Abstract
Background
Despite advances in prevention and treatment of cardiovascular disease, sudden cardiac death (SCD) remains a clinical challenge. Risk stratification in the general population is needed.
Methods and Results
Beat‐to‐beat spatiotemporal variability in the T vector was measured as the mean angle between consecutive T‐wave vectors (mean TT′ angle) on standard 12‐lead ECGs in 14 024 participants in the Atherosclerosis Risk in Communities (ARIC) study. Subjects with left ventricular hypertrophy, atrial arrhythmias, frequent ectopy, ventricular pacing, or QRS duration ≥120 ms were excluded. The mean spatial TT′ angle was 5.21±3.55°. During a median of 14 years of follow‐up, 235 SCDs occurred (1.24 per 1000 person‐years). After adjustment for demographics, coronary heart disease risk factors, and known ECG markers for SCD, mean TT′ angle was independently associated with SCD (hazard ratio 1.089; 95% CI 1.044 to 1.137; P<0.0001). A mean TT′ angle >90th percentile (>9.57°) was associated with a 2‐fold increase in the hazard for SCD (hazard ratio 2.01; 95% CI 1.28 to 3.16; P=0.002). In a subgroup of patients with T‐vector amplitude ≥0.2 mV, the association with SCD was almost twice as strong (hazard ratio 3.92; 95% CI 1.91 to 8.05; P<0.0001). A significant interaction between mean TT′ angle and age was found: TT′ angle was associated with SCD in participants aged <55 years (hazard ratio 1.096; 95% CI 0.043 to 1.152; P<0.0001) but not in participants aged ≥55 years (Pinteraction=0.009).
Conclusions
In a large, prospective, community‐based cohort of left ventricular hypertrophy–free participants, increased beat‐to‐beat spatiotemporal variability in the T vector, as assessed by increasing TT′ angle, was associated with SCD.
Keywords: atherosclerosis, electrocardiography, electrophysiology, epidemiology, sudden cardiac death, TT′ angle
Introduction
Although cardiovascular mortality has declined by ≈30% in the past decade, the incidence of sudden cardiac death (SCD) remains high.1 An estimated 80% of SCDs are associated with coronary heart disease (CHD),2 but despite significant advances in the management of cardiovascular risk factors, approximately half of all SCDs may be the first clinical manifestation of CHD. Consequently, there is a critical need to develop noninvasive, readily available, and inexpensive tools to identify persons with an elevated risk of SCD in the absence of manifest CHD so they can be targeted with preventative therapies.
Ventricular tachycardia and ventricular fibrillation, which cause of more than half of SCDs, require an abnormal myocardial electrophysiological substrate to be sustained; this could be manifested by enhanced temporal and spatial variability in cardiac repolarization.3–4 Surface electrocardiogram (ECG) measures of beat‐to‐beat variability of repolarization such as QT variability,5 T‐wave variability,6 T‐wave alternans,7 and T peaks cloud volume8 have been associated with ventricular arrhythmias in heart failure (HF) patients.5–6,5–10 Beat‐to‐beat temporal and spatial variability in cardiac repolarization has not been studied in a general population with and without manifest cardiovascular disease, and the implications of abnormal repolarization in this population is unknown.
We recently developed a novel dynamic vectorcardiograph (VCG) approach for assessing beat‐to‐beat temporal and spatial variability in the spatial T vector.8,11–12 We found that the spatial TT′ angle (angle between consecutive spatial T vectors) was minimally influenced by heart rate,13 showed good reproducibility14 when measured on routine 12‐lead ECGs, and was associated with ventricular tachycardia and ventricular fibrillation in HF patients. We hypothesized that increasing beat‐to‐beat temporal and spatial variability in the spatial T vector, as measured by increasing mean TT′ angle on a resting 12‐lead ECG, is associated with SCD in a large, prospective, community‐based cohort.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) study is a prospective cohort study designed to identify risk factors, progression, and outcomes of atherosclerosis in the community. Between 1987 and 1989, 15 792 male and female volunteers aged 45 to 64 years were recruited by probability sampling from 4 communities (Forsyth County, NC; suburban Minneapolis, MN; Washington County, MD; and Jackson, MS). Details of enrollment and study procedures have been described previously.15 The study was approved by institutional review committees of all participating institutions. All study participants gave informed consent before entering the study.
Participants were excluded from this analysis if their race was neither black nor white (n=47), if their race was black in the Minnesota and Maryland cohorts (n=55), if they were not eligible for the beat‐to‐beat variability assessment because of cardiac rhythm other than sinus (ventricular pacing or atrial tachyarrhythmias), or if they had ≥2 premature atrial or ventricular contractions (n=281), QRS duration ≥120 ms (n=415), or poor quality ECG recordings (n=277). Because left ventricular hypertrophy affects ventricular repolarization,16–18 participants with signs of left ventricular hypertrophy on ECG, defined as a sex‐adjusted Cornell product19 >2440 mm×ms (n=743), were also excluded. After exclusion, 14 024 participants remained.
Definitions
History of myocardial infarction (MI) was defined as self‐reported MI or ECG evidence of MI, as defined by the Minnesota code.20 CHD was defined as a history of claudication, angina, or MI, diagnosed by the Rose questionnaire21; a physician's diagnosis of MI or stroke; a history of coronary revascularization; or ECG evidence of prior MI. HF was defined by self‐reported use of HF medications or evidence of symptomatic HF, as defined by stage 3 of the Gothenburg criteria.22 Stroke was diagnosed by the ARIC stroke and transient ischemic attack diagnostic algorithm.23 Cardiovascular disease (CVD) was defined as the presence of CHD, HF, MI, or stroke (as defined above). Diabetes mellitus (DM) was defined as a nonfasting glucose level of ≥140 mg/dL, a history of DM, or current use of DM medications.
