Abstract
Background
The relationship between resting heart rate (RHR) and incident heart failure (HF) has been questioned.
Methods and Results
RHR was assessed at baseline in 7073 participants in 3 prospective cohorts (Cardiovascular Health Study, Health ABC study and Kuopio Ischemic Heart Disease Study) that recorded 1189 incident HF outcomes during 92 702 person‐years of follow‐up. Mean age of participants was 67 (9.9) years and mean RHR was 64.6 (11.1) bpm. Baseline RHR correlated (P<0.001) positively with body mass index (r=0.10), fasting glucose (r=0.18), and C‐reactive protein (r=0.20); and inversely with serum creatinine (r=−0.05) and albumin (r=−0.05). Baseline RHR was non‐linearly associated with HF risk. The age and sex‐adjusted hazard ratio for HF comparing the top (>72 bpm) versus the bottom (<57 bpm) quartile of baseline RHR was 1.48 (95% confidence interval [CI] 1.26 to 1.74) and was modestly attenuated (1.30, 95% CI 1.10 to 1.53) with further adjustment for body mass index, history of diabetes, hypertension, smoking status, serum creatinine, and left ventricular hypertrophy. These findings remained consistent in analyses accounting for incident coronary heart disease, excluding individuals with prior cardiovascular events, or those taking beta‐blockers; and in subgroups defined by several individual participant characteristics. In a pooled random effects meta‐analysis of 7 population‐based studies (43 051 participants and 3476 HF events), the overall hazard ratio comparing top versus bottom fourth of RHR was 1.40 (95% CI: 1.19 to 1.64).
Conclusions
There is a non‐linear association between RHR and incident HF. Further research is needed to understand the physiologic foundations of this association.
Keywords: heart failure, heart rate, risk factor
Introduction
Aging of the population, worsening risk factor profile, and improved care of acute cardiovascular disease (CVD) have all led to an increase in the prevalence of heart failure (HF).1 Heart failure is associated with an unacceptably high morbidity and mortality2 and imposes a significant economic burden, underscoring the need to identify high‐risk individuals in order to tailor preventive and therapeutic measures. Resting heart rate (RHR) is independently associated with prognosis among patients with prevalent HF and RHR lowering has been demonstrated to benefit patients with HF and reduced ejection fraction.3–5 Whereas, some studies have suggested a positive association between baseline RHR and incident HF risk,6–7 others have only shown a sex‐differentiated association,8 with uncertainties remaining about the shape of this association due to the small size of the prior investigations. Most studies have included individuals from select population9 or patients with pre‐existing disease3 or using drugs that modify heart rate.10 It is important to assess the association of RHR with risk of HF prospectively in order to also avoid the potential bias of reverse causality. In this study, we sought to assess the association between RHR and incident HF in 3 population‐based cohort studies by performing an individual participant meta‐analysis of these cohorts. To contextualize our findings, we also performed a systematic review and meta‐analysis of the published prospective evidence on the association of RHR and risk of incident HF.
Methods
Study Populations
The 3 cohort studies that were included were the Heath Aging and Body Composition (Health ABC) study, the Cardiovascular Health Study (CHS), and the Kuopio Ischemic Heart Disease (KIHD) study. The Health ABC Study is a community‐based cohort of 3075 individuals aged 70 to 79 years at inception.11–12 Participants were identified from a random sample of white Medicare beneficiaries and all age‐eligible black residents in designated zip codes surrounding Pittsburgh, PA and Memphis, TN. To be eligible, participants had to report no difficulty walking one‐quarter of a mile or climbing 10 stairs without resting. Exclusion criteria included difficulties with activities of daily living, obvious cognitive impairment, or intention of moving within 3 years. Baseline data were collected in 1997–1998.11–12 The design and rationale of CHS has been previously published.13 Briefly, non‐institutionalized individuals 65 to 100 years old were recruited from Medicare eligibility lists and examined at 4 field centres in Forsyth County, NC; Sacramento County, CA; Allegheny County, PA; and Washington County, MD). An original cohort of 5201 participants was recruited in 1989–1990 and a second cohort of 687 black participants was recruited in 1992–1993 (total, 5888 participants; 2495 men, 3393 women). KIHD study is a population‐based study, representative of men living in the city of Kuopio and its surrounding rural communities in Eastern Finland.18 The men were 42 to 61 years of age during baseline examinations performed between March 20, 1984 and December 5, 1989. Of 3235 potentially randomly selected eligible men, 2682 (83%) volunteered to participate in this study, 186 did not respond to the invitation, and 367 declined to give informed consent.18 Each cohort study was approved by its Institutional Research Ethics Committee, and each participant gave written informed consent.11–12,18
For the current analysis, we created an individual‐level pooled dataset from CHS, Health ABC study, and KIHD study for participants without prevalent HF who did not have any major electrocardiographic abnormalities at baseline (ventricular conduction defect, major Q‐wave abnormality, isolated ST‐T wave abnormalities, atrial fibrillation, or atrio‐ventricular blocks). Prevalent HF was adjudicated on the basis of self‐report, medications, and review of medical records. From the 5888 CHS participants we excluded those with prevalent HF (n=275) and individuals with major ECG abnormalities (N=1550). From the 3075 Health ABC participants, we excluded those with prevalent HF (n=95) or inadequate data to adjudicate HF (n=45) and those with major ECG abnormalities (n=1102) at baseline. From KIHD we excluded those with prevalent HF (n=194) and those with major ECG abnormalities (n=164). The final cohort for this analysis comprised 7073 participants with non‐missing data at baseline on several HF risk factors including age, sex, body mass index, diabetes status, history of hypertension, left ventricular hypertrophy, and serum creatinine levels.
