Abstract
Background
Pneumonia frequently complicates stroke and has a major impact on outcome. We derived and internally validated a simple clinical risk score for predicting stroke‐associated pneumonia (SAP), and compared the performance with an existing score (A2DS2).
Methods and Results
We extracted data for patients with ischemic stroke or intracerebral hemorrhage from the Sentinel Stroke National Audit Programme multicenter UK registry. The data were randomly allocated into derivation (n=11 551) and validation (n=11 648) samples. A multivariable logistic regression model was fitted to the derivation data to predict SAP in the first 7 days of admission. The characteristics of the score were evaluated using receiver operating characteristics (discrimination) and by plotting predicted versus observed SAP frequency in deciles of risk (calibration). Prevalence of SAP was 6.7% overall. The final 22‐point score (ISAN: prestroke Independence [modified Rankin scale], Sex, Age, National Institutes of Health Stroke Scale) exhibited good discrimination in the ischemic stroke derivation (C‐statistic 0.79; 95% CI 0.77 to 0.81) and validation (C‐statistic 0.78; 95% CI 0.76 to 0.80) samples. It was well calibrated in ischemic stroke and was further classified into meaningful risk groups (low 0 to 5, medium 6 to 10, high 11 to 14, and very high ≥15) associated with SAP frequencies of 1.6%, 4.9%, 12.6%, and 26.4%, respectively, in the validation sample. Discrimination for both scores was similar, although they performed less well in the intracerebral hemorrhage patients with an apparent ceiling effect.
Conclusions
The ISAN score is a simple tool for predicting SAP in clinical practice. External validation is required in ischemic and hemorrhagic stroke cohorts.
Keywords: clinical risk score; pneumonia; stroke, acute; stroke‐associated pneumonia
Introduction
Pneumonia frequently complicates stroke and has a major impact on outcome. It occurs in approximately 10% of patients hospitalized with stroke, most frequently during the first week after stroke, and particularly the first 3 days after stroke onset.1 In those at greatest risk, the incidence of stroke‐associated pneumonia (SAP) may be as high as 40%.2 Several studies have highlighted the adverse impact of infections on the outcome of stroke. SAP independently increases in‐hospital mortality 2‐ to 6‐fold, and increases hospitalization costs, length of stay, and likelihood of poor outcome in survivors.3–10 As SAP is a potentially modifiable cause of morbidity and mortality, identifying patients at greatest risk in the acute phase of stroke is of fundamental importance in approaching prevention and treatment.
Baseline clinical factors such as dysphagia/aspiration, age, and stroke severity are consistently associated with SAP, and several recent studies have developed clinical scores to predict SAP with the aim of improving risk‐stratification in patients with acute ischemic stroke.2,11–14 However, the utility of such scores in routine clinical care or research remains unclear. The simplest score, the A2DS2 (Age, Atrial fibrillation [AF], Dysphagia, Sex, Stroke Severity using the National Institutes of Health Stroke Scale [NIHSS] score), was derived in a German cohort,2 and subsequently validated using German2 and Chinese registry data.14 Refining prediction scores to make application easier, while improving reliability and validity in acute stroke patients, is essential prior to consideration of implementation in research or clinical practice. We aimed to explore whether a simpler scoring system could be derived in a cohort of unselected stroke patients, and compare its performance with that of the A2DS2 score.
Patients and Methods
We undertook secondary analyses of a multicenter UK cohort study using data collected prospectively in the Sentinel Stroke National Audit Programme (SSNAP) registry core‐dataset. SSNAP is a UK‐wide resource with the objective of improving the quality of stroke care by auditing stroke services against evidence‐based standards. Ethical approval of the SSNAP audit and associated data linkage was granted by the Ethics and Confidentiality Committee of the National Information Governance Board. SSNAP is administered centrally by the Royal College of Physicians Stroke Working Party and has been collecting data since January 2013. Participating hospitals in England, Wales, and Northern Ireland enter data for all confirmed stroke admissions into a secure online database, covering a range of prespecified domains of care (imaging; stroke unit care; thrombolysis; specialist assessments; occupational therapy; physiotherapy; speech and language therapy; multidisciplinary teamworking; and discharge processes). Case ascertainment was estimated through linkage with administrative data. The mortality status of patients was determined through linkage to the national statutory register of deaths in England. Data linkage was carried out by a secure third party (the Health and Social Care Information Centre), and the investigators were provided with an anonymized dataset following removal of patient identifiers.