ECG Data Recording and Analysis
At the time of enrollment in the ARIC study, a digital 10‐second, 12‐lead, resting ECG was obtained using the 12SL algorithm (Marquette, GE Electronics). The digital ECG (sampling rate 500 Hz, amplitude resolution 1 μV) was then analyzed using customized Matlab software (MathWorks, Inc) and transformed into orthogonal ECGs (x‐, y‐, and z‐axes) and VCGs using the inverse Dower transformation matrix.24 Spatial peak QRS‐T angle was measured, as described previously.13,25
Dynamic VCGs: Removal of Respiration Effects and Measurement of the Mean Spatial TT′ Angle
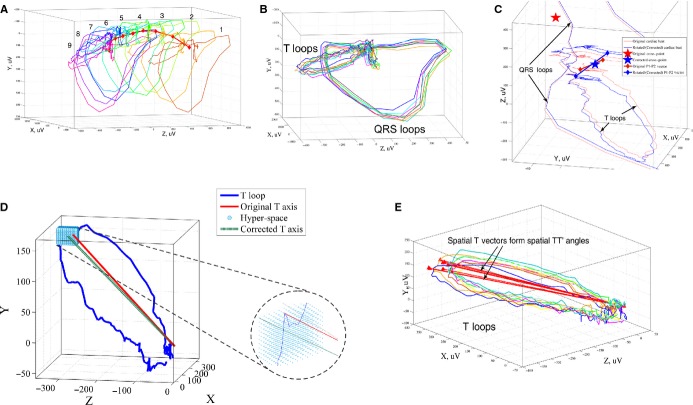
VCG fiducial points (the origin point and the peak of spatial T‐wave vector) were detected automatically. The gross effect of respiration was removed by aligning and rotating VCG loops in space,26–27 as shown in Figure 1A–1C. To accomplish this, the cross‐point between QRS and T‐wave loops was determined for each beat after detection of P1 and P2 points, as described previously,13 and all beats were translated in space so that the cross‐point of each beat was located at the origin (0, 0, 0). This translation allowed preservation of spatial angles and minimized the effects of baseline wandering and respiration. Rotation of VCG loops, another important method of respiration removal, was performed by aligning the P1–P2 vectors, as shown in Figure 1C.
Figure 1.
Measurement of spatial TT′ angle. A, Each sinus beat of the original ECG signal is transformed into a VCG loop. Due to respiration and slow baseline wander, the VCG loops are separated in 3‐dimensional space. QRS and T loops with detected origin cross‐points (red diamonds) for 9 consecutive sinus beats are shown. B, VCG loops are rotated around the cross‐point and aligned by P1–P2 vector and the main plane of the QRS loop. C, VCG loops after correction for respiration by translation and alignment of the origin cross‐points and P1–P2 vectors. D, To further remove the effects of noise, a hyperspace of points can be used to locate the peak of the spatial T vector. E, Spatial TT′ angle is measured as the angle between 2 consecutive spatial T vectors (red lines). The mean spatial TT′ angle is averaged across all pairs of consecutive spatial T vectors during the 10‐second ECG. ECG indicates electrocardiogram; TT′ angle, angle between consecutive T‐wave vectors; VCG, vectorcardiograph.
The spatial TT′ angle was calculated using the definition of the inner product:
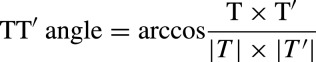 |
It was measured for consecutive sinus beats recorded during the 10‐second ECG. The mean spatial TT′ angle was then calculated by averaging all individual TT′ angles (Figure 1E).
Validation of Respiration and Noise‐Effect Removal
To ensure that we compensated for the effect of respiration on T axis and TT′ angle measurement27–30 and minimized the potential effect of noise on detection of the spatial T‐vector peak, we applied additional corrections to the VCG loops. To provide additional correction for respiration, we reanalyzed 12‐lead ECG data with an additional respiration‐removal approach described previously by Lazaro et al.31 To correct for the potential effect of noise on measurement of the T vector (and thus TT′ angle), we used a hyperspace of points around the peak of the T loop to find the best fit for the peak of the spatial T vector (rather than relying solely on the point furthest from the T‐wave loop origin) (Figure 1D).
Patient Follow‐up and End Point Definitions and Adjudication
Study participants were followed with annual telephone calls, local hospital surveillance, 3 triennal visits until the end of 2002, and querying of the National Death Index. Details of follow‐up have been reported previously.32 The primary outcome in this study was SCD, which was adjudicated in a 2‐step process involving 2 independent death adjudication committees, as described previously.33 All deaths were reviewed and adjudicated by the ARIC morbidity and mortality classification committee using established criteria to determine whether or not a death was attributed to CHD.32 Definite and possible CHD deaths were then reviewed by an independent SCD adjudication committee to determine whether a death was attributable to SCD, as described previously.34
Statistical Analysis
Linear regression (with adjustment for age, sex, race, and study center) was used to determine the association between demographic, clinical, and ECG characteristics and spatial TT′ angle. Cox proportional hazards analyses were used to quantify associations between the mean TT′ angle as a continuous variable and SCD. Participants were considered at risk from enrollment until death or censoring on December 31, 2002, whichever came first. We constructed 3 Cox proportional hazards models to adjust for covariates associated with TT′ angle and SCD by including variables with P<0.1 in linear regression. Model 1 adjusted for age and sex and was stratified by race and study center. Model 2 was further adjusted for the presence of CVD, use of β‐blockers, use of antihypertensive medications, traditional cardiovascular risk factors (systolic blood pressure, DM, smoking status, total cholesterol, high‐density lipoprotein cholesterol, triglycerides, albumin, body mass index, and leisure activity index), and SCD risk factors (use of alcohol, sodium level, and magnesium level). Model 3 incorporated all variables in models 1 and 2 plus the ECG characteristics of heart rate, corrected QT interval, spatial QRS‐T angle, QRS axis, T‐wave axis, and spatial T‐vector magnitude. Schoenfeld residuals confirmed that the proportional hazards assumption was valid in all models.
The association between mean spatial TT′ angle as a continuous variable and SCD was further evaluated through the use of fully adjusted Cox regression models incorporating quadratic splines with 4 knots at 1.8°, 3.4°, 5.2°, and 12.5°.
To develop and validate a clinically meaningful threshold of mean TT′ angle, we randomly divided the study population into 2 groups matched for age, sex, race, and study center. We then chose empirical cutoffs for mean TT′ angle (at the 75th, 90th, and 95th percentiles of mean TT′ angle values in the derivation cohort) and compared the strength of associations at these thresholds in the validation cohort.
To compare the predictive value of mean TT′ angle with other ECG metrics, we calculated the C statistic of model 2 (without any ECG parameters) and compared incremental changes in the C statistic with the incremental addition of each ECG parameter individually. To allow comparison of the relative hazards of different ECG parameters with different units, we also calculated hazard ratios (HRs) for SCD based on a 1‐SD increase in each ECG parameter in the fully adjusted Cox proportional hazards model (model 3).
It is known that patients with small T‐wave amplitudes are at higher risk of mortality.35 Consequently, to test for robustness of the findings across different spatial T‐vector amplitudes, we conducted a sensitivity analysis in a subgroup of participants with spatial T‐vector amplitude ≥0.2 mV.
Data were independently analyzed by the authors using STATA 13 software (StataCorp LP).
Results
The average participant age (n=14 024) was 54.0±5.8 years. Women composed 56.2% of the study population (n=7880), and 75.0% of participants (n=10 515) self‐identified as white. The mean TT′ angle was 5.21±3.55°. Mean TT′ angle was larger in women than in men (5.6±3.8° versus 4.6±3.1°; P<0.0001), in black participants compared with white participants (6.2±4.3° versus 4.9±3.2°; P<0.0001), and in participants aged ≥55 years compared with participants aged <55 years (5.4±3.6° versus 5.1±3.5°, P<0.0001).