Study Outcomes
We used 10 plus‐year adjudicated outcomes from all 3 prospective studies for this analysis. In the Health ABC and CHS studies, all participants were asked to report any hospitalizations every 6 months and were asked direct questions about interim events. Medical records for overnight hospitalizations were reviewed at each site. All first admissions with an overnight stay that was confirmed as related to HF were classified as incident HF. Heart failure diagnosis was adjudicated based on symptoms, signs, chest radiograph results, and echocardiographic findings.13 The criteria required at least HF diagnosis from a physician and treatment for HF.12 In addition, data on left ventricular ejection fraction during the index HF hospitalization was available in a subset of HF cases. All deaths were reviewed by each study's diagnosis and disease ascertainment committee and underlying causes of death were determined by central adjudication. In the KIHD study, participants are under continuous surveillance for the development of new CVD events, including new incident HF cases18. The sources of information on HF were based on hospital records and medico‐legal reports. The diagnostic classification of HF cases were coded according to the International Classification of Disease, Tenth Revision (ICD‐10) codes (I00 to I99) and (I50.0 to I50.9, I11, I42.0 to I42.9). The diagnosis of HF was based on a previous history of heart disease, physical examination by a doctor, laboratory investigations including the determination of natriuretic peptides, echocardiography, as well as electrocardiographic findings. Data on incident acute coronary events and deaths were obtained by computer linkage to the national hospital discharge and death certificate registers.9
Study Definitions
Resting heart rate (bpm) was automatically measured from a 12‐lead electrocardiogram recorded in the morning as part of the baseline enrollment visit, along with fasting venous blood sample collection. Race was self‐reported. Hypertension was defined as self‐reported history of physician diagnosis accompanied by use of antihypertensive medications. Diabetes mellitus was considered present if the participant reported a history of diabetes mellitus or use of anti‐hyperglycemic medication. Smoking was defined as current, past (≥100 lifetime cigarettes), or never. Left ventricular hypertrophy was diagnosed based on the following criteria; R amplitude >26 mm in either V5 or V6, or >20 mm in any of leads I, II, III, aVF, or >12 mm in lead aVL or R in V5 or V6 plus S amplitude in V1 >35 mm. Prevalent HF was based on hospital records and medico‐legal reports. Prevalent coronary artery disease (CAD) was defined as: (1) history of surgical or percutaneous revascularization; or (2) electrocardiographic evidence of myocardial infarction; or (3) self‐reported history of myocardial infarction or angina accompanied by use of anti‐anginal medications. Incident CAD was defined as hospitalization for myocardial infarction or angina pectoris, or elective revascularization. Prevalent vascular disease was defined as prevalent: (1) CAD; (2) cerebrovascular disease (history of stroke, transient ischemic attack, or carotid endarterectomy); or (3) PVD (history of intermittent claudication or vascular bypass or angioplasty).14–15 Incident vascular disease was defined as incident (1) CAD; (2) cerebro‐vascular disease (stroke, transient ischemic attack, or symptomatic carotid artery disease); (3) PVD; or (4) death due to cardiovascular causes.
Statistical Analysis
The principal analyses were pre‐specified to exclude participants with a history of HF and major ECG abnormalities at baseline. Cross‐sectional associations of RHR with various risk factors were assessed using linear regression models adjusted for cohort, age, and sex. The primary outcome was incident HF, defined as first‐ever nonfatal hospital admission for HF. Participants contributed only follow‐up time to recorded first HF outcome. Time‐to‐event analyses were conducted using Cox proportional hazard models, stratified by cohort and sex. The proportional hazards assumptions were tested as previously described and satisfied.16 To characterize shapes of associations, multivariate fractional polynomial models were fitted to data. In secondary analyses of the individual studies, the hazards were further adjusted for several potential confounders, including glucose, loge triglycerides, cholesterol, HDL‐c, albumin, loge C‐reactive protein (CRP). Subgroup analyses were conducted using interaction tests to assess statistical evidence of any differences in hazards across levels of pre‐specified individual level characteristics, including age at survey, smoking, history of diabetes mellitus, history of hypertension, history of cardiovascular disease, left ventricular hypertrophy, history of anti‐hypertensive medication use, body mass index, and systolic blood pressure.
Meta‐Analysis
A systematic review was conducted using a predefined protocol and in accordance with the PRISMA and MOOSE guidelines17–18 (Appendices S1 and S2). Prospective (cohort or nested case‐control) studies of the association between resting heart rate and incident heart failure that were published up to March 2014 were sought using computer‐based databases (MEDLINE, EMBASE, and Science Citation Index). We crossed the term “heart rate” (and similar) with HF, left ventricular dysfunction (and similar terms) without any language restrictions. Reference lists of the retrieved articles were searched for additional articles. Studies were eligible for inclusion if they had at least 1 year of follow‐up and had recruited participants from approximately general populations (ie, did not select participants on the basis of pre‐existing disease at baseline). Hazard ratios and risk ratios were assumed to approximate the same underlying measure of relative risk (RR),19 henceforth referred to as risk ratios (RRs). Reported study‐specific RRs were converted to a consistent comparison as described previously.20 Note the log risk ratio for a 1 SD change is equivalent to the log risk ratio for a comparison of extreme quarters divided by 2.54 assuming a normal distribution or that a transformation of the explanatory variable for which the risk ratio is based was normally distributed. Risk estimates were transformed and pooled to involve comparisons between the top fourth and bottom fourth of the baseline levels of RHR. Study‐specific RRs were combined using a random‐effects meta‐analysis (subsidiary analyses used a fixed effect meta‐analysis). Standard errors of the log risk estimates were calculated using published confidence limits and were standardized in the same way. Consistency of findings among studies was assessed by Cochran's Q statistic (χ2 test) and the I2 statistic.21–22 Several study‐level characteristics were pre‐specified as characteristics for assessment of heterogeneity, which was conducted using stratified analyses and random effects meta‐regression.22 All statistical analyses were conducted using Stata version 13 (Stata Corp, College Station, TX).