Eligibility, Data Collection, and Definitions
Patients with index ischemic stroke or intracerebral hemorrhage (ICH) entered into the SSNAP database between January 1 and September 31, 2013 with complete data at baseline through day 7 were eligible for inclusion (69% of SSNAP database entries). The primary outcome measure was clinician diagnosis of SAP based on initiation of antibiotic therapy occurring within the first 7 days after admission as recorded by the participating centers. The following routine clinical baseline factors were included: age; sex; prestroke modified Rankin Scale (score 0 to 5); index stroke subtype (cerebral infarction or ICH determined by clinician diagnosis and baseline imaging); presentation stroke severity (NIHSS); baseline comorbidities: hypertension, diabetes mellitus, congestive heart failure (reported or documented past history and/or receiving relevant prescribed medication at admission), AF (reported or documented past history and/or documentation of standard electrocardiographic findings at admission), and previous stroke or transient ischemic attack (reported or documented past history preceding the index admission stroke). Dysphagia status was derived from the baseline swallow screen (within 4 hours of admission) and formal swallow assessment by a speech and language therapist (SALT) within 72 hours as follows (Figure 1): no dysphagia if did not receive a SALT assessment because baseline swallow screen was passed; dysphagia if received a SALT assessment or if did not receive a SALT assessment as a result of organizational reasons, patient refusal, or being medically unwell. “Organizational reasons” refers to reasons for not receiving a SALT assessment that are not related to patient factors—such as lack of availability of appropriately trained staff or that this was omitted in error as a result of suboptimal care. Patients were excluded if no reason was given for not having a SALT assessment. Level of consciousness and other baseline neurological impairments were retrieved from the complete baseline NIHSS record comprising 11 categories.15 The 10‐point A2DS2 score was assigned as described previously.2
Figure 1.
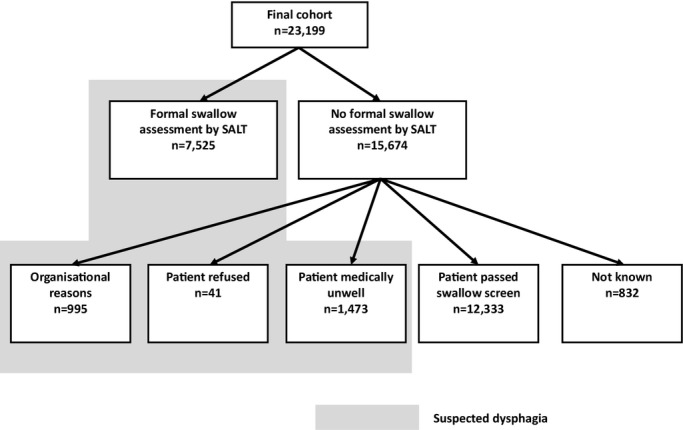
Flow chart defining allocation of dysphagia status. SALT indicates speech and language therapist.
Statistical Analyses
The data were split at random into derivation and validation samples of patients with ischemic stroke or ICH. Univariate analyses were undertaken to determine the clinical factors associated with SAP in the derivation cohort. As dysphagia status was derived indirectly from swallow screen and SALT assessment, it was not included in the uni‐ or multivariable analyses. Univariable hypothesis tests used were χ2 for categorical variables and Mann–Whitney U test for continuous variables.
A risk score for SAP was derived by fitting a multivariable logistic regression model to the derivation data. There were no missing data in the study sample, and models were fitted to the complete sample. Initial variables were selected based on the results of the univariate analyses, clinical experience, and previously published literature on SAP risk factors. Variables were selected by a process of backward elimination, keeping covariables significant to P<0.1. Nonsignificant covariables were removed sequentially, aiming to derive a parsimonious model with the highest predictive characteristics. Variable selection took into account the need for the final risk score to be useful in a clinical setting and be simple to calculate. Model fitting explored various strategies to include continuous and ordinal variables, either natively, or grouped into categorical variables. Where nonlinear associations were identified, these variables were included as categorical variables.
The coefficients from the final model were used to derive an integer‐based risk score. The characteristics of the novel and A2DS2 risk scores were evaluated in the validation sample using receiving operating characteristic analysis (discrimination) and by plotting predicted versus observed for each level of the risk score (calibration). The primary analyses were restricted to ischemic stroke. Secondary analyses were undertaken for those with ICH. Sensitivity analyses were carried out in the ICH group, excluding patients who died within 3 days of admission, and also after excluding any patients receiving palliative care. This was to examine the effect of the competing risk of death of ICH patients or those patients receiving palliative care estimated to be at high risk of SAP.
Results
Baseline Characteristics of the Derivation and Validation Cohorts
Data were available for 23 199 patients with acute stroke admitted to 218 stroke services. The characteristics of the patients were well balanced between the derivation and validation cohorts (Table 1). The median age of the cohort was 76 years, with ischemic stroke in 92% and ICH in 8%. Overall prevalence of SAP within the first week of admission was 6.7%, with a prevalence of 6.5% in the patients with ischemic stroke and 8.5% in patients with ICH. Both 7‐day and 30‐day all‐cause mortality rates were similar between the derivation and validation cohorts. Patients excluded because of incomplete data at baseline (incomplete NIHSS) tended to be older, more dependent prestroke, with more frequent ICH and higher mortality (Table 2).
Table 1.