Validation of Respiration and Noise‐Effect Removal
Additional corrections for the removal of residual respiration effects31 and use of a hyperspace of points to define the peak of the spatial T vector did not change the mean TT′ angle (paired t test 5.21±3.55° versus 5.26±4.65°; P=0.318).
Correlates of Spatial TT′ Angle
The associations between clinical and demographic characteristics of participants and mean spatial TT′ angle determined through linear regression are shown in Table 1. In general, increasing comorbidities and cardiovascular risk factors including MI, HF, CVD, CHD, DM, and current smoking were associated with increasing mean TT′ angle. Female sex and black race were associated with larger mean TT′ angles. Increasing age, systolic blood pressure, body mass index, heart rate, QT interval, and QRS‐T angle were associated with increasing mean TT′ angle (Figure 2). Increases in T‐wave vector amplitude, serum magnesium, and serum potassium were associated with smaller mean TT′ angles. Of note, older participants (aged ≥55 years) had smaller spatial T‐vector amplitude (0.30±0.13 versus 0.32±0.14 mV; P<0.0001). A significant association was noted between cholesterol and TT′ angle, but the magnitude of this association was quite low.
Table 1.
Associations of Baseline Clinical and ECG Characteristics With Spatial TT′ Angle
| Characteristic | Difference (95% CI) in TT′ Angle, ° | P Value |
|---|---|---|
| Age, per 10 y | 0.44 (0.34 to 0.54) | <0.0001 |
| Women | 0.99 (0.87 to 1.10) | <0.0001 |
| White | −1.20 (−1.33 to −1.07) | <0.0001 |
| History of myocardial infarction | 1.10 (0.78 to 1.42) | <0.0001 |
| Prevalent heart failure | 1.23 (0.95 to 1.52) | <0.0001 |
| Prevalent stroke | 0.84 (0.36 to 1.32) | 0.001 |
| Prevalent CVD | 1.15 (0.94 to 1.35) | <0.0001 |
| Prevalent CHD | 1.26 (0.97 to 1.56) | <0.0001 |
| Use of β‐blockers | 0.60 (0.39 to 0.80) | <0.0001 |
| Systolic BP, per 10 mm Hg | 0.17 (0.13 to 0.20) | <0.0001 |
| Prevalent hypertension | 0.82 (0.69 to 0.95) | <0.0001 |
| Hypertension medications | 0.84 (0.71 to 0.97) | <0.0001 |
| Prevalent diabetes | 0.61 (0.43 to 0.80) | <0.0001 |
| Current smoking | 0.32 (0.17 to 0.46) | <0.0001 |
| BMI, per 1 kg/m2 | 0.10 (0.09 to 0.12) | <0.0001 |
| Leisure activity index | −0.25 (−0.35 to −0.14) | <0.0001 |
| Total cholesterol, per 10 mg/dL | 0.03 (0.02 to 0.05) | <0.0001 |
| HDL cholesterol, per 1 mg/dL | −0.02 (−0.02 to −0.2) | <0.0001 |
| Triglycerides, per 10 mg/dL | 0.04 (0.04 to 0.05) | <0.0001 |
| Lipid‐lowering medications | 0.45 (0.10 to 0.80) | 0.011 |
| Former alcohol abuse | 0.09(−0.10 to 0.27) | 0.35 |
| Current alcohol abuse | −0.13 (−0.28 to 0.02) | 0.098 |
| Serum albumin, per 1 g/dL | 0.33 (0.11 to 0.55) | 0.004 |
| Serum creatinine, per 1 mg/dL | −0.05(−0.24 to 0.15) | 0.63 |
| Magnesium, per 1 mmol/L | −1.84 (−2.59 to −1.09) | <0.0001 |
| Sodium, per 10 mEq/L | 0.47 (0.23 to 0.71) | <0.0001 |
| Potassium, per 1 mEq/L | −0.73 (−0.86 to −0.61) | <0.0001 |
| QT‐prolonging medications | 0.47 (0.27 to 0.67) | <0.0001 |
| Heart rate, per 10 bpm | 0.30 (0.25 to 0.36) | <0.0001 |
| Corrected QT interval, per 10 ms | 0.22 (0.19 to 0.25) | <0.0001 |
| QRS duration, per 10 ms | 0.02 (−0.05 to 0.08) | 0.55 |
| QRS axis, per 10° | −0.05 (−0.07 to −0.04) | <0.0001 |
| T‐axis, per 10° | 0.03 (0.01 to 0.05) | 0.009 |
| QRS‐T angle, per 10° | 0.16 (0.14 to 0.18) | <0.0001 |
| T‐vector amplitude, per 1 mV | −15.42 (−15.79 to −15.05) | <0.0001 |
All models are general linear models. All models are adjusted for age, race, sex, and study center. BMI indicates body mass index; BP, blood pressure; bpm, beats per minute; CHD, coronary heart disease; CVD, cardiovascular disease; HDL, high‐density lipoprotein; TT′ angle, angle between consecutive T‐wave vectors.
Figure 2.
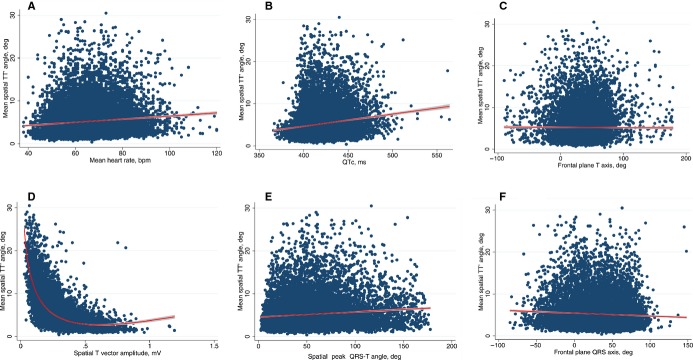
A, Scatterplot of mean spatial TT′ angle (y‐axis) against mean heart rate (x‐axis). B, Scatterplot of mean spatial TT′ angle (y‐axis) against QTc (x‐axis). C, Scatterplot of mean spatial TT′ angle (y‐axis) against frontal plane T axis (x‐axis). D, Scatterplot of mean spatial TT′ angle (y‐axis) against spatial T‐vector amplitude (x‐axis). E, Scatterplot of mean spatial TT′ angle (y‐axis) against spatial peak QRS‐T angle (x‐axis). F, Scatterplot of mean spatial TT′ angle (y‐axis) against frontal plane QRS axis (x‐axis). A fractional polynomial fit is used in panel D, and a linear fit is used in all other panels. bpm indicates beats per minute; deg, degress; QTc, corrected QT interval; TT′ angle, angle between consecutive T‐wave vectors.