Results
Study Population
Table 1 summarizes baseline characteristics of the 4095 participants in the CHS, 1269 participants in Health ABC Study and 1709 participants in the KIHD Study. The mean age of participants was 67 (9.9) years and 3815 (53.9%) were males. Mean RHR was 64.6 (11.1) bpm. During 92 702 person‐years at risk, there were 1189 incident HF events. Of these, 693 (58.3%) has had ejection fraction determined and documented at the time of HF diagnosis. The median ejection fraction was 50%. Overall 294 (42.4%) had preserved ejection fraction (≥45%) and 399 (57.6%) had reduced ejection fraction (<45%).
Table 1.
Baseline Characteristics of the Study Participants
| Overall (N=7073) | Cardiovascular Health Study (N=4095) | Health ABC Study (N=1269) | Kuopio Ischemic Heart Disease (N=1709) | |
|---|---|---|---|---|
| Mean (SD)* or n (%) | Mean (SD)* or n (%) | Mean (SD)* or n (%) | Mean (SD)* or n (%) | |
| Heart rate, bpm | 64.6 (11.1) | 65.1 (11.5) | 66.0 (10.0 | 62.6 (10.6) |
| Age at baseline, y | 67.8 (9.9) | 72.4 (5.4) | 73.4 (2.9) | 52.7 (4.9) |
| Males, % | 3815 (53.9%) | 1591 (38.5%) | 515 (40.7%) | 1709 (100.1%) |
| Current cigarette smoker | 1029 (14.3%) | 492 (12.1%) | 537 (41.8%) | 560 (33%) |
| Body mass index, kg/m2 | 26.9 (4.5) | 26.6 (4.7) | 27.7 (4.9) | 26.9 (3.5) |
| History of hypertension | 3439 (48.4%) | 2278 (56.1%) | 661 (51.7%) | 500 (29.7%) |
| History of diabetes | 836 (12.1%) | 562 (13.2%) | 194 (15.4%) | 80 (4.4%) |
| History of cardiovascular disease | 1491 (20.9%) | 707 (17.6%) | 238 (18.7%) | 546 (31.9%) |
| Left ventricular hypertrophy | 416 (5.5%) | 248 (6.6%) | 149 (12.1%) | 19 (1.1%) |
| Use of anti‐hypertensive agents | 2417 (34.2%) | 1739 (42.5%) | 668 (52.6) | 10 (0.6%) |
| Total cholesterol, mg/dL | 215.8 (40.4) | 213.0 (38.6) | 207.3 (38.9) | 228.9 (42.5) |
| HDL cholesterol, mg/dL | 54.2 (15.4) | 55.4 (16.0) | 55.9 (17.1) | 50.0 (11.5) |
| Triglycerides, mg/dL* | 113.0 (86.0, 156.0) | 119.0 (92.0, 163.0) | 119.0 (91.0, 162.5) | 94.7 (69.0, 131.9) |
| Creatinine, mg/dL | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) |
| Fasting glucose, mg/dL* | 97.0 (90.0, 107.0) | 100.0 (94.0, 110.0) | 94.0 (87.0, 106.0) | 96.0 (22.0) |
| Albumin, g/dL | 4.0 (0.3) | 4.0 (0.3) | 4.0 (0.3) | N/A |
| C‐reactive protein, mg/dL* | 2.2 (1.1, 4.0) | 2.4 (1.2, 4.3) | 1.7 (1.0, 3.2) | N/A |
ABC indicates Aging and Body Composition; HDL, high‐density lipoprotein.
Median (IQR) is presented for skewed variables.
Correlates of Resting Heart Rate
Heart rate was found to be significantly lower (2.6 bpm) in males than in females. After adjusting for age and sex, there remained modestly weak positive correlation between RHR and body mass index (r=0.10), fasting glucose (r=0.18) and loge C‐reactive protein (r=0.20), and weaker association with loge triglycerides (r=0.08) and total cholesterol (r=0.05). Negative correlations were observed with serum albumin (r=−0.05), and creatinine levels (r=−0.05, Table 2). Resting heart rate was 4.8 bpm higher in individuals with diabetes mellitus, and 1 bpm higher in those with hypertension (P<0.001). Individuals receiving beta‐blockers, on average had 5 bpm lower RHR than those not receiving beta‐blockers.
Table 2.