Characteristics of the Derivation and Validation Cohorts
| Derivation | Validation | P Value | |
|---|---|---|---|
| N | 11 551 | 11 648 | |
| Female (%) | 5425 (47.0) | 5594 (48.0) | 0.003 |
| Median age (IQR) | 76 (65 to 84) | 76 (65 to 84) | 0.46 |
| Age group, n (%) (y) | 0.881 | ||
| <60 | 1909 (16.5) | 1884 (16.2) | |
| 60 to 69 | 2029 (17.6) | 2044 (17.6) | |
| 70 to 79 | 3167 (27.4) | 3167 (27.2) | |
| 80 to 89 | 3355 (29.1) | 3442 (29.6) | |
| 90+ | 1091 (9.5) | 1110 (9.5) | |
| Stroke type, n (%) | 0.39 | ||
| Ischemic | 10 635 (92.1) | 10 699 (91.9) | |
| ICH | 916 (7.9) | 949 (8.2) | |
| Median NIHSS (IQR) | 4 (2 to 9) | 4 (2 to 9) | 0.34 |
| Dependent prestroke, n (%) | 2761 (23.9) | 2793 (24.0) | 0.8 |
| Comorbidity, n (%) | |||
| Congestive heart failure | 609 (5.3) | 630 (5.4) | 0.09 |
| Atrial fibrillation | 2213 (19.2) | 2186 (18.8) | 0.53 |
| Hypertension | 6228 (53.9) | 6389 (54.9) | 0.88 |
| Diabetes mellitus | 2271 (19.7) | 2224 (19.1) | 0.27 |
| Previous stroke or TIA | 3072 (26.6) | 3073 (26.4) | 0.62 |
| Dysphagia, n (%) | 4976 (44.7) | 5058 (45.0) | 0.69 |
| Pneumonia, n (%) | 772 (6.7) | 751 (6.7) | 0.78 |
| 7‐day mortality, n (%) | 407 (3.5) | 403 (3.5) | 0.27 |
| 7‐day mortality—ischemic stroke, n (%) | 307 (2.9) | 292 (2.7) | 0.85 |
| 7‐day mortality—ICH, n (%) | 100 (10.9) | 111 (11.7) | 0.77 |
| 30‐day mortality, n (%) | 853 (7.4) | 839 (7.2) | 0.04 |
| 30‐day mortality—ischemic stroke, n (%) | 707 (6.7) | 671 (6.3) | 0.63 |
| 30‐day mortality—ICH, n (%) | 146 (15.9) | 168 (17.7) | 0.51 |
ICH indicates intracerebral hemorrhage; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
Table 2.
Characteristics of the SSNAP Patients Excluded and Included
| Excluded (NIHSS Incomplete) | Included (NIHSS Complete) | |
|---|---|---|
| N | 10 229 | 23 199 |
| Median age (IQR) | 80 (69 to 86) | 76 (65 to 84) |
| Female (%) | 5639 (54) | 11 260 (48) |
| Stroke type, n (%) | ||
| Ischemic | 8756 (86) | 21 334 (92) |
| ICH | 1473 (14) | 1865 (8) |
| Dependent prestroke (%) | 3504 (34) | 5639 (24) |
| Comorbidity, n (%) | ||
| CCF | 660 (6.3) | 1250 (5.3) |
| Atrial fibrillation | 2357 (23) | 4452 (19) |
| Hypertension | 5301 (51) | 12 788 (54) |
| Diabetes mellitus | 1960 (19) | 4553 (19) |
| Previous stroke or TIA | 3096 (30) | 6234 (27) |
| 7‐day mortality, n (%) | 1108 (10.6) | 820 (3.5) |
| 30‐day mortality, n (%) | 2034 (19.5) | 1708 (7.3) |
CCF indicates congestive cardiac failure; ICH, intracerebral hemorrhage; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; SSNAP, Sentinel Stroke National Audit Programme; TIA, transient ischemic attack.
Predictors of SAP and Derivation of the ISAN Clinical Risk Score
Baseline factors associated with SAP in the uni‐ and multivariable analyses are presented in Tables 3 and 4. The integer‐based pneumonia risk score (ISAN ) derived from the validation cohort ranged from 0 to 21 (Table 5), and consists of 4 variables available on admission: prestroke Independence (modified Rankin Scale 0 to 1 versus 2 to 5); Sex; Age (5 categories); NIHSS (4 categories). The greatest weightings were for older age and higher stroke severity on the NIHSS.
Table 3.
Univariable Predictors of Pneumonia in the Derivation Cohort
| Item | OR | 95% CI |
|---|---|---|
| Male | 1.23 | 1.10 to 1.37 |
| Age group, y | ||
| <60 | Ref | |
| 60 to 69 | 1.50 | 1.11 to 2.02 |
| 70 to 79 | 2.64 | 2.04 to 3.43 |
| 80 to 89 | 4.29 | 3.34 to 5.50 |
| 90+ | 6.46 | 4.95 to 8.42 |
| Stroke subtype | ||
| Ischemic | Ref | |
| ICH | 1.44 | 1.22 to 1.70 |
| Comorbidities | ||
| CCF | 2.07 | 1.72 to 2.49 |
| Hypertension | 1.02 | 0.91 to 1.14 |
| Atrial fibrillation | 1.9 | 1.69 to 2.15 |
| Diabetes mellitus | 0.97 | 0.85 to 1.12 |
| Stroke/TIA | 1.23 | 1.09 to 1.38 |
| Dependent prestroke | 2.80 | 2.51 to 3.13 |
| NIHSS on admission | ||
| 0 to 4 | Ref | |
| 5 to 15 | 3.38 | 2.77 to 4.13 |
| 6 to 20 | 9.58 | 7.50 to 12.2 |
| 21+ | 13.2 | 10.5 to 16.7 |
CCF indicates congestive cardiac failure; ICH, intracerebral hemorrhage; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
Table 4.