Sudden Cardiac Death and Mean TT′ Angle
During median follow‐up of 14 years, there were 235 SCDs (event rate 1.24 per 1000 person‐years; 95% CI 1.09 to 1.41). Mean TT′ angle was independently associated with SCD in all 3 Cox proportional hazards models, as shown in Table 2. In model 1, each 1° increase in mean TT′ angle was associated with an 11.7% risk increment for SCD. With additional adjustment for CVD and CHD risk factors in model 2, the association between mean TT′ angle and SCD was mildly attenuated but remained significant: Each 1° increment in mean TT′ angle was associated with a 9.0% risk increment for SCD. In model 3, after further adjustment for ECG markers of SCD, the association between mean TT′ angle and SCD remained similar (each 1° increment in mean TT′ angle was associated with an 8.9% risk increment for SCD).
Table 2.
Risk for Sudden Cardiac Death, Associated With Mean Spatial TT′ Angle
| Model | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| 1 | 1.117 (1.075 to 1.129) | <0.0001 |
| 2 | 1.090 (1.049 to 1.133) | <0.0001 |
| 3 | 1.089 (1.044 to 1.137) | <0.0001 |
Model 1 adjusted for age, sex, race, and study center. Model 2 additionally adjusted for cardiovascular disease, use of β‐blockers, use of antihypertensive medications, systolic blood pressure, diabetes, smoking status, total cholesterol, high‐density lipoprotein cholesterol, triglycerides, albumin, body mass index, leisure activity index, alcohol use, sodium level, and magnesium level. Model 3 additionally adjusted for heart rate, corrected QT interval, spatial QRS‐T angle, QRS axis, T‐wave axis, and spatial T‐vector magnitude. TT′ angle indicates angle between consecutive T‐wave vectors.
Subgroup Analyses
Table 3 shows fully adjusted HRs for SCD based on mean TT′ angle after stratification into clinically relevant subgroups. There was evidence of an association between mean TT′ angle and SCD only among participants who were aged <55 years (Pinteraction<0.0001). When participants were stratified by sex, there was also a trend of mean TT′ angle being more strongly associated with SCD among women than among men, although the Pinteraction for sex was just above statistical significance. No statistically significant differences were noted in the association between mean TT′ angle and SCD when participants were stratified by race or by the presence of CVD, hypertension, or DM.
Table 3.
Risk for SCD Associated With Mean Spatial TT′ Angle in Clinically Relevant Subgroups
| Subgroup | Hazard Ratio (95% CI) | P Value | P Interaction |
|---|---|---|---|
| Male (146 SCDs/n=6028) | 1.056 (0.987 to 1.130) | 0.11 | 0.091 |
| Female (83 SCDs/n=7669) | 1.131 (1.063 to 1.203) | <0.0001 | |
| Aged ≥55 years (145 SCDs/n=6318) | 1.001 (0.949 to 1.057) | 0.96 | 0.009 |
| Aged <55 years (84 SCDs/n=7379) | 1.096 (1.043 to 1.152) | <0.0001 | |
| White (139 SCDs/n=10 420) | 1.056 (0.985 to 1.131) | 0.13 | 0.54 |
| Black (90 SCDs/n=3277) | 1.115 (1.054 to 1.179) | <0.0001 | |
| CVD, Yes (74 SCDs/n=1160) | 1.083 (1.010 to 1.162) | 0.027 | 0.35 |
| CVD, No (155 SCDs/n=12 537) | 1.092 (1.032 to 1.157) | 0.002 | |
| Diabetes, Yes (71 SCDs/n=1499) | 1.104 (1.014 to 1.201) | 0.022 | 0.23 |
| Diabetes, No (158 SCDs/n=12 198) | 1.082 (1.029 to 1.137) | 0.002 | |
| Hypertension, Yes (128 SCDs/n=4368) | 1.102 (1.045 to 1.162) | <0.0001 | 0.86 |
| Hypertension, No (101 SCDs/n=9275) | 1.056 (0.975 to 1.144) | 0.18 |
All models are fully adjusted (model 3 of Table 2) Cox regression models. CVD indicates cardiovascular disease; SCD, sudden cardiac death; TT′ angle, angle between consecutive T‐wave vectors.
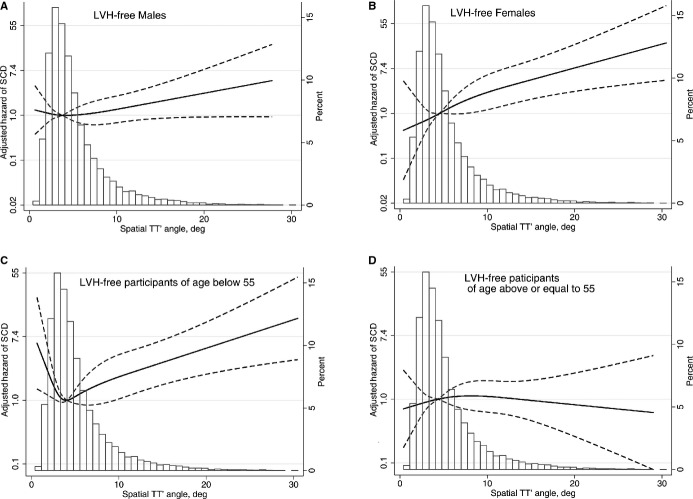
The relationships between mean TT′ angle and SCD evaluated through the use of fully adjusted Cox regression models incorporating quadratic splines in all participants and in participants stratified by age and sex are shown in Figures 3 and 4. Among all participants and in the subgroup of women there was a linearly increasing relationship between mean TT′ angle and the HR of SCD. Among men, the association had a similar direction but was significantly attenuated. Participants aged <55 years showed a J‐shaped relationship between mean TT′ angle and the HR for SCD, although a predominantly linear relationship existed between TT′ angle and the HR for SCD for TT′ angles above the mean. There was no relationship between TT′ angle and SCD for participants aged >55 years.
Figure 3.
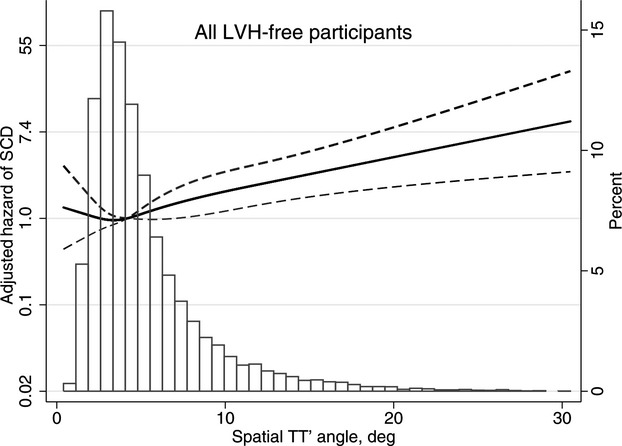
Multivariate adjusted hazard ratio with 95% CI for SCD associated with mean spatial TT′ angle, modeled as a continuous variable using quadratic splines for SCD in all study participants. deg indicates degrees; LVH, left ventricular hypertrophy; SCD, sudden cardiac death; TT′ angle, angle between consecutive T‐wave vectors.