Correlation Between Resting Heart Rate and Baseline Characteristics
| Characteristics | Resting Heart Rate |
|---|---|
| Continuous variable | Partial correlation (95% CI) or change relative to ref category* |
| Age, y | 0.01 (−0.05 to 0.06) |
| Body mass index, kg/m2 | 0.10 (0.03 to 0.15) |
| Systolic blood pressure, mm Hg | 0.11 (−0.03 to 0.24) |
| Diastolic blood pressure, mm Hg | 0.16 (0.05 to 0.27) |
| Triglycerides, mg/dL | 0.08 (0.06 to 0.11) |
| Serum total cholesterol, mg/dL | 0.05 (0.02 to 0.07) |
| Serum HDL‐cholesterol, mg/dL | 0.00 (−0.06 to 0.06) |
| Fasting blood glucose, mg/dL | 0.18 (0.14 to 0.21) |
| Creatinine, mg/dL | −0.05 (−0.07 to −0.02) |
| Albumin, g/dL | −0.05 (−0.07 to −0.02) |
| C‐reactive protein, mg/dL | 0.20 (0.17 to 0.22) |
| Categorical variable | Change relative to ref. category*,* |
| Males | −2.68 (−3.37 to −1.99) |
| Current smoking | 1.16 (−0.98 to 3.30) |
| History of diabetes | 4.75 (3.86 to 5.64) |
| Hypertension | 1.00 (0.45 to 1.55) |
| Left ventricular hypertrophy | 0.82 (−1.05 to 2.69) |
| History of coronary heart disease | −1.44 (−2.34 to −0.54) |
| History of cardiovascular disease | −0.64 (−1.30 to 0.03) |
| Regular use of medication | |
| Antihypertensive drugs | 0.14 (−0.64 to 0.91) |
| β‐blockers | −5.04 (−6.24 to −3.84) |
HDL indicates high‐density lipoprotein.
Age adjusted partial correlation coefficient (95% confidence interval, CI) or change relative to the reference category for categorical variables.
Reference is the category without the characteristic: eg, non‐smokers for smoking.
Resting Heart Rate and Risk for Heart Failure
In analyses adjusted for conventional risk factors (age, sex, smoking, body mass index, diabetes, history of hypertension, left ventricular hypertrophy, and systolic blood pressure), there was a nonlinear association between RHR and incident HF (Figure 1). The age and sex‐adjusted hazard ratio (HR) comparing the top (>72 bpm) versus the bottom (<57 bpm) fourth of RHR was 1.48 (1.26 to 1.74), which attenuated to 1.30 (1.10 to 1.53) with further adjustment for other conventional risk factors (Table 3). Beyond a RHR of 60 bpm, a linear association was observed with an overall hazard ratio of 1.13 (1.07 to 1.18, P<0.001 per 10 bpm). Hazards did not vary significantly by levels of several conventional cardiovascular risk factors (Figure 2), and were similar in analyses that adjusted for incident Coronary Heart disease (CHD) events (Table 3), excluding HF events recorded in the first 2 years of follow‐up, excluding individuals with a history of CVD at baseline, or those individuals who were using beta‐blockers (Table 3). The association of RHR with incident HF was similar in those with preserved (HR: 1.10, 95% CI 0.96 to 1.26, P=0.182) versus reduced (HR: 1.33, 95% CI 1.13 to 1.56, P=0.001) ejection fraction per 10 bpm increase in resting heart rate, P value of heterogeneity=0.121). Among 4095 participants (857 HF cases) in the CHS, the hazard ratio was 1.36 (1.12 to 1.64); in 1269 participants (177 HF cases) in the Health ABC study, the hazard ratio was 1.23 (0.82 to 1.84); and in 1709 participants in the KIHD study (171 HF cases), the hazard ratio was 1.32 (0.83 to 2.08) comparing top versus bottom fourth of resting heart rate levels.
Figure 1.
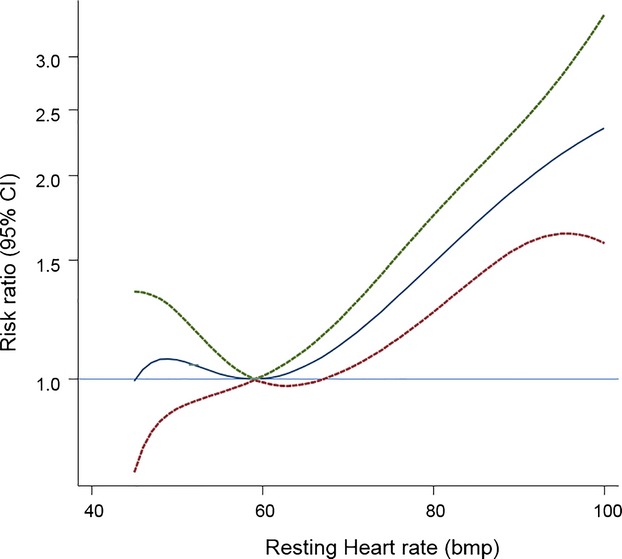
Hazard ratios for heart failure by heart rate using fractional polynomials model. Analyses involved data from 7073 participants (1181 incident heart failure events) with complete information on relevant factors. Hazard ratios were adjusted for age, sex, smoking status, history of diabetes, hypertension, body mass index, serum creatinine, fasting glucose, left ventricular hypertrophy, history of cardiovascular disease, use of anti‐hypertensive drugs and stratified by cohort. The HR (95% CI) using fractional polynomial models were plotted by solid and 95% CI by dashed lines.
Table 3.
