However, these studies have been observational and confined to a single time point of measurement of natriuretic peptides. To our knowledge, only one previous study has examined the association of obesity with salt‐induced natriuretic peptide concentrations. Licata and colleagues found reduced, salt‐loaded plasma ANP concentrations in 9 obese individuals compared with 10 lean controls.21 They did not examine the influence of weight loss on the natriuretic peptide system. Thus, the present study is the first to provide serial, physiologic data from the same individuals over time.
One proposed mechanism for reduced natriuretic peptide concentrations in obesity is the relative abundance of natriuretic peptide clearance receptors (NPR‐C) in adipose tissue.13,22 Elevated insulin has also been linked to increased expression of NPR‐C in obese subjects.23 On the other hand, plasma Nt‐proANP and Nt‐proBNP levels are reduced in obesity to a comparable degree as the mature peptides. Because the pro‐peptides are not known to bind to NPR‐C, impaired synthesis or secretion likely plays a role in obesity.
Strengths of our study include the serial physiologic assessments before and after bariatric surgery. The gastric bypass procedure ensured a large degree of weight loss (≈27% mean change in BMI), while the administration of normal saline provided an acute stimulus for eliciting acute natriuretic peptide responses. Thus, we were able to compare the relative effects of weight loss and saline infusion, with each individual serving as his or her own control. This study design minimizes confounding from sources of natriuretic peptide variation that might correlate with BMI. We performed the post‐surgical assessment 6 months after surgery to ensure that acute hemodynamic changes from surgery had resolved and patients had attained most of their expected weight loss. Mitral annular early diastolic (e′) velocity at the lateral annulus has been accepted as an index of diastolic function24–25 and we had significant improvement in e′ suggesting improvement in myocardial relaxation. Our echocardiographic findings are in accordance with the recently published meta‐analysis demonstrating benefits of bariatric surgery on diastolic function.26
There are several limitations of our study. Given the nature of our physiologic protocols, which required two large volume saline infusions in obese patients before and after surgery, our sample size was modest. Nonetheless, we were able to elicit significant relationships of all four natriuretic peptides (ANP, Nt‐proANP, BNP, and Nt‐proBNP) across a variety of salt conditions before and after surgical weight loss. Our study population consisted of primarily females. We do not believe from prior epidemiologic studies looking at resting natriuretic peptide levels in obese individuals12 that having more men in our cohort would have modified our findings. Prior epidemiologic studies do not suggest that gender modifies the association between obesity and natriuretic peptide concentrations. We did not examine short‐term changes in the natriuretic peptide system, as a physiologic assessment immediately after surgery would have been impractical and potentially confounded by post‐operative shifts in volume or nutrition. We also focused on surgical weight loss because weight loss with non‐surgical treatments is less consistent. Thus, we cannot exclude any surgery‐specific effects. Because the saline infusion was indexed to BSA, less saline was given at the post‐weight loss visit. This could have created a “conservative” bias, eg, toward observing a smaller natriuretic peptide response after surgery. Indexing was performed to ensure that the amount of saline relative to plasma volume was relatively constant. Lastly, we did not perform a complete assessment of the renin‐angiotensin‐aldosterone system and the sympathetic nervous system, all of which could also be primarily affected resulting in the observed responses of the natriuretic peptide system after weight loss and/or saline loading.
In summary, our study provides evidence of an alteration in the natriuretic peptide “set point” with weight loss. These findings highlight the potential role of a “natriuretic peptide deficiency” in obesity‐related conditions such as hypertension and heart failure. One can further speculate that reversal of the “natriuretic peptide deficiency” could play a role in the improvement of blood pressure and cardiac function after weight loss.
Sources of Funding
This study has been supported by the following grants: R01 HL102780, R21 DK092909, and 1 UL1 RR025758‐01 from the National Institutes of Health and the National Center for Research Resources. The research was also supported by a grant from the Foundation LeDucq.
Disclosures
None.