Figure 4.
Multivariate adjusted hazard ratio with 95% CI for SCD associated with mean spatial TT′ angle, modeled as a continuous variable using quadratic splines in left ventricular hypertrophy–free (A) men, (B) women, (C) participants aged <55 years, and (D) participants aged ≥55 years. deg indicates degrees; SCD, sudden cardiac death; TT′ angle, angle between consecutive T‐wave vectors.
Clinical Applicability of a Mean TT′ Angle Cutoff for Risk Stratification for SCD
The study population without missing variables (n=13,697) was randomly divided into 2 groups matched for age, sex, race, and study center (6847 participants in the derivation cohort and 6850 participants in the validation cohort). The 90th percentile of mean of TT′ angle (9.57°) was associated with a statistically significant increase in the fully adjusted risk of SCD (model 3) in the derivation cohort (HR 1.91, 95% CI 1.02 to 3.58, P=0.043). When the same mean TT′ angle cutoff of 9.57° was applied to the validation cohort, a similar adjusted HR was obtained (HR 2.02, 95% CI 1.03 to 3.98, P=0.041) (Table 4). Consequently, a mean TT′ angle >9.57° was associated with a 2‐fold increase in the hazard for SCD (including all participants: HR 2.01, 95% CI 1.28 to 3.16, P=0.002). Unadjusted Kaplan–Meier curves for SCD based on a mean TT′ angle cutoff of 9.57° are illustrated in Figure 5.
Table 4.
Results From Derivation and Validation Cohorts for the Risk for Sudden Cardiac Death Associated With Mean Spatial TT′ Angle of >9.57°
| Hazard Ratio (95% CI) | P Value | |
|---|---|---|
| Derivation cohort (n=6847) | 1.91 (1.02 to 3.58) | 0.043 |
| Validation cohort (n=6850) | 2.02 (1.03 to 3.98) | 0.041 |
| All participants (n=13 697) | 2.01 (1.28 to 3.16) | 0.002 |
Model adjusted for age, sex, race, study center, cardiovascular disease, use of β‐blockers, use of antihypertensive medications, systolic blood pressure, diabetes, smoking status, total cholesterol, high‐density lipoprotein cholesterol, triglycerides, albumin, body mass index, leisure activity index, alcohol use, sodium level, and magnesium level, heart rate, corrected QT interval, spatial QRS‐T angle, QRS axis, T‐wave axis, and spatial T‐vector magnitude. TT′ angle indicates angle between consecutive T‐wave vectors.
Figure 5.
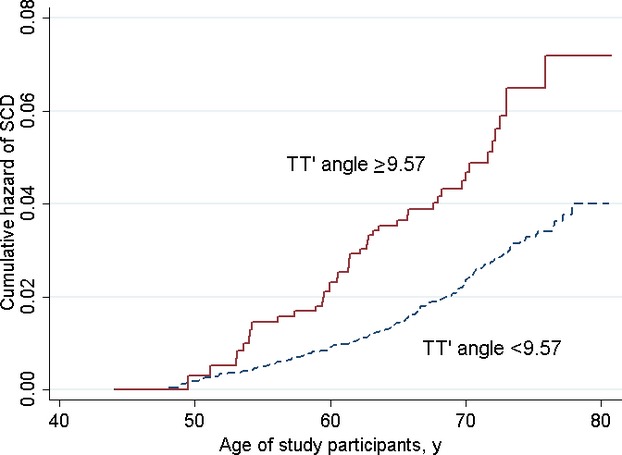
Unadjusted Kaplan–Meier curves for the probabilities of sudden cardiac death in participants with TT′ angles ≥9.57° and <9.57°. TT′ angle indicates angle between consecutive T‐wave vectors.
Comparison of the Predictive Value of Mean TT′ Angle and Other ECG Parameters for SCD Risk Stratification
HRs for a 1‐SD increase in each ECG parameter in the fully adjusted multivariable Cox proportional hazards model (model 3) are shown in Table 5. A 1‐SD increase in mean TT′ angle was associated with a 36% increase in the hazard of SCD. Of all ECG parameters evaluated, a 1‐SD increase in mean TT′ angle was associated with the largest observed increase in SCD risk. Heart rate, T axis, and spatial T‐vector magnitude were not associated with SCD after multivariable adjustment.
Table 5.
Comparison of the Risk of Sudden Cardiac Death Associated With Spatial TT′ Angle and Other ECG Parameters in Fully Adjusted Model 3
| ECG Parameter | HR (95% CI) Per 1‐SD Increase | P Value |
|---|---|---|
| Spatial TT′ angle, ° | 1.36 (1.16 to 1.58) | <0.0001 |
| Heart rate, bpm | 1.01 (0.88 to 1.16) | 0.867 |
| QTc, ms | 1.16 (1.02 to 1.32) | 0.022 |
| QRS axis, ° | 0.81 (0.71 to 0.92) | 0.001 |
| T axis, ° | 1.02 (0.93 to 1.11) | 0.717 |
| Spatial peak QRS‐T angle, ° | 1.23 (1.10 to 1.38) | <0.0001 |
| Spatial T‐vector magnitude, mV | 1.06 (0.90 to 1.25) | 0.496 |
Model adjusted for age, sex, race, study center, cardiovascular disease, use of β‐blockers, use of antihypertensive medications, systolic blood pressure, diabetes, smoking status, total cholesterol, high‐density lipoprotein cholesterol, triglycerides, albumin, body mass index, leisure activity index, alcohol use, sodium level, and magnesium level, heart rate, corrected QT interval, spatial QRS‐T angle, QRS axis, T‐wave axis, and spatial T‐vector magnitude. bpm indicates beats per minute; HR, hazard ratio; TT′ angle, angle between consecutive T‐wave vectors.
Table 6 shows the calculated C statistics of Cox proportional hazards model 2 (without any ECG parameters) and the incremental change in C statistic with 1‐by‐1 addition of ECG parameters. The addition of mean spatial TT′ angle to model 2 resulted in a borderline increase in the C statistic by 0.06 (P=0.053). No other ECG parameters achieved a statistically significant or even borderline increase in C statistic when added to multivariable model 2. Consequently, spatial TT′ angle demonstrated the strongest predictive value in comparison to other studied ECG parameters. Of note, the fully adjusted Cox proportional hazards model (model 3) had a C statistic of 0.822, implying excellent discrimination of the model.