Multivariable Predictors of Pneumonia in the Derivation Cohort
| Item | aOR | 95% CI |
|---|---|---|
| Age group, y | ||
| <60 | Ref | |
| 60 to 69 | 2.24 | 1.41 to 3.55 |
| 70 to 79 | 3.11 | 2.03 to 4.76 |
| 80 to 89 | 4.72 | 3.12 to 7.16 |
| 90+ | 5.96 | 3.82 to 9.28 |
| Sex | ||
| Female | Ref | |
| Male | 1.23 | 1.04 to 1.46 |
| NIHSS on admission | ||
| 0 to 4 | Ref | |
| 5 to 15 | 2.87 | 2.33 to 3.54 |
| 16 to 20 | 6.47 | 4.93 to 8.48 |
| 21+ | 9.38 | 7.31 to 12.05 |
| mRS prestroke | ||
| Independent | Ref | |
| Not independent | 1.62 | 1.36 to 1.93 |
aOR indicates adjusted odds ratio; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale.
Table 5.
ISAN Score Components and Weightings
| Item | Score |
|---|---|
| Age, y | |
| <60 | 0 |
| 60 to 69 | 3 |
| 70 to 79 | 4 |
| 80 to 89 | 6 |
| 90+ | 8 |
| Sex | |
| Female | 0 |
| Male | 1 |
| NIHSS on admission | |
| 0 to 4 | 0 |
| 5 to 15 | 4 |
| 16 to 20 | 8 |
| 21+ | 10 |
| mRS prestroke | |
| Independent | 0 |
| Not independent | 2 |
ISAN indicates prestroke Independence (modified Rankin scale), Sex, Age, National Institutes of Health Stroke Scale; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale.
Discrimination of the ISAN Score and Comparison With the A2DS2 Score
The discrimination of the ISAN score was good in both the derivation and validation cohorts of ischemic stroke patients, with a C‐statistic of 0.78 (95% CI 0.76 to 0.80) in the validation cohort (Table 6). The C‐statistic for the A2DS2 score was very similar (0.79, 0.77 to 0.81). Discrimination of the ISAN score was less good in patients with ICH, with a C‐statistic of 0.71 (0.66 to 0.77), while the C‐statistic for the A2DS2 score was 0.72 (0.67 to 0.77). Both scores also performed similarly in the subgroup of ICH patients who survived the first 3 days of admission. In the validation sample, 5.2% of those patients received palliative care. Excluding these patients had a similar effect on discrimination in the ICH patients (Table 6).
Table 6.
C‐Statistics (95% CI) for the ISAN and A2DS2 Scores in the Derivation and Validation Samples and Sensitivity Analyses
| Derivation | Validation | P Value | |
|---|---|---|---|
| Ischemic stroke | |||
| ISAN | 0.79 (0.77 to 0.81) | 0.78 (0.76 to 0.80) | 0.008 |
| A2DS2 | — | 0.79 (0.77 to 0.81) | |
| Ischemic stroke (excluding palliative care) | |||
| ISAN | — | 0.78 (0.76 to 0.80) | 0.01 |
| A2DS2 | — | 0.79 (0.77 to 0.81) | |
| ICH | |||
| ISAN | — | 0.71 (0.66 to 0.77) | 0.32 |
| A2DS2 | — | 0.72 (0.67 to 0.77) | |
| ICH (excluding patients dying within 3 days) | |||
| ISAN | — | 0.75 (0.69 to 0.80) | 0.20 |
| A2DS2 | — | 0.76 (0.71 to 0.81) | |
| ICH (excluding palliative care) | |||
| ISAN | — | 0.76 (0.71 to 0.82) | 0.30 |
| A2DS2 | — | 0.77 (0.72 to 0.83) |
A2DS2 indicates age, atrial fibrillation, dysphagia, sex, stroke severity (National Institutes of Health Stroke Scale); ICH, intracerebral hemorrhage; ISAN, prestroke Independence (modified Rankin scale), Sex, Age, National Institutes of Health Stroke Scale.
Calibration of the ISAN Score
In the validation cohort, the ISAN score was well calibrated across each of the 22 levels in ischemic stroke patients (Figure 2A). The prevalence of SAP was 1.3% for patients with a score of 0, rising to >30% in patients with an ISAN score of >18. The calibration plot for patients with ICH (Figure 2B) shows evidence of a ceiling effect, with scores above 15 tending to overestimate the risk of SAP in these patients.
Figure 2.
