Abstract
Background
We aimed to investigate the incidence and risk factors for ventricular fibrillation (VF) before primary percutaneous coronary intervention (PPCI) among patients with ST‐segment elevation myocardial infarction (STEMI) in a prospective nationwide setting.
Methods and Results
In this case‐control study, patients presenting within the first 12 hours of first STEMI who survived to undergo angiography and subsequent PPCI were enrolled. Over 2 years, 219 cases presenting with VF before PPCI and 441 controls without preceding VF were enrolled. Of the 219 case patients, 182 (83%) had STEMI with out‐of‐hospital cardiac arrest due to VF, and 37 (17%) had cardiac arrest upon arrival to the emergency room. Medical history was collected by standardized interviews and by linkage to national electronic health records. The incidence of VF before PPCI among STEMI patients was 11.6%. Multivariable logistic regression analysis identified novel associations between atrial fibrillation and alcohol consumption with VF. Patients with a history of atrial fibrillation had a 2.80‐fold odds of experiencing VF before PPCI (95% CI 1.10 to 7.30). Compared with nondrinkers, patients who consumed 1 to 7 units, 8 to 14 units, or >15 units of alcohol per week had an odds ratio (OR) of 1.30 (95% CI, 0.80 to 2.20), 2.30 (95% CI, 1.20 to 4.20), or 3.30 (95% CI, 1.80 to 5.90), respectively, for VF. Previously reported associations for preinfarction angina (OR 0.46; 95% CI 0.32 to 0.67), age of <60 years (OR 1.75; 95% CI 1.20 to 2.60), anterior infarction (OR 2.10; 95% CI 1.40 to 3.00), preprocedural thrombolysis in myocardial infarction flow grade 0 (OR 1.65; 95% CI 1.14 to 2.40), and family history of sudden death (OR 1.60; 95% CI 1.10 to 2.40) were all associated with VF.
Conclusion
Several easily assessed risk factors were associated with VF occurring out‐of‐hospital or on arrival at the emergency room before PPCI in STEMI patients, thus providing potential avenues for investigation regarding improved identification and prevention of life‐threatening ventricular arrhythmias.
Keywords: myocardial infarction, sudden death, ventricular fibrillation
Introduction
Coronary artery disease (CAD) and its ultimate consequence, myocardial infarction (MI), is believed to underlie 75% of deaths in patients with sudden cardiac death (SCD), especially among men, and is the most common cause of death in Western countries.1–4 It has been estimated that in more than half of SCD cases, CAD is not recognized clinically and SCD occurs as its first symptom.2,5 In a sizable proportion of these patients, ventricular fibrillation (VF) is the primary rhythm resulting in SCD.3 To date, clinically utilized risk stratifiers for SCD, such as left ventricular ejection fraction, are not specific for arrhythmic death and similarly predict other modes of death from MI or CAD.5 Consequently, better understanding of the factors that predispose patients with CAD and/or MI to present with arrhythmic death or VF as the first manifestation are needed.
Toward this end, retrospective analyses of several clinical trials and observational studies have sought to identify risk factors that predict VF in the setting of acute MI6–10; however, the majority of VF events in these studies occurred during or after revascularization and may represent a different pathophysiologic process, as suggested by animal studies.11–12 Results from these studies may not be generalizable to the majority of SCDs or VF arrests due to MI that occurs outside the hospital.
Despite advances in delivery of primary percutaneous coronary intervention (PPCI) for ST‐segment elevation MI (STEMI), an unknown proportion of patients with STEMI experience VF prior to PPCI and thus may die before revascularization. Three prospective case–control studies of patients with STEMI and VF before PPCI identified associations between family history of sudden death, cumulative ST elevation, left coronary artery culprit lesions, and absence of preinfarction angina with VF.13–15 By design, these studies included only VF survivors; therefore, the patients enrolled in these studies may be less reflective of the general population of STEMI patients with out‐of‐hospital cardiac arrest due to VF. Moreover, in these studies, cases with STEMI were matched to controls by age and sex; therefore, the contribution of these factors to VF risk could not be investigated,13–15 and no study has examined whether alcohol intake might contribute to VF risk in the setting of MI despite data suggesting important proarrhythmic properties.16–18
To address these gaps in our knowledge, the present study aimed to investigate the incidence and risk factors for VF before PPCI among survivors and nonsurvivors of STEMI in a prospective nationwide setting.