Association Between Heart Rate and Heart Failure
| Resting Heart Rate (bpm) | ||||
|---|---|---|---|---|
| Q1 (<57) | Q2 (58 to 63) | Q3 (64 to 71) | Q4 (>72) | |
| 3 studies, 7073 participants 1189 cases | ||||
| Age, sex | 1.00 (Ref) | 0.87 (0.73 to 1.03) | 1.12 (0.96 to 1.32) | 1.48 (1.26 to 1.74) |
| + Body mass index, history of diabetes, hypertension, smoking status, creatinine & left ventricular hypertrophy | 1.00 (Ref) | 0.83 (0.70 to 0.99) | 1.12 (0.96 to 1.32) | 1.30 (1.10 to 1.53) |
| 3 studies, 7049 participants 1186 cases | ||||
| Basic model* | 1.00 (Ref) | 0.83 (0.70 to 0.99) | 1.05 (0.89 to 1.23) | 1.29 (1.10 to 1.52) |
| + History of CVD | 1.00 (Ref) | 0.84 (0.71 to 1.00) | 1.07 (0.91 to 1.26) | 1.34 (1.14 to 1.58) |
| 3 studies, 7040 participants 1185 cases | ||||
| Basic model* | 1.00 (Ref) | 0.84 (0.71 to 1.00) | 1.06 (0.90 to 1.25) | 1.32 (1.12 to 1.56) |
| + Fasting glucose | 1.00 (Ref) | 0.84 (0.71 to 1.00) | 1.06 (0.90 to 1.24) | 1.28 (1.09 to 1.50) |
| 3 studies, 7066 participants 1188 cases | ||||
| Basic model* | 1.00 (Ref) | 0.83 (0.70 to 0.99) | 1.05 (0.89 to 1.23) | 1.30 (1.10 to 1.53) |
| + Anti‐hypertensive use | 1.00 (Ref) | 0.83 (0.70 to 0.98) | 1.06 (0.90 to 1.24) | 1.31 (1.11, 1.54) |
| 3 studies, 7055 participants, 1185 cases | ||||
| Basic model* | 1.00 (Ref) | 0.83 (0.70 to 0.99) | 1.05 (0.89 to 1.23) | 1.30 (1.10 to 1.53) |
| + log triglycerides, total cholesterol, & HDL‐c | 1.00 (Ref) | 0.82 (0.69 to 0.97) | 1.04 (0.89 to 1.22) | 1.28 (1.09 to 1.51) |
| 2 studies, 5364 participants 1038 cases | ||||
| Basic model* | 1.00 (Ref) | 0.82 (0.68 to 0.98) | 1.06 (0.89 to 1.26) | 1.26 (1.06 to 1.51) |
| + Albumin | 1.00 (Ref) | 0.82 (0.68 to 0.98) | 1.08 (0.91 to 1.29) | 1.30 (1.09 to 1.55) |
| 2 studies, 5345 participants 1036 cases | ||||
| Basic model* | 1.00 (Ref) | 0.81 (0.68 to 0.98) | 1.05 (0.89 to 1.25) | 1.26 (1.06 to 1.50) |
| + log CRP | 1.00 (Ref) | 0.80 (0.67 to 0.97) | 1.01 (0.85 to 1.20) | 1.15 (0.96 to 1.37) |
| 3 studies, 7073 participants 1189 cases | ||||
| Basic model | 1.00 (Ref) | 0.83 (0.70 to 0.99) | 1.12 (0.96 to 1.32) | 1.30 (1.10 to 1.53) |
| + Incident CHD as a time varying covariate | 1.00 (Ref) | 0.82 (0.68 to 0.99) | 1.13 (0.95 to 1.35) | 1.38 (1.16 to 1.65) |
| 3 studies, 5528 participants 826 cases | ||||
| Excluding people with hx of CVD* | 1.00 (Ref) | 0.78 (0.63 to 0.97) | 1.12 (0.93 to 1.36) | 1.33 (1.09 to 1.62) |
| 3 studies, 6188 participants 992 cases | ||||
| Excluding people using beta blockers* | 1.00 (Ref) | 0.79 (0.65 to 0.96) | 1.04 (0.87 to 1.24) | 1.31 (1.10 to 1.57) |
| 3 studies, 6838 participants 1085 cases | ||||
| Excluding first 2 years of follow‐up* | 1.00 (Ref) | 0.84 (0.70 to 1.00) | 1.03 (0.87 to 1.22) | 1.27 (1.07 to 1.50) |
CVD indicates cardiovascular disease; HDL‐c, high‐density lipoprotein cholesterol.
Basic model incudes: age, sex, body mass index, history of diabetes, history of hypertension, smoking status, serum creatinine, and left ventricular hypertrophy.
Figure 2.