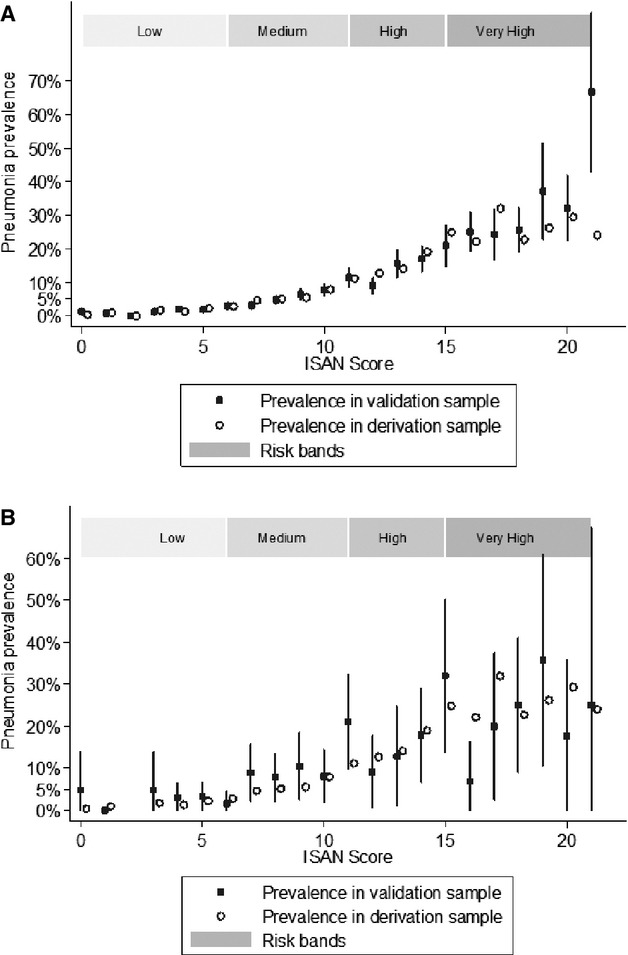
Calibration plots of the ISAN score in patients with (A) ischemic stroke and (B) intracerebral hemorrhage. Vertical lines are 95% CIs. ISAN indicates prestroke Independence (modified Rankin scale), Sex, Age, National Institutes of Health Stroke Scale.
ISAN Score Risk Categories
Aggregation of the ISAN score showed that 4 risk groupings meaningfully identified populations at high or low risk of SAP (Table 7). The low (score 0 to 5) and medium (score 6 to 10) risk groups together accounted for 77% of the validation sample, and the prevalence of SAP in these groups was 1.6% and 4.9%, respectively. 15.5% of the patients were in the high (score 11 to 14) risk category, and had a 12.6% (ischemic stroke) or 15.7% (ICH) prevalence of SAP. The group at highest predicted risk (score 15+) accounted for 8.3% of the stroke population and had a prevalence of SAP of 26.4% (ischemic stroke) and 21.9% (ICH). The relative risk of SAP in ischemic stroke was 7.9 (95% CI 6.0 to 10.5) and 16.5 (95% CI 12.6 to 21.9) in the high and very high categories, respectively, compared to patients in the low‐risk category. Similarly, comparing to the low‐risk group in ICH, the relative risk of SAP was 5.6 (95% CI 2.6 to 12.4) and 7.8 (95% CI 3.6 to 17.2) in the high and very high categories, respectively.
Table 7.
Prevalence of Pneumonia and Mortality by ISAN Risk Group in the Validation Cohort
| Risk Category | Score | % of Cohort | 7‐Day Mortality (%) | 30‐Day Mortality (%) | Ischemic Stroke (N=10 699) | ICH (n=949) | ||
|---|---|---|---|---|---|---|---|---|
| SAP Prevalence (%, 95% CI) | Relative Rate (95% CI) | SAP Prevalence (%, 95% CI) | Relative Rate (95% CI) | |||||
| Low | 0 to 5 | 38.5 | 0.3 | 0.8 | 1.6 (1.2 to 5.5) | 1 | 2.8 (1.2 to 5.5) | 1 |
| Medium | 6 to 10 | 37.8 | 1.6 | 4.2 | 4.9 (4.2 to 5.6) | 3.1 (2.3 to 4.1) | 7.4 (4.8 to 10.8) | 2.6 (1.2 to 5.9) |
| High | 11 to 14 | 15.5 | 6.3 | 13.4 | 12.6 (11.0 to 14.5) | 7.9 (6.0 to 10.5) | 15.7 (10.3 to 22.8) | 5.6 (2.6 to 12.4) |
| Very high | 15+ | 8.3 | 21.4 | 40.0 | 26.4 (22.9 to 30.1) | 16.5 (12.6 to 21.9) | 21.9 (14.8 to 21.3) | 7.8 (3.6 to 17.2) |
ICH indicates intracerebral hemorrhage; ISAN, prestroke Independence (modified Rankin scale), Sex, Age, National Institutes of Health Stroke Scale; SAP, stroke‐associated pneumonia.
Discussion
Despite improvements in acute stroke care, the impact of SAP on mortality may have changed little in recent years.9 Prediction of patients at greatest risk of SAP could be of major importance in individualized monitoring and prognostication, and stratifying inclusion in clinical trials of preventative therapies (eg, antibiotics). We undertook a model development study with the aim of deriving and internally validating a novel clinical risk score for predicting SAP in a large national cohort of contemporary stroke patients. The ISAN score exhibited good discrimination and was well calibrated in ischemic stroke, and identified a clinically meaningful spectrum of SAP risk when aggregated into 4 scoring categories.
An important consideration is how the ISAN score performs in comparison to existing SAP prediction scores developed for ischemic stroke (Table 8). In terms of discrimination, the ISAN score performed comparably to the A2DS2 score in our validation sample and at least as well as the A2DS2 and AIS‐APS scores in the previous studies.2,14 We were unable to externally validate the AIS‐APS in our cohort as several components of the score (ie, current smoking status, chronic obstructive pulmonary disease, Glasgow Coma Scale score, blood glucose) are not recorded in the SSNAP dataset.
Table 8.