Table 6.
Comparison of C Statistic for Model 2 (Without Any ECG Parameters) and Changed Area Under the Receiver Operating Characteristic Curve After Addition of 1‐by‐1 ECG Parameters
| Parameter | C Statistic (95% CI) | P Value |
|---|---|---|
| Model 2 (without any ECG markers) | 0.809 (0.782 to 0.836) | Reference |
| Spatial TT′ angle, ° | 0.815 (0.788 to 0.842) | 0.053 |
| Heart rate, bpm | 0.809 (0.782 to 0.836) | 0.882 |
| QTc interval, ms | 0.809 (0.781 to 0.836) | 0.953 |
| QRS axis, ° | 0.812 (0.785 to 0.839) | 0.284 |
| T axis, ° | 0.809 (0.782 to 0.836) | 0.694 |
| Spatial peak QRS‐T angle, ° | 0.814 (0.788 to 0.841) | 0.100 |
| Spatial T‐vector magnitude, mV | 0.809 (0.782 to 0.837) | 0.669 |
| Model 3 (with all ECG markers) | 0.822 (0.796 to 0.849) | 0.021 |
Model 2 adjusted for age, sex, race, study center, cardiovascular disease, use of β‐blockers, use of antihypertensive medications, systolic blood pressure, diabetes, smoking status, total cholesterol, high‐density lipoprotein cholesterol, triglycerides, albumin, body mass index, leisure activity index, alcohol use, sodium level, and magnesium level. Model 3 additionally adjusted for heart rate, corrected QT interval, spatial QRS‐T angle, QRS axis, T‐wave axis, and spatial T‐vector magnitude. TT′ angle indicates angle between consecutive T‐wave vectors.
Predictive Value of TT′ Angle in Participants With Spatial T‐Vector Amplitude ≥0.2 mV
After exclusion of 2963 participants with a spatial T‐vector amplitude <0.2 mV, the strength of association between TT′ angle and SCD increased. In a fully adjusted Cox regression model (model 3 in Table 2), each 1° increment in mean TT′ angle was associated with a 14% risk increment for SCD (HR 1.14, 95% CI 1.04 to 1.24, P=0.03). Remarkably, participants with a T‐vector amplitude ≥0.2 mV and a TT′ angle >90th percentile (>9.57°) had nearly quadruple risk for SCD (HR 3.92, 95% CI 1.91 to 8.05, P<0.0001).
Discussion
In this large, community‐based cohort of >14 000 participants without left ventricular hypertrophy on ECG, increased beat‐to‐beat temporal and spatial variability in the spatial T vector, as assessed by increased mean TT′ angle, was independently associated with an arrhythmogenic substrate and SCD, even after adjusting for multiple cardiovascular and SCD risk factors. Each 1° increase in mean TT′ angle was associated with a 9% increase in the risk of SCD, and a mean TT′ angle >9.57° was associated with a 2‐fold increase in the hazard for SCD. When compared with multiple other ECG parameters, TT′ angle had the strongest association with SCD. Mean TT′ angle had significant interactions with age, and the strongest associations between mean TT′ angle and SCD were present in participants aged <55 years. The presence of CVD had no significant influence on the association between mean TT′ angle and SCD.
The Association Between Beat‐to‐Beat Temporal and Spatial Variability in the Spatial T Vector and SCD
The spatial TT′ angle, a novel electrocardiographic marker of beat‐to‐beat temporal and spatial variability in the spatial T vector, has been evaluated previously only in a study of 414 patients with HF and an elevated risk for ventricular arrhythmias who received implantable cardioverter‐defibrillators for primary prevention of SCD.8,11,36 Within this population, each 1° increase in TT′ angle was associated with a 2.5% increase in the risk of ventricular tachycardia and ventricular fibrillation detected on follow‐up implantable cardioverter‐defibrillator interrogations.36 Although implantable cardioverter‐defibrillator therapy for ventricular tachycardia and ventricular fibrillation is not equivalent to aborted SCD, TT′ angle was associated with a proarrhythmic substrate in these patients.
Our current results demonstrate that even in a population with significantly lower SCD risk, increasing TT′ angle was associated with SCD after adjustment for known SCD risk markers including factors specifically characterizing ventricular repolarization (T‐axis and T‐vector amplitude). Consequently, TT′ angle represents a novel electrocardiographic characteristic that is independently associated with SCD risk.
Mechanisms of SCD Associated With Increased Beat‐to‐Beat Temporal and Spatial Variability in Cardiac Repolarization
Stochastic variation in L‐type calcium channel,4,37 IKs and IKr potassium channels,3–4 and late INa4 function are crucial determinants of beat‐to‐beat changes in ventricular repolarization. Variations in channel gating and density are important determinants of short‐term changes in single‐cell and cell‐to‐cell action potential duration. In healthy myocardium, intracellular coupling minimizes variation in repolarization and action potential duration; however, in diseased myocardium, ischemia, infarction, or other injury results in cell‐to‐cell decoupling and increased variability in cardiac repolarization, which can result in frequent early and delayed afterdepolarizations.3 Under certain circumstances, early and delayed afterdepolarizations may propagate through cardiac tissue and initiate ventricular tachycardia and ventricular fibrillation, which can lead to SCD. We speculate that increased beat‐to‐beat temporal and spatial variability in the spatial T vector, as measured by TT′ angle, allows identification of a myocardial substrate with less “repolarization reserve” and more frequent triggers that can initiate malignant ventricular arrhythmias.
The genetic and environmental determinants of TT′ angle variation are unknown, but in people without cardiac disease, increased TT′ angle could be related to genetically determined increased stochasticity of ion channel gating, which subsequently produces a myocardial substrate primed for induction of ventricular arrhythmias. Future discovery and study of genes associated with elevated beat‐to‐beat temporal and spatial variability in the spatial T vector in healthy populations might lead to improved methods of identifying healthy people at risk for SCD and novel therapeutic methods to reduce this risk.
Influence of Sex on Repolarization and TT′ Angle
The effect of sex hormones on repolarizing currents is well known,38 and sex‐specific variations in ventricular repolarization have been described previously.39 In general, the QT interval is shorter in men than in women after adolescence40 because of an earlier onset of phase 3 of the action potential41 and more rapid repolarization dynamics.42 Our results, which demonstrated that mean spatial TT′ angle was larger in women than in men, are consistent with previously reported data.13 This sex‐specific variation in TT′ angle, and the further observation that mean TT′ angle had a trend toward a stronger association with SCD in women than in men, is possibly mediated by sex‐specific variation in sex hormones. Further studies are needed to test this hypothesis.