Methods
Study Population
This study was designed as a nationwide prospective case–control study among patients with first STEMI between the ages of 18 and 80 years recruited at all 4 percutaneous coronary intervention (PCI) centers in Denmark. Patients with out‐of‐hospital cardiac arrest were included on admission to PCI sites after resuscitation by trained emergency medical service personnel. To avoid heterogeneity of underlying causes of VF, we limited our phenotype to include only patients with first STEMI, which also provided a discrete population with similar pathology of underlying CAD for the selection of both cases and controls. To qualify for the study, all patients had to have cardiac symptoms lasting ≤12 hours and acute ST‐segment elevation on ECG. All cases and controls enrolled in the study underwent coronary angiography and had either an acute coronary occlusion documented at angiography or typical elevations of biomarkers. The majority underwent subsequent PPCI, and a few underwent coronary artery bypass grafting. In patients with diffuse or distal disease for which PCI was not technically feasible, typical elevations of biomarkers were required to ensure that the cause of ST elevation and arrest was acute MI. Acute STEMI on ECG was defined as (1) persistent ST‐segment elevation in 2 adjacent ECG leads of >0.1 mV in V4 through V6 or limb leads II, III, and aVF or (2) >0.2 mV in lead V1 through V3 or (3) new left bundle branch block. The culprit lesion was classified according to the judgment of the PCI operator and, in the case of >1 lesion, the 1 that corresponded with the location of ST elevation on the ECG was chosen.
The case group was composed of patients who experienced VF within the first 12 hours of symptoms of STEMI before PPCI, and the control group did not have VF during this time period. VF before PPCI was defined as VF occurrence before guiding catheter insertion for PCI procedure. Cases and controls who developed VF during PPCI were both excluded because VF during PPCI is believed to be reperfusion induced19–22; however, case patients with VF before PPCI who continued to have VF during PPCI were enrolled in the study. Other exclusions included VF after PPCI, congenital heart defects, known structural heart disease, use of class I and III antiarrhythmic drugs, recent cancer, major surgery or trauma within 4 weeks, and presentation with potassium concentration of <3 or >5 mmol/L. Because we also planned to identify genetic markers associated with VF, and because >90% of the Danish populations are white Danes, we excluded nonwhite Danes to reduce the risk of population stratification for future genetic analysis.
To avoid unintended loss of patients, particularly in the case group, the study had permission from the National Committee on Health Research Ethics to include patients (both cases and controls) who were unconscious and who did not have a family member available to provide informed consent (protocol number: H‐3‐2010‐133). Informed consent for unconscious or dead patients was subsequently obtained from the next of kin on arrival at the PCI center and subsequently from patients who regained consciousness when deemed competent to make decisions. Signed informed consent is available for all patients enrolled in this study. Procedures are in accordance with the ethical standards of the national ethics committee on human research and with the Helsinki Declaration of 1975, as revised in 1983. Permission from the Danish Data Protection Agency was also obtained before the study was initiated (Jr.nr. 2010‐41‐5688).
Data Collection
All individuals with permanent residence in Denmark receive a unique Civil Registration Number that enables linkage among nationwide registers at an individual level for various sources and that is used for all healthcare‐related services from birth.