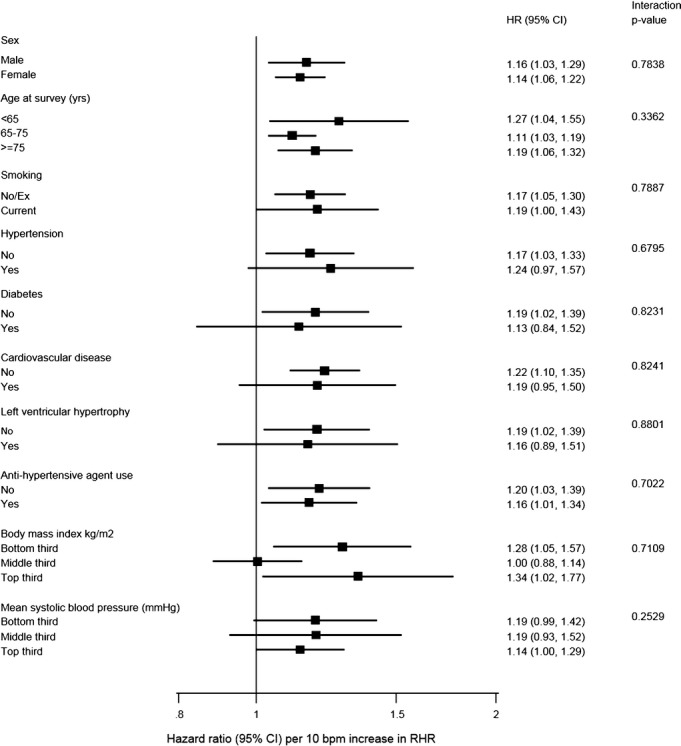
Hazard ratio for heart failure by heart rate by participant‐level characteristics. Participants below resting heart rate (RHR) of 60 bpm were excluded. HR are calculated for a per‐10 bpm increase in RHR. P values for interaction were calculated from analyses using continuous variables where appropriate. Analyses were conducted using studies with information across all levels of each subgroup variable.
Meta‐Analysis
Including the current 3 studies, we identified 7 population‐based prospective studies (Figure 3), reporting on the association between RHR and HF risk (Table 4). In a pooled analysis of 43 051 participants and 3476 HF events the combined RR for HF comparing the top versus the bottom fourth baseline RHR, typically adjusted for several conventional risk factors, was 1.40 (1.19 to 1.64) (Figure 4). There was moderate evidence of heterogeneity among the contributing studies (I2=54%, 95% CI: 0 to 80; P=0.04), which was not explained by any study‐level characteristics assessed. A significant proportion of the heterogeneity came from the MESA study, which observed the smallest number of events. There was no evidence of heterogeneity in pooled analysis excluding this study I2=0%, 95% CI: 0 to 75; P=0.78). Among the remaining studies, the pooled RR for HF was 1.31 (95% CI: 1.19 to 1.44), which was similar to the overall combined RR.
Figure 3.
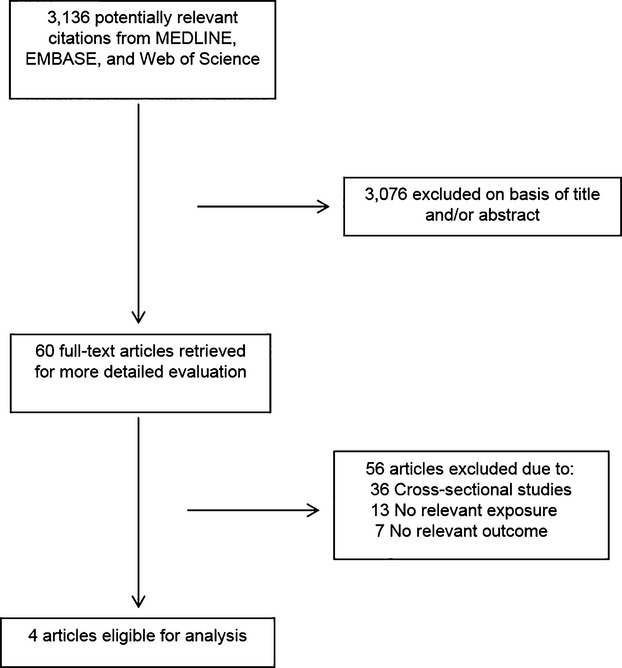
Literature search strategy used for the meta‐analysis.
Table 4.
Prospective Studies Resting Heart Rate and Incident Heart Failure Contributing to Pooled Analyses
| Study | Location | Population Source | Year of Baseline Survey | Age Range at Baseline (y) | Male (%) | Mean Follow‐Up (y) | No. of Cases | No. of Non‐Cases |
|---|---|---|---|---|---|---|---|---|
| CHS | USA | Population register | 1989–1990 | 65 to 100 | 38.9 | 11.2 | 857 | 3238 |
| Health ABC | USA | Population register | 1997–1998 | 68 to 80 | 40.7 | 9.6 | 177 | 1092 |
| KIHD | Finland | Population register | 1984–1989 | 42 to 61 | 100 | 20.4 | 151 | 1558 |
| MESA | USA | Population register | 2000–2002 | 45 to 84 | 47.0 | 9.0 | 112 | 4888 |
| PROSPER | Netherlands, Ireland, Scotland | Clinical trial | 1997–1999 | 70 to 82 | 49.0 | 3.2 | 167 | 3917 |
| EPIC NORFOLK | UK | Population register | 1993–1997 | 39 to 79 | 44.3 | 12.9 | 1356 | 20 770 |
| ROTTERDAM | Netherlands | Population register | 1990–1993 | ≥55 | 38 | 14.6 | 656 | 4112 |
| Total | 3476 | 39 575 |
ABC indicates Aging and Body Composition; CHS, Cardiovascular Health Study; KIHD, Kuopio Ischemic Heart Disease; MESA, Multi‐Ethnic Study of Atherosclerosis.
Figure 4.