Comparison of Cohort Characteristics and Discrimination of ISAN, A2DS2, and AIS‐APS Scores
| Cohort | |||||||
|---|---|---|---|---|---|---|---|
| SSNAP | Berlin | NWSR | CNSR | CICAS | |||
| Derivation | Internal Validation | Derivation | External Validation | Derivation | Validation | External Validation | |
| N | 11 551 | 11 648 | 15 335 | 45 085 | 8820 | 5882 | 3037 |
| Rate of pneumonia (%) | 6.7 | 6.7 | 7.2 | 7.8 | 11.4 | 11.3 | 7.3 |
| Diagnosis of pneumonia | Clinician‐reported diagnosis | Clinician‐reported diagnosis | CDC criteria | CDC criteria | |||
| ICH (%) | 916 (8) | 948 (8) | |||||
| Median age (y, IQR) | 76 (65 to 84) | 76 (65 to 84) | 72 (64 to 81) | 74 (65 to 81) | 66 (56 to 74) | 66 (57 to 75) | 64 (54 to 72) |
| Male sex (%) | 6126 (53) | 6054 (52) | 7759 (51) | 22 801 (51) | 5430 (62) | 3675 (63) | 1997 (66) |
| Median NIHSS score (IQR) | 4 (2 to 9) | 4 (2 to 9) | 4 (2 to 10) | 5 (2 to 10) | 5 (2 to 9) | 5 (2 to 9) | 4 (2 to 7) |
| Dysphagia (%) | 4976 (45) | 5058 (45) | 3507 (23) | 10 465 (24) | 840 (10) | 529 (9) | 236 (8) |
| Prestroke dependent (%) | 2761 (24) | 2793 (24) | 2866 (21) | 6754 (19) | 809 (9) | 535 (9) | 0 (0) |
| Previous stroke (%) | 3072 (27) | 3073 (26) | 4320 (28) | 11 800 (27) | 2795 (32) | 1822 (31) | 777 (26) |
| Atrial fibrillation (%) | 2213 (19) | 2186 (19) | 4139 (27) | 12 337 (28) | 643 (7) | 415 (7) | 180 (6) |
| Diabetes mellitus (%) | 2271 (20) | 2224 (19) | 4783 (31) | 13 146 (30) | 1834 (21) | 1287 (22) | 742 (24) |
| Hypertension (%) | 6228 (54) | 6389 (55) | 11 358 (86) | 30 978 (87) | 5601 (64) | 3683 (63) | 2016 (66) |
| ISAN (C‐statistic; 95% CI) | 0.79 (0.77 to 0.81) | 0.78 (0.76 to 0.80) | |||||
| A2DS2 (C‐statistic; 95% CI) | 0.79 (0.77 to 0.81) | 0.84 (0.83 to 0.85) | 0.84 (0.83 to 0.84) | 0.75 (0.74 to 0.75) | 0.73 (0.72 to 0.74) | 0.76 (0.74 to 0.77) | |
| AIS‐APS (C‐statistic; 95% CI) | 0.80 (0.78 to 0.81) | 0.79 (0.77 to 0.80) | 0.79 (0.76 to 0.82) | ||||
A2DS2 indicates age, atrial fibrillation, dysphagia, sex, stroke severity (National Institutes of Health Stroke Scale); AIS‐APS, acute ischemic stroke–associated pneumonia score; CDC, Centers for Disease Control and Prevention; CICAS, Chinese Intracranial Atherosclerosis Study; CNSR, Chinese National Stroke Registry; ICH, intracerebral hemorrhage; IQR, interquartile range; ISAN, prestroke Independence (modified Rankin scale), Sex, Age, National Institutes of Health Stroke Scale; NIHSS, National Institutes of Health Stroke Scale; NWSR, North‐West Germany Stroke Register; SSNAP, Sentinel Stroke National Audit Programme.
A second consideration is the potential usefulness of prediction scores in clinical practice and research. As SAP develops most frequently in the first days after stroke, preventative strategies need to be administered as soon as possible after stroke onset. To facilitate such early risk‐stratification of patients at greatest risk, prediction scores should be simple, based on valid, reliable, readily available clinical parameters, and quick to apply. The ISAN score is simple, incorporating only 4 clinical factors that are available at emergency presentation. By contrast, definitive past medical history (eg, AF, chronic obstructive pulmonary disease, congestive heart failure) may not always be available without some delay. Detection of AF in the acute phase of stroke is influenced by the frequency of electrocardiography and duration of inpatient telemetry.16 Dysphagia screening techniques and the incidence of dysphagia reported in acute stroke are also variable.17–19 Since the availability of past medical history and recognition of AF and dysphagia may vary between different units, the ISAN score may be both simpler to apply and less prone to interobserver variation than other scores. The 4 risk‐categories of the ISAN score potentially increase its usefulness in both clinical trial and routine care settings.
To the best of our knowledge, this is the first study to apply SAP prediction scores to patients with ICH. While rates of pneumonia are reportedly higher in patients with ICH than ischemic stroke,20–21 early neurological deterioration and death resulting from intracranial sequelae of the initial hematoma occur frequently.22–24 This may lead to under‐reporting of SAP in ICH either because early neurological deterioration confounds clinical diagnosis of evolving SAP, or by decisions not to treat SAP based on perceived futility. The ISAN and A2DS2 scores performed similarly for patients with ICH but less well than for ischemic stroke. There was evidence of a ceiling effect at the higher end of the ISAN scoring range, which is most likely attributable to the competing risk of early death in ICH.