The Civil Registration Number was used to ascertain hospitals records and discharge summaries from the prehospital trauma and emergency doctors who, in most cases, escort ambulances to the catheterization laboratory and document the exact time of VF. Furthermore, the Civil Registration Number was used to ascertain previous medical history (eg, previous MI) for every patient from the national electronic health records, which are linked to the national patient registry. The national patient registry contains information on all in‐ and outpatient activity in Danish hospitals and emergency departments since 1978. Diagnoses are coded according to the International Classification of Diseases (ICD), with ICD‐8 codes used from 1977 to 1993 and ICD‐10 codes used since 1994.23
Baseline Demographics, Medical History, and Lifestyle Information
Previous medical history was also collected by research coordinators at the study sites using a predesigned standardized questionnaire including all baseline characteristics such as family history of sudden death that was similarly administered to cases and controls. All questions in this questionnaire (Tables 1 and 2) were considered predefined research questions. The interview was carried out in a similar fashion for cases and controls by trained study personnel. If the patient was unable to provide us with information, the next of kin was contacted. The questionnaire collected information on baseline demographics (Table 1), education, prior medical history, smoking status, alcohol intake, and previous medications. For alcohol intake, we asked the patients, “How many units of alcohol do you drink each week?” One unit of alcohol was defined as a beer, a single measure of spirits, or a glass of wine (≈12 g=1 unit). We also collected information on family history of sudden death, MI, and stroke (as reported by the patients, their families, or as determined from their previous medical records). Family history of sudden death in first‐degree relatives (biological parents, brothers, sisters) was defined as sudden, natural, unexpected death from an unknown or cardiac cause in parents or siblings aged <80 years. In witnessed cases, the family member died within 1 hour of an acute change in cardiovascular status. For unwitnessed cases, the person was last seen alive and functioning normally within 24 hours of being found dead.24 Additional detailed information regarding cardiac symptoms (angina, dyspnea, palpitations, presyncope, and syncope) experienced within the last 12 months and contact with the healthcare system on the basis of these symptoms was also collected (Table 2). Presenting clinical characteristics at the time of STEMI were collected from medical records (Table 3).
Table 1.
Baseline Characteristics
| Variables | Cases (n=219) | Controls (n=441) | P Value |
|---|---|---|---|
| Female sex, n (%) | 30 (14) | 102 (23) | 0.004 |
| Median age at index infarction, years (IQR) | 59 (53 to 68) | 61 (52 to 66) | 0.020 |
| Cardiovascular risk profile | |||
| Body mass index, kg/m2 (IQR) | 27.5 (25 to 29) | 26.7 (24 to 29) | 0.300 |
| Smoking, pack‐year (IQR) | 25 (11 to 41) | 23 (5 to 40) | 0.200 |
| Smoking, n (%) | |||
| Never | 35 (16) | 91 (21) | 0.300 |
| Past | 66 (30) | 116 (26) | |
| Current | 116 (54) | 233 (53) | |
| Alcohol per week, unit* (IQR) | 7 (1 to 15) | 3 (1 to 9) | <0.001 |
| Alcohol units per week (categorized), n (%) | |||
| Nondrinkers | 36 (17) | 117 (27) | <0.001 |
| Normal (1 to 7) | 81 (38) | 202 (46) | |
| Moderate high (8 to 14) | 37 (18) | 62 (14) | |
| High (>15) | 58 (27) | 58 (13) | |
| Diabetes, n (%) | 23 (10) | 38 (8) | 0.400 |
| Hypertension, n (%) | 93 (42) | 153 (35) | 0.052 |
| COPD, n (%) | 10 (5) | 25 (6) | 0.600 |
| Hypercholesterolemia, n (%) | 88 (40) | 143 (32) | 0.049 |
| Stroke, n (%) | 16 (7) | 23 (5) | 0.300 |
| Atrial fibrillation, n (%) | 14 (7) | 10 (3) | 0.020 |
| Depression, n (%) | 23 (11) | 53 (12) | 0.600 |
| Epilepsy, n (%) | 4 (2) | 5 (1) | 0.500 |