Influence of Age on Repolarization and TT′ Angle
We demonstrated that older participants had larger TT′ angles. The relationship between age and increasing TT′ angle is not surprising, given that the aging process results in cardiac atrophy, fibrosis,43 and loss of gap junctions,44 which reduce repolarization reserve and create a myocardial environment favorable to arrhythmogenesis. The mechanisms underlying our observation that mean TT′ angle was associated only with SCD in patients aged <55 years are less clear. Age is a well‐known risk factor for SCD because of its association with CHD,45 but it is also associated with an increased risk for nonsudden modes of death, and competing risks for sudden and nonsudden death may at least partially explain the lack of association between TT′ angle and SCD in participants aged >55 years. Alternatively, spatial T‐vector amplitude was significantly smaller in older participants, but the predictive value of TT′ angle was much stronger in participants with larger spatial T‐vector amplitudes (≥0.2 mV), which could explain the observed interaction. The mechanisms underlying SCD in younger and older participants also likely differ and manifest at different points in life. Further study of the interplay between age and beat‐to‐beat temporal and spatial variability in the spatial T vector is required.
Strengths and Limitations
The ARIC study provided high‐quality digital ECGs and long‐term follow‐up with rigorous adjudication of cause of death in a large, prospective, community‐based cohort, but these analyses have limitations. ECG data were collected at the time of enrollment; therefore, we are unable to determine whether and how TT′ angle varied over the follow‐up period, whether short‐ or long‐term changes were prognostically important, and what the TT′ angle was just prior to SCD. In addition, although we adjusted for multiple traditional and ECG‐based confounders, residual confounding could have contributed to our observed results.
Although we made multiple corrections to minimize the effect of respiration, we did not perform rescaling of VCG loops. VCG loops can expand or contract with respiration due to changes in tissue conductivity; however, Sornmo and associates26–27 demonstrated that VCG loops expand and contract symmetrically. Consequently, this should not affect the main plane of VCG loops or the measurement of the spatial T‐loop vector or TT′ angle. Future studies are needed to evaluate the effect of VCG rescaling on TT′ angle. It is also possible that the applied corrections for respiration were suboptimal, and further studies with respiration signals recorded simultaneously with the ECG are needed to better study the effect of respiration on spatial TT′ angle. Simultaneous recording of repolarization ion channel gating and TT′ angle might be needed to provide definite proof of the mechanism underlying beat‐to‐beat temporal and spatial variability in the spatial T vector. Unmeasured autonomic influences could have influenced the measurement of TT′ angle and thus been correlated with SCD.
Conclusions
In a large, prospective, community‐based cohort study of >14 000 participants without left ventricular hypertrophy on ECG, increased beat‐to‐beat temporal and spatial variability in the spatial T vector—as measured by increased mean TT′ angle on a 12‐lead ECG—was independently associated with an increased risk of SCD, even after adjusting for multiple cardiovascular and SCD risk factors. Validation of our findings in another independent cohort is needed. Further study of the electrophysiological mechanisms underlying increased beat‐to‐beat temporal and spatial variability in the spatial T vector and variations in TT′ angle are warranted and may lead to the development of effective, noninvasive SCD screening tools and/or novel therapies to reduce the risk of SCD.
Acknowledgments
The authors thank Muammar Kabir, PhD, and Elyar Ghafoori, MS, for helping with the data analysis. The authors also thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by the National Institutes of Health (NIH) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was supported by the NIH (1R01HL118277) and the Johns Hopkins Institute for Clinical and Translational Research grant (Tereshchenko).
Disclosures
Johns Hopkins University (Tereshchenko) holds the patent on dynamic VCG technology (not licensed).
References
- Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al‐Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011; 57:794-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012; 125:1043-1052. [DOI] [PubMed] [Google Scholar]
- Pueyo E, Corrias A, Virag L, Jost N, Szel T, Varro A, Szentandrassy N, Nanasi PP, Burrage K, Rodriguez B. A multiscale investigation of repolarization variability and its role in cardiac arrhythmogenesis. Biophys J. 2011; 101:2892-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijman J, Zaza A, Johnson DM, Rudy Y, Peeters RL, Volders PG, Westra RL. Determinants of beat‐to‐beat variability of repolarization duration in the canine ventricular myocyte: a computational analysis. PLoS Comput Biol. 2013; 9:e1003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhoff P, Tereshchenko LG, van der Heyden MA, Ghanem RN, Fetics BJ, Berger RD, Vos MA. Short‐term variability of repolarization predicts ventricular tachycardia and sudden cardiac death in patients with structural heart disease: a comparison with QT variability index. Heart Rhythm. 2011; 8:1584-1590. [DOI] [PubMed] [Google Scholar]
- Couderc JP, Zareba W, McNitt S, Maison‐Blanche P, Moss AJ. Repolarization variability in the risk stratification of MADIT II patients. Europace. 2007; 9:717-723. [DOI] [PubMed] [Google Scholar]
- Verrier RL, Kumar K, Nearing BD. Basis for sudden cardiac death prediction by T‐wave alternans from an integrative physiology perspective. Heart Rhythm. 2009; 6:416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereshchenko LG, Han L, Cheng A, Marine JE, Spragg DD, Sinha S, Dalal D, Calkins H, Tomaselli GF, Berger RD. Beat‐to‐beat three‐dimensional ECG variability predicts ventricular arrhythmia in ICD recipients. Heart Rhythm. 2010; 7:1606-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereshchenko LG, Fetics BJ, Domitrovich PP, Lindsay BD, Berger RD. Prediction of ventricular tachyarrhythmias by intracardiac repolarization variability analysis. Circ Arrhythm Electrophysiol. 2009; 2:276-284. [DOI] [PubMed] [Google Scholar]
- Chen X, Tereshchenko LG, Berger RD, Trayanova NA. Arrhythmia risk stratification based on QT interval instability: an intracardiac electrocardiogram study. Heart Rhythm. 2013; 10:875-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Tereshchenko LG. Lability of R‐ and T‐wave peaks in three‐dimensional electrocardiograms in implantable cardioverter defibrillator patients with ventricular tachyarrhythmia during follow‐up. J Electrocardiol. 2010; 43:577-582. [DOI] [PubMed] [Google Scholar]