Strengths of our study include model development within a large, multicenter national registry representative of real‐world UK stroke care, with prospectively collected data. However, our study also has limitations. First, nearly one third of SSNAP database patient entries were excluded from the analyses due to incomplete baseline data. This may have underestimated prevalence of pneumonia, particularly as the excluded patients tended to be older, with ICH, and had higher mortality. Second, other baseline factors not recorded in the SSNAP registry, for example, medications (eg, statins, proton‐pump inhibitors, or angiotensin‐converting enzyme inhibitors), baseline vital signs, or intubation/ventilation may influence pneumonia risk. Third, as dysphagia status was derived indirectly, it was not incorporated into the model‐building. Patients who refused a SALT assessment, were too unwell, or did not receive an assessment due to organizational reasons were allocated dysphagic status by default, which may have overestimated the frequency of dysphagia. However, as the dysphagia rates in the German and Chinese cohorts varied between 8% and 24% (Table 8), it is unlikely that this alone accounted for the higher frequency of dysphagia in our study. As the NIHSS score is correlated with dysphagia and age,25–26 exclusion of dysphagia from the ISAN score is unlikely to have adversely influenced face validity. Indeed, admission NIHSS exhibited better test characteristics for predicting dysphagia than a dysphagia screening tool in 1 study.27 Fourth, while the modified Rankin Scale is widely used in research and clinical practice, the interobserver reliability of assigning prestroke modified Rankin Scale is moderate, and may lack validity in rating prior independence.28 Finally, our definition of SAP was based on individual clinician‐based diagnosis. Diagnosis of SAP is challenging as presentation may be nonspecific, cough is impaired, fever occurs frequently without infection, microbiological samples have limited practical value, and chest radiography, a central component of diagnostic criteria, may have limited utility in the early stages.29 Use of standardized diagnostic criteria with independent adjudication might provide a more rigorous approach to diagnosing SAP, but there are currently no universally agreed or validated diagnostic criteria for SAP. Interestingly, the prevalence of SAP was similar in the SSNAP and German cohorts where clinician‐based diagnosis was used, but higher in the Chinese Registry where the Centers for Disease Control and Prevention criteria were prospectively applied (Table 8). These variations could relate to underlying population differences, but may also reflect different thresholds for initiation of antibiotics when pneumonia is clinically suspected, or underdiagnosis in the absence of a standardized diagnostic approach.
In conclusion, the ISAN score, which is simple to use and performed similarly to previously described scores, is a promising tool for predicting SAP in acute stroke care. The ISAN score requires external validation and refinement, preferably in large prospective multicenter studies with adjudicated and standardized definition of SAP, plus evaluation of clinician behaviors in using the score, relative to usual practice or clinical judgment.
Sources of Funding
SSNAP is commissioned by the Healthcare Quality Improvement Partnership on behalf of the Department of Health in England. No specific funding from any source was sought for this study. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London.
Disclosures
None.
Acknowledgments
The authors thank the many hundreds of individuals who have contributed to SSNAP. The authors also thank the Intercollegiate Stroke Working Party for their guidance and support.
References
- 1.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post‐stroke infection: a systematic review and meta‐analysis. BMC Neurol. 2011; 11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann S, Malzahn U, Harms H, Koennecke HC, Berger K, Kalic M, Walter G, Meisel A, Heuschmann PUBerlin Stroke Register and the Stroke Register of Northwest Germany. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. 2012; 43:2617-2623. [DOI] [PubMed] [Google Scholar]
- 3.Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003; 60:620-625. [DOI] [PubMed] [Google Scholar]
- 4.Ovbiagele B, Hills NK, Saver JL, Johnston SCCalifornia Acute Stroke Prototype Registry Investigators. Frequency and determinants of pneumonia and urinary tract infection during stroke hospitalization. J Stroke Cerebrovasc Dis. 2006; 15:209-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik GInvestigators of the Registry of the Canadian Stroke Network. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011; 77:1338-1345. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RD. Mortality and cost of pneumonia after stroke for different risk groups. J Stroke Cerebrovasc Dis. 2012; 21:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslanyan S, Weir CJ, Deiner HC, Kaste M, Lees KGAIN International Steering Committee and Investigators. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN international trial. Eur J Neurol. 2004; 11:49-53. [DOI] [PubMed] [Google Scholar]