| Family history, n (%) | |||
| Sudden death | 81 (38%) | 112 (26%) | 0.001 |
| MI | 82 (41%) | 162 (38%) | 0.500 |
| Stroke | 31 (16%) | 57 (14%) | 0.500 |
| Medication before MI, n (%) | |||
| β‐blockers | 17 (8) | 39 (9) | 0.700 |
| Statins | 49 (23) | 55 (12) | 0.001 |
| ACE/ARB blockers | 40 (19) | 75 (17) | 0.500 |
| Aspirin | 21 (10) | 35 (8) | 0.400 |
| Location of cardiac arrest, n (%) | |||
| Out‐of‐hospital cardiac arrest | 182 (83) | — | |
| On arrival at the ER before PPCI | 37 (17) | — | |
| Coma at arrival to PCI center | 82 (37%) | — | |
| ST elevation on ECG after cardiac arrest | 139 | — | |
| In‐hospital death | 38 (17) | 3 (1) | <0.001 |
| Education after primary school n (%) | Cases (n=205) | Controls (n=438) | |
| None | 28 (14) | 80 (18) | 0.400 |
| High school | 15 (7) | 30 (7) | |
| Trade school | 95 (46) | 192 (44) | |
| University (≤3 years) | 39 (19) | 93 (22) | |
| University (>3 years) | 28 (14) | 43 (9) |
ST elevation on ECG after cardiac arrest is defined as patients with out‐of‐hospital cardiac arrest due to ventricular fibrillation who developed ST‐segment elevation MI on ECG after the arrest. ACE indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; ER, emergency room; MI, myocardial infarction; PCI, percutaneous coronary intervention; PPCI, primary percutaneous coronary intervention.
1 unit of alcohol=12 g (1 drink).
Table 2.
Symptoms Prior to STEMI and Ventricular Fibrillation
| Cardiac Symptoms Within 1 Year Prior to STEMI, n (%) | Cases (n=219) | Controls (n=441) | P Value |
|---|---|---|---|
| Angina | 105 (49) | 284 (64) | <0.001 |
| Dyspnea | 52 (25) | 123 (28) | 0.400 |
| Palpitations | 15 (7) | 41 (9) | 0.400 |
| Syncope* | 3 (2) | 4 (1) | 0.400 |
| Presyncope* | 2 (1) | 14 (4) | 0.200 |
| Healthcare contact within 1 year prior to STEMI due to cardiac symptoms | 37 (18) | 61 (14) | 0.200 |
STEMI indicates ST‐segment elevation myocardial infarction.
Missing value for syncope or presyncope (15%).
Table 3.
Presenting Characteristics
| Variables | Cases (n=219) | Controls (n=441) | P Value |
|---|---|---|---|
| Time from symptom to PPCI, min (IQR) | 145 (107 to 207) | 187 (127 to 296) | <0.001 |
| LVEF after PCI, mean±SD (%)* | 41.65±11.9% | 46.2±10.45% | <0.001 |
| CK‐MB max, μg/L (IQR) | 232 (76 to 393) | 138 (51 to 277) | <0.001 |
| Time to peak, min (IQR) | 783 (552 to 967) | 660 (497 to 880) | <0.001 |
| Acute CABG, n (%) | 18 (9) | 13 (3) | 0.002 |
| Culprit lesion, n (%) | |||
| LAD | 118 (54) | 202 (46) | 0.001 |
| RCA | 71 (33) | 185 (42) | |
| CX | 23 (11) | 54 (12) | |
| >1 | 5 (2) | 0 (0) | |
| Infarct location, n (%) | |||
| Anterior | 123 (57) | 202 (46) | 0.009 |
| Nonanterior | 94 (43) | 239 (54) | |
| Preprocedural TIMI flows, n (%) | |||
| TIMI 0 | 126 (58) | 221 (50) | 0.080 |
| TIMI I | 17 (8) | 45 (10) | |
| TIMI II | 19 (9) | 65 (15) | |
| TIMI III | 56 (25) | 108 (25) | |
| Postprocedural TIMI flows, n (%) | |||
| TIMI 0 | 8 (4) | 8 (2) | 0.140 |
| TIMI I | 0 (0) | 0 (0) | |
| TIMI II | 9 (4) | 10 (2) | |
| TIMI III | 201 (92) | 421 (96) | |
| ECG* | Cases (n=209) | Controls (n=401) | |
| PQ interval, milliseconds (IQR) | 164 (146 to 192) | 164 (148 to 182) | 0.300 |
| QRS interval, milliseconds (IQR) | 96 (88 to 108) | 97 (88 to 106) | 0.700 |
| QTc interval, milliseconds (IQR) | 415 (404 to 440) | 416 (406 to 432) | 0.700 |
| LBBB, n (%) | 21 (10) | 9 (2) | <0.001 |
| RBBB, n (%) | 30 (14) | 17 (4) | <0.001 |
| ST deviation, mm (IQR) | 4 (2 to 6) | 2 (1 to 4) | <0.001 |
| Killip class, n (%)* | Cases (n=171) | Controls (n=355) | |
| I | 145 (85) | 343 (97) | <0.001 |
| II | 9 (5) | 12 (3) | |
| III | 7 (4) | 0 (0) | |
| IV | 10 (6) | 0 (0) |
CABG indicates coronary artery bypass grafting; CK‐MB, creatine kinase‐MB; CX, circumflex artery; LAD, left anterior descending; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PPCI, primary percutaneous coronary intervention; RBBB, right bundle branch block; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction.