- Han L, Cheng A, Sur S, Tomaselli GF, Berger RD, Tereshchenko LG. Complex assessment of the temporal lability of repolarization. Int J Cardiol. 2013; 166:543-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur S, Han L, Tereshchenko LG. Comparison of sum absolute QRST integral and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS One. 2013; 8:e57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeny A, Han L, Tereshchenko LG. Repolarization lability measured on 10‐second ECG by spatial TT’ angle: reproducibility and agreement with QT variability. J Electrocardiol. 2014; 47:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ARIC Investigators. The Atherosclerosis Risk in Community (ARIC) study: design and objectives. Am J Epidemiol. 1989; 129:687-702. [PubMed] [Google Scholar]
- Marionneau C, Brunet S, Flagg TP, Pilgram TK, Demolombe S, Nerbonne JM. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing K+ currents with left ventricular hypertrophy. Circ Res. 2008; 102:1406-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rials SJ, Wu Y, Salata JJ, Liu T, Bharucha DB, Marinchak RA, Kowey PR. Left ventricular hypertrophy decreases slowly but not rapidly activating delayed rectifier potassium currents of epicardial and endocardial myocytes in rabbits. Circulation. 2001; 103:1585-1590. [DOI] [PubMed] [Google Scholar]
- Yang KC, Foeger NC, Marionneau C, Jay PY, McMullen JR, Nerbonne JM. Homeostatic regulation of electrical excitability in physiological cardiac hypertrophy. J Physiol. 2010; 588:5015-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okin PM, Roman MJ, Devereux RB, Borer JS, Kligfield P. Electrocardiographic diagnosis of left ventricular hypertrophy by the time‐voltage integral of the QRS complex. J Am Coll Cardiol. 1994; 23:133-140. [DOI] [PubMed] [Google Scholar]
- Blackburn H, Keys A, Simonson E, Rautaharju P, Punsar S. The electrocardiogram in population studies. A classification system. Circulation. 1960; 21:1160-1175. [DOI] [PubMed] [Google Scholar]
- Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968; 56:1-188. [PubMed] [Google Scholar]
- Eriksson H, Caidaul K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svärdsudd K. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical‐epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987; 8:1007-1014. [DOI] [PubMed] [Google Scholar]
- Toole JF, Chambless LE, Heiss G, Tyroler HA, Paton CC. Prevalence of stroke and transient ischemic attacks in the Atherosclerosis Risk in Communities (ARIC) study. Ann Epidemiol. 1993; 3:500-503. [DOI] [PubMed] [Google Scholar]
- Edenbrandt L, Pahlm O. Vectorcardiogram synthesized from a 12‐lead ECG: superiority of the inverse Dower matrix. J Electrocardiol. 1988; 21:361-367. [DOI] [PubMed] [Google Scholar]
- Oehler A, Feldman T, Henrikson CA, Tereshchenko LG. QRS‐T angle: a review. Ann Noninvasive Electrocardiol. 2014; 19:534-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornmo L. Vectorcardiographic loop alignment and morphologic beat‐to‐beat variability. IEEE Trans Biomed Eng. 1998; 45:1401-1413. [DOI] [PubMed] [Google Scholar]
- Astrom M, Carro Santos E, Sornmo L, Laguna P, Wohlfart B. Vectorcardiographic loop alignment and the measurement of morphologic beat‐to‐beat variability in noisy signals. IEEE Trans Biomed Eng. 2000; 47:497-506. [DOI] [PubMed] [Google Scholar]
- Bailon R, Sornmo L, Laguna P. A robust method for ECG‐based estimation of the respiratory frequency during stress testing. IEEE Trans Biomed Eng. 2006; 53:1273-1285. [DOI] [PubMed] [Google Scholar]
- Noriega M, Martinez JP, Laguna P, Romero D, Bailon R, Almeida R. Respiration effect on single and multi lead ECG delineation strategies. Conf Proc IEEE Eng Med Biol Soc. 2010; 2010:3575-3578. [DOI] [PubMed] [Google Scholar]
- Noriega M, Martinez JP, Laguna P, Bailon R, Almeida R. Respiration effect on wavelet‐based ECG T‐wave end delineation strategies. IEEE Trans Biomed Eng. 2012; 59:1818-1828. [DOI] [PubMed] [Google Scholar]
- Lazaro J, Alcaine A, Romero D, Gil E, Laguna P, Pueyo E, Bailon R. Electrocardiogram derived respiratory rate from QRS slopes and R‐wave angle. Ann Biomed Eng. 2014; 42:2072-2083. [DOI] [PubMed] [Google Scholar]
- White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol. 1996; 49:223-233. [DOI] [PubMed] [Google Scholar]
- Soliman EZ, Prineas RJ, Case LD, Russell G, Rosamond W, Rea T, Sotoodehnia N, Post WS, Siscovick D, Psaty BM, Burke GL. Electrocardiographic and clinical predictors separating atherosclerotic sudden cardiac death from incident coronary heart disease. Heart. 2011; 97:1597-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WH, Arking DE, Post W, Rea TD, Sotoodehnia N, Prineas RJ, Bishe B, Doan BQ, Boerwinkle E, Psaty BM, Tomaselli GF, Coresh J, Siscovick DS, Marban E, Spooner PM, Burke GL, Chakravarti A. Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community‐based populations. Circulation. 2009; 119:940-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker JM, Schouten EG, Klootwijk P, Pool J, Kromhout D. ST segment and T wave characteristics as indicators of coronary heart disease risk: the Zutphen Study. J Am Coll Cardiol. 1995; 25:1321-1326. [DOI] [PubMed] [Google Scholar]
- Tereshchenko LG. Repolarization lability measured by spatial TT’ angle. Comput Cardiol. 2014; 41:181-184. [PMC free article] [PubMed] [Google Scholar]
- Tanskanen AJ, Greenstein JL, O'Rourke B, Winslow RL. The role of stochastic and modal gating of cardiac L‐type Ca2+ channels on early after‐depolarizations. Biophys J. 2005; 88:85-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TV, Robinson RB, Danilo P, Jr, Rosen MR. Effects of gonadal steroids on gender‐related differences in transmural dispersion of L‐type calcium current. Cardiovasc Res. 2002; 53:752-762. [DOI] [PubMed] [Google Scholar]
- Surawicz B, Parikh SR. Differences between ventricular repolarization in men and women: description, mechanism and implications. Ann Noninvasive Electrocardiol. 2003; 8:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992; 8:690-695. [PubMed] [Google Scholar]
- Rautaharju PM, Zhou SH, Gregg RE, Startt‐Selvester RH. Electrocardiographic estimates of action potential durations and transmural repolarization time gradients in healthy subjects and in acute coronary syndrome patients—profound differences by sex and by presence vs absence of diagnostic ST elevation. J Electrocardiol. 2011; 44:309-319. [DOI] [PubMed] [Google Scholar]
- Lehmann MH, Yang H. Sexual dimorphism in the electrocardiographic dynamics of human ventricular repolarization: characterization in true time domain. Circulation. 2001; 104:32-38. [DOI] [PubMed] [Google Scholar]
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008; 358:1370-1380. [DOI] [PubMed] [Google Scholar]
- Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009; 2:185-194. [DOI] [PubMed] [Google Scholar]
- Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998; 98:2334-2351. [DOI] [PubMed] [Google Scholar]