- 8.Katzan IL, Dawson NV, Thomas CL, Votruba ME, Cebul RD. The cost of pneumonia after acute stroke. Neurology. 2007; 68:1938-1943. [DOI] [PubMed] [Google Scholar]
- 9.Tong X, Kuklina EV, Gillespie C, George MG. Medical complications among hospitalizations for ischemic stroke in the United States from 1998 to 2007. Stroke. 2010; 41:980-986. [DOI] [PubMed] [Google Scholar]
- 10.Koennecke HC, Belz W, Berfelde D, Endres M, Fitzek S, Hamilton F, Kreitsch P, Mackbert BM, Nabavi DG, Nolte CH, Pöhls W, Schmehl I, Schmitz B, Brevern M, Walter G, Heuschmann PUBerlin Stroke Register Investigators. Factors influencing in‐hospital mortality and morbidity in patients treated on a stroke unit. Neurology. 2011; 77:965-972. [DOI] [PubMed] [Google Scholar]
- 11.Kwon HM, Jeong SW, Lee SH, Yoon BW. The pneumonia score: a simple grading scale for prediction of pneumonia after acute stroke. Am J Infect Control. 2006; 34:64-68. [DOI] [PubMed] [Google Scholar]
- 12.Chumbler NR, Williams LS, Wells CK, Lo AC, Nadeau S, Peixoto AJ, Gorman M, Boice JL, Concato J, Bravata DM. Derivation and validation of a clinical system for predicting and validating pneumonia in acute stroke. Neuroepidemiology. 2010; 34:193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms H, Grittner U, Dröge H, Meisel A. Predicting post‐stroke pneumonia: the PANTHERIS score. Acta Neurol Scand. 2013; 128:178-184. [DOI] [PubMed] [Google Scholar]
- 14.Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y, Li H, Wang YChina National Stroke Registry Investigators. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. 2013; 44:1303-1309. [DOI] [PubMed] [Google Scholar]
- 15.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, Haley EC, Grotta J, Marler JNINDS TPA Stroke Study Group. Improved reliability of the NIH stroke scale using video training. Stroke. 1994; 25:2220-2226. [DOI] [PubMed] [Google Scholar]
- 16.Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ, Smith CJ. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta‐analysis. Stroke. 2014; 45:520-526. [DOI] [PubMed] [Google Scholar]
- 17.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005; 36:2756-2763. [DOI] [PubMed] [Google Scholar]
- 18.Daniels SK, Anderson JA, Willson PC. Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012; 43:892-897. [DOI] [PubMed] [Google Scholar]
- 19.Donovan NJ, Daniels SK, Edmiaston J, Weinhardt J, Summers D, Mitchell PHAmerican Heart Association Council on Cardiovascular Nursing and Stroke Council. Dysphagia screening: state of the art. Invitational conference proceeding from the state‐of‐the‐art nursing symposium, international stroke conference 2012. Stroke. 2013; 44:e24-e31. [DOI] [PubMed] [Google Scholar]
- 20.Ji R, Wang D, Shen H, Pan Y, Liu G, Wang P, Wang Y, Li H, Wang YChina National Stroke Registry (CNSR) Investigators. Interrelationship among common medical complications after acute stroke: pneumonia plays an important role. Stroke. 2013; 44:3436-3444. [DOI] [PubMed] [Google Scholar]
- 21.Kwan J, Pickering RM, Kunkel D, Fitton C, Jenkinson D, Perry VH, Ashburn AMStroke Association Rehabilitation Research Centre. Impact of stroke‐associated infection on long‐term survival: a cohort study. J Neurol Neurosurg Psychiatry. 2013; 84:297-304. [DOI] [PubMed] [Google Scholar]
- 22.Moon JS, Janjua N, Ahmed S, Kirmani JF, Harris‐Lane P, Jacob M, Ezzedine MA, Qureshi AL. Prehospital neurologic deterioration in patients with intracerebral hemorrhage. Crit Care Med. 2008; 36:172-175. [DOI] [PubMed] [Google Scholar]
- 23.Ali M, Lyden P, Sacco RL, Shuaib A, Lees KRVISTA investigators. Natural history of complications after intracerebral haemorrhage. Eur J Neurol. 2009; 16:624-630. [DOI] [PubMed] [Google Scholar]
- 24.Di Napoli M, Parry‐Jones AR, Smith CJ, Hopkins SJ, Slevin M, Masotti L, Campi V, Singh P, Papa F, Popa‐Wagner A, Tudorica V, Godoy DA. C‐reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke. 2014; 45:59-65. [DOI] [PubMed] [Google Scholar]
- 25.Okubo PC, Fábio SR, Domenis DR, Takayanagui OM. Using the National Institute of Health stroke scale score to predict dysphagia in acute ischemic stroke. Cerebrovasc Dis. 2012; 33:501-507. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Doughty C, Doros G, Selim M, Lahoti S, Gokhale S, Schlaug G. Recovery of swallowing after dysphagic stroke: an analysis of prognostic factors. J Stroke Cerebrovasc Dis. 2014; 23:56-62. [DOI] [PubMed] [Google Scholar]
- 27.Bravata DM, Daggett VS, Woodward‐Hagg H, Damush T, Plue L, Russell S, Allen G, Williams LS, Harezlak J, Chumbler NR. Comparison of two approaches to screen for dysphagia among acute ischemic stroke patients: nursing admission screening tool versus National Institutes of Health Stroke Scale. J Rehabil Res Dev. 2009; 46:1127-1134. [DOI] [PubMed] [Google Scholar]
- 28.Fearon P, McArthur KS, Garrity K, Graham LJ, McGroarty G, Vincent S, Quinn TJ. Prestroke modified Rankin scale has moderate interobserver reliability and validity in an acute stroke setting. Stroke. 2012; 43:3184-3188. [DOI] [PubMed] [Google Scholar]
- 29.Harms H, Hoffman S, Malzahn U, Ohlraun S, Heuschmann P, Meisel A. Decision‐making in the diagnosis and treatment of stroke‐associated pneumonia. J Neurol Neurosurg Psychiatry. 2012; 83:1225-1230. [DOI] [PubMed] [Google Scholar]