Acknowledgments
The authors thank the Massachusetts General Hospital Clinical Research Center staff.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the united states, 2009–2010. NCHS Data Brief. 0000; 000:1-8. [PubMed] [Google Scholar]
- 2.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990; 322:882-889. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the framingham experience. Arch Intern Med. 2002; 162:1867-1872. [DOI] [PubMed] [Google Scholar]
- 4.Dyer AR, Elliott P. The INTERSALT study: relations of body mass index to blood pressure. INTERSALT Co‐Operative Research Group. J Hum Hypertens. 1989; 3:299-308. [PubMed] [Google Scholar]
- 5.Chirinos JA, Franklin SS, Townsend RR, Raij L. Body mass index and hypertension hemodynamic subtypes in the adult us population. Arch Intern Med. 2009; 169:580-586. [DOI] [PubMed] [Google Scholar]
- 6.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002; 360:1903-1913. [DOI] [PubMed] [Google Scholar]
- 7.Hall JE, Brands MW, Henegar JR. Mechanisms of hypertension and kidney disease in obesity. Ann N Y Acad Sci. 1999; 892:91-107. [DOI] [PubMed] [Google Scholar]
- 8.Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989; 321:580-585. [DOI] [PubMed] [Google Scholar]
- 9.Johnston CI, Hodsman PG, Kohzuki M, Casley DJ, Fabris B, Phillips PA. Interaction between atrial natriuretic peptide and the renin angiotensin aldosterone system. Endogenous antagonists. Am J Med. 1989; 87:24S-28S. [DOI] [PubMed] [Google Scholar]
- 10.Clerico A, Iervasi G, Mariani G. Pathophysiologic relevance of measuring the plasma levels of cardiac natriuretic peptide hormones in humans. Horm Metab Res. 1999; 31:487-498. [DOI] [PubMed] [Google Scholar]
- 11.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998; 339:321-328. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004; 109:594-600. [DOI] [PubMed] [Google Scholar]
- 13.Dessi‐Fulgheri P, Sarzani R, Tamburrini P, Moraca A, Espinosa E, Cola G, Giantomassi L, Rappelli A. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J Hypertens. 1997; 15:1695-1699. [DOI] [PubMed] [Google Scholar]
- 14.Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, Canham RM, Chung AK, Leonard D, Wians FH, Jr, de Lemos JA. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the dallas heart study. Circulation. 2005; 112:2163-2168. [DOI] [PubMed] [Google Scholar]
- 15.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous doppler‐catheterization study. Circulation. 2000; 102:1788-1794. [DOI] [PubMed] [Google Scholar]
- 16.Heringlake M, Heide C, Bahlmann L, Eichler W, Pagel H, Schmucker P, Wergeland R, Armbruster FP, Klaus S. Effects of tilting and volume loading on plasma levels and urinary excretion of relaxin, NT‐pro‐ANP, and NT‐pro‐BNP in male volunteers. J Appl Physiol. 2004; 97:173-179. [DOI] [PubMed] [Google Scholar]
- 17.Arora P, Wu C, Khan AM, Bloch DB, Davis‐Dusenbery BN, Ghorbani A, Spagnolli E, Martinez A, Ryan A, Tainsh LT, Kim S, Rong J, Huan T, Freedman JE, Levy D, Miller KK, Hata A, Del Monte F, Vandenwijngaert S, Swinnen M, Janssens S, Holmes TM, Buys ES, Bloch KD, Newton‐Cheh C, Wang TJ. Atrial natriuretic peptide is negatively regulated by microrna‐425. J Clin Invest. 2013; 123:3378-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takemura G, Takatsu Y, Doyama K, Itoh H, Saito Y, Koshiji M, Ando F, Fujiwara T, Nakao K, Fujiwara H. Expression of atrial and brain natriuretic peptides and their genes in hearts of patients with cardiac amyloidosis. J Am Coll Cardiol. 1998; 31:754-765. [DOI] [PubMed] [Google Scholar]
- 19.Wambach G, Koch J. Bnp plasma levels during acute volume expansion and chronic sodium loading in normal men. Clin Exp Hypertens. 1995; 17:619-629. [DOI] [PubMed] [Google Scholar]
- 20.Lang CC, Choy AM, Turner K, Tobin R, Coutie W, Struthers AD. The effect of intravenous saline loading on plasma levels of brain natriuretic peptide in man. J Hypertens. 1993; 11:737-741. [DOI] [PubMed] [Google Scholar]
- 21.Licata G, Volpe M, Scaglione R, Rubattu S. Salt‐regulating hormones in young normotensive obese subjects. Effects of saline load. Hypertension. 1994; 23:I20-I24. [DOI] [PubMed] [Google Scholar]
- 22.Sarzani R, Dessi‐Fulgheri P, Paci VM, Espinosa E, Rappelli A. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest. 1996; 19:581-585. [DOI] [PubMed] [Google Scholar]
- 23.Pivovarova O, Gogebakan O, Kloting N, Sparwasser A, Weickert MO, Haddad I, Nikiforova VJ, Bergmann A, Kruse M, Seltmann AC, Bluher M, Pfeiffer AF, Rudovich N. Insulin up‐regulates natriuretic peptide clearance receptor expression in the subcutaneous fat depot in obese subjects: a missing link between CVD risk and obesity? J Clin Endocrinol Metab. 2012; 97:E731-E739. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa H, Little WC, Ohno M, Brucks S, Morimoto A, Cheng HJ, Cheng CP. Diastolic mitral annular velocity during the development of heart failure. J Am Coll Cardiol. 2003; 41:1590-1597. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009; 22:107-133. [DOI] [PubMed] [Google Scholar]
- 26.Cuspidi C, Rescaldani M, Tadic M, Sala C, Grassi G. Effects of bariatric surgery on cardiac structure and function: a systematic review and meta‐analysis. Am J Hypertens. 2014; 27:146-156. [DOI] [PubMed] [Google Scholar]