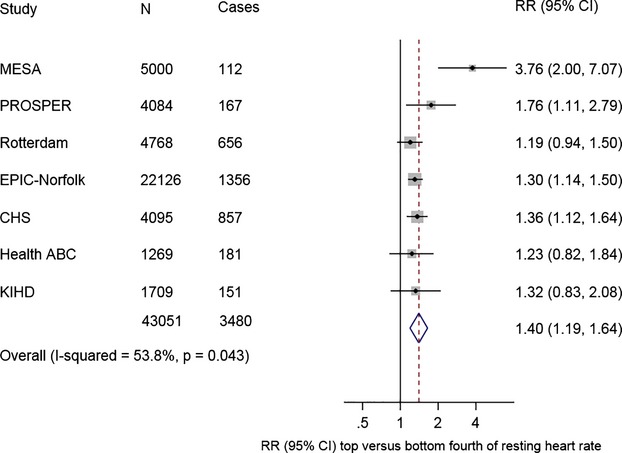
Relative risks for heart failure comparing top vs bottom fourth of heart rate. CHS indicates Cardiovascular Health Study; Health ABC, Heath Aging and Body Composition; KIHD, Kuopio Ischemic Heart Disease; MESA, Multi‐Ethnic Study of Atherosclerosis; RR, relative risk.
Discussion
In this population of middle‐aged to older individuals without HF at baseline, there were weak to modest associations of RHR with several conventional HF risk factors, and resting RHR was non‐linearly associated with risk of incident HF independent of other known risk factors. These findings remained consistent across several subgroups, including the presence or absence of pre‐existing cardiovascular disease or use of beta‐blockers, and were similar in analyses that adjusted for CHD events during follow‐up, excluded HF events recorded in the first 2 years of follow‐up, people with a history of CVD at baseline, or individuals who were using beta‐blockers. Pooled findings from the systematic meta‐analysis of 7 studies reinforces the validity and generalizability of these data suggesting that individuals in the top quartile of RHR had about a 30% higher risk of HF compared with individuals in the lower quartile of baseline RHR levels.
Elevated RHR has been previously recognized as a risk factor for HF in high‐risk individuals such as those with prevalent CHD and hypertension.3,5,23 Limited prospective evidence has been available for healthy middle‐aged adults. Whereas, a few prospective studies have shown a positive association of RHR with incident HF, these have failed to delineate in detail the nature and shape of this association. In contrast to prior evidence, we observed a non‐linear J‐shaped association of RHR with HF risk. An increased risk of HF was observed at both low and high levels of RHR, with risk rising progressively beyond a heart rate of 60 bpm. In line with the epidemiological evidence, findings from clinical trials of an RHR‐lowering agent have shown the benefit of lowering RHR.5,23 In SHIFT (Systolic Heart failure treatment Trial)5 the role of a pure heart rate‐lowering agent, ivabradine was investigated in HF patients with RHR >70 bpm despite beta‐blockade. It was observed that additional heart rate reduction was beneficial in reducing HF re‐hospitalizations and improves overall survival, providing further evidence that elevated RHR may be causally linked to HF risk and outcomes. However, evidence showing benefit of further lowering RHR to levels as low as 60 bpm while also taking into account the risks associated with lower heart rates needs to be further ascertained.
Several mechanistic pathways have been implicated in the causal link between elevated RHR and the development of HF. Among these, systemic inflammation and endothelial dysfunction have been commonly suggested.9 However, findings from the study by Nanchen and colleagues8 observed that these factors do not fully explain the risk of HF associated with elevated RHR. Higher resting heart rate may reflect decreased levels physical fitness and/or higher sympathetic tone relative to parasympathetic tone, which in turn are associated with HF.24 Although, it has been suggested that this association might be mediated by CHD, adjustment for either preceding CHD and incident CHD in our study did not attenuate the association between RHR and incident HF, which is similar to findings from previous studies.7–8 A chronic increase in RHR may lead to an increase in myocardial oxygen requirements and may contributes to ventricular remodeling and subsequently to regional and global left ventricular dysfunction,6 though this currently remains speculative.7 Further work is needed to help identify key underlying biological mechanisms through which RHR may play a role in the development of HF. Irrespective of whether RHR has a causal role in the etiology of HF, its potential utility for HF risk assessment warrants consideration, particularly as its measurement involves a simple, non‐invasive, and routine procedure in clinical practice.
These analyses have several strengths and limitations that merit consideration. Participants in the Health ABC, CHS, and KIHD studies were identified from general populations, are well characterized, involved high response and follow‐up rates, and have been prospectively monitored using established databases for hospital admissions and causes of death. Individuals with manifest HF or major rhythm disorders (ventricular conduction defect, major Q‐wave abnormality, isolated ST‐T wave abnormalities, atrial fibrillation, or atrio‐ventricular blocks) at baseline were excluded from the analyses, reducing the effects of any pre‐existing disease on RHR. As findings from these studies were reinforced by a meta‐analysis of 4 other long‐term population‐based prospective studies in Western countries, it increases the likelihood that these results can be generalized, to Western populations in general. Further studies are, however, needed in ethnic sub populations of Western countries and populations in non‐Western countries. Repeat measurements of RHR were not available, preventing the quantification of the extent of within‐person variability in RHR measurements. Also, individual patient‐level data was only available for 3 of the 7 cohorts, further limiting the participant‐level analysis that could be shown across all cohorts.
In conclusion, in Western populations there is a non‐linear association between resting heart rate and risk of incident HF, which is independent of conventional HF risk factors. Further assessment of the possible role of RHR reduction in HF prevention is warranted.
Author Contributions
Dr(s) Butler and Khan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Khan, Kalogeropoulos, Laukkanen, Butler. Acquisition of data: Butler, Harris, Kritchevsky, Khan, Kunutsor, Laukkanen. Analysis and interpretation of data: Kalogeropoulos, Kunutsor, Khan, Laukkanen, Butler. Drafting of the manuscript: Butler, Khan, Kunutsor. Critical revision of the manuscript for important intellectual content: Bibbins‐Domingo, Butler, Fonarow, Gheorghiade, Georgiopoulou, Harris, Kalogeropoulos, Kauhanen, Khan, Kunutsor, Laukkanen, Newman. Statistical analysis: Khan. Obtained funding: Butler, Kritchevsky, Laukkanen. Study supervision: Butler, Laukkanen.
Supplementary Material
Appendix 1. PRISMA 2009 check-list.
Appendix 2. Baseline and study-end levels for changes in LDL-C and HDL-C stratified by baseline HDL-P tertiles.
Sources of Funding
Cardiovascular Health Study: This research was supported by contract numbers N01‐HC‐85079 through N01‐HC‐85086, N01‐HC‐35129, N01 HC‐15103, N01 HC‐55222, N01‐HC‐75150, N01‐HC‐45133, grant U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of the Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Health ABC study: This research was supported by National Institute on Aging (NIA) Contracts N01‐AG‐6‐2101; N01‐AG‐6‐2103; N01‐AG‐6‐2106; NIA grant R01‐AG028050, NINR grant R01‐NR012459 and supported in part by the Intramural Research Program of the NIH, National Institute on Aging. Kuopio Ischemic Heart Disease study (KIHD): This research was supported by Academy of Finland, Helsinki, Finland; City of Kuopio, Kuopio, Finland; Finnish Medical Foundation, Helsinki, Finland, and Finnish Cultural Foundation, Helsinki, Finland https://www.uef.fi/en/nutritionepidemiologists/kihd. The contents of this paper are solely the responsibility of the authors and do not represent the views of the Sponsors. The sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Disclosures
None.
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd‐Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011; 123:933-944. [DOI] [PubMed] [Google Scholar]
- 2.Bleumink GS, Knetsch AM, Sturkenboom MCJM, Straus SMJM, Hofman A, Deckers JW, Witteman JCM, Stricker BHC. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004; 25:1614-1619. [DOI] [PubMed] [Google Scholar]
- 3.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PWF, Kritchevsky SB. Incident heart failure prediction in the elderly: the Health ABC Heart Failure Score. Circ Heart Fail. 2008; 1:125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari R. The story of the heartbeat, I: part I—heart rate: the rhythm of life. Eur Heart J. 2012; 33:4-5. [PubMed] [Google Scholar]
- 5.Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi LSHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet. 2010; 376:886-894. [DOI] [PubMed] [Google Scholar]
- 6.Opdahl A, Ambale Venkatesh B, Fernandes VRS, Wu CO, Nasir K, Choi E‐Y, Almeida ALC, Rosen B, Carvalho B, Edvardsen T, Bluemke DA, Lima JAC. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2014; 63:1182-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw K‐T. Resting heart rate and incident heart failure in apparently healthy men and women in the EPIC‐Norfolk study. Eur J Heart Fail. 2012; 14:1163-1170. [DOI] [PubMed] [Google Scholar]
- 8.Nanchen D, Leening MJG, Locatelli I, Cornuz J, Kors JA, Heeringa J, Deckers JW, Hofman A, Franco OH, Stricker BHC, Witteman JCM, Dehghan A. Resting heart rate and the risk of heart failure in healthy adults: the Rotterdam Study. Circ Heart Fail. 2013; 6:403-410. [DOI] [PubMed] [Google Scholar]
- 9.Diaz A, Bourassa MG, Guertin M‐C, Tardif J‐C. Long‐term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005; 26:967-974. [DOI] [PubMed] [Google Scholar]
- 10.Rautaharju PM, Prineas RJ, Wood J, Zhang Z‐M, Crow R, Heiss G. Electrocardiographic predictors of new‐onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study). Am J Cardiol. 2007; 100:1437-1441. [DOI] [PubMed] [Google Scholar]
- 11.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins‐Domingo K, Smith AL, Wilson PWF, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the Health, Aging, and Body Composition study. Arch Intern Med. 2009; 169:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005; 165:2460-2466. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991; 1:263-276. [DOI] [PubMed] [Google Scholar]
- 14.Cesari M, Penninx BWJH, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton‐Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study). Am J Cardiol. 2003; 92:522-528. [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long‐distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006; 295:2018-2026. [DOI] [PubMed] [Google Scholar]
- 16.Thompson S, Kaptoge S, White I, Wood A, Perry P, Danesh J. Statistical methods for the time‐to‐event analysis of individual participant data from multiple epidemiological studies. Int J Epidemiol. 2010; 39:1345-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008-2012. [DOI] [PubMed] [Google Scholar]
- 19.Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002; 55:893-899. [DOI] [PubMed] [Google Scholar]
- 20.Chêne G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996; 144:610-621. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003; 327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Stat Med. 1999; 18:2693-2708. [DOI] [PubMed] [Google Scholar]
- 23.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari RBEAUTIFUL investigators. Heart rate as a prognostic risk factor in patients with coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008; 372:817-821. [DOI] [PubMed] [Google Scholar]
- 24.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif J‐C, Tavazzi L, Tendera MHeart Rate Working Group. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007; 50:823-830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. PRISMA 2009 check-list.
Appendix 2. Baseline and study-end levels for changes in LDL-C and HDL-C stratified by baseline HDL-P tertiles.