LVEF cases, n=184; LVEF controls, n=429.
Missing value for Killip class (20%), for ECG (8%) and for LVEF (7%).
ECG Measurements
ECGs were taken at the moment of hospital admission for the index STEMI. ST deviation, PQ, QRS, and QTc were calculated. QTc was calculated using Bazett's formula, and ECGs with left and right bundle branch blocks were excluded (n=77) from the QTc measurements. For ST‐segment deviation analysis, ECGs with left bundle branch block were excluded (n=30).
STEMI Registry
To evaluate the representativeness of our case–control population for all STEMI patients presenting at the 4 PCI centers in Denmark, we performed the following screening procedures.
To evaluate the representativeness of our case population and to estimate the incidence of VF before PPCI, we screened all STEMI patients admitted to 1 of the 4 centers, Rigshospitalet, where information on VF has been collected systematically on consecutive patients admitted for STEMI in a local hospital registry (WEB‐PATS registry) since October 1999 (Figure 1). Rigshospitalet is the PPCI center for all of eastern Denmark, with a catchment area of 2.5 million inhabitants, corresponding to 45% of the entire Danish population.
Figure 1.
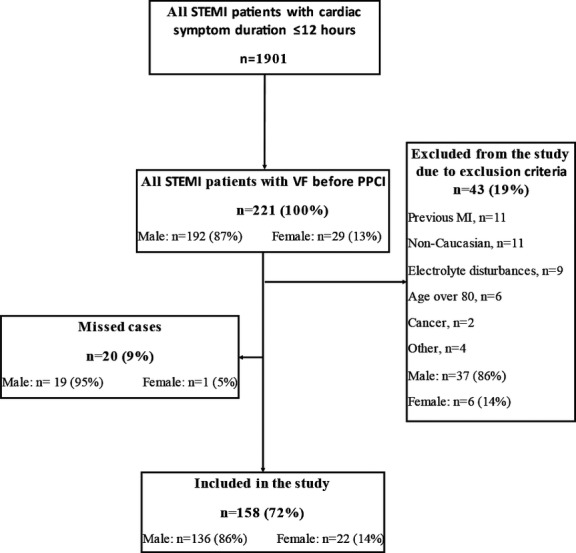
Flow diagram demonstrating screening of STEMI patients for VF at 1 center (June 1, 2011 to May 31, 2013): All STEMI patients presenting to the largest center (Rigshospitalet) were screened to estimate the incidence of VF prior to PPCI in STEMI and to evaluate the representativeness of our case population to all STEMI patients presenting to percutaneous coronary intervention centers. PPCI indicates primary percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction; VF, ventricular fibrillation.
To evaluate the representativeness of the control population, we also screened all STEMI patients admitted to 2 of the 4 PPCI centers (Rigshospitalet and Aalborg University Hospital) during the 8‐month inclusion period of the control participants. These 2 centers serve all of eastern and northern Denmark, with a catchment area of 3.1 million persons, corresponding to 56% of the entire Danish population (Figure 2).
Figure 2.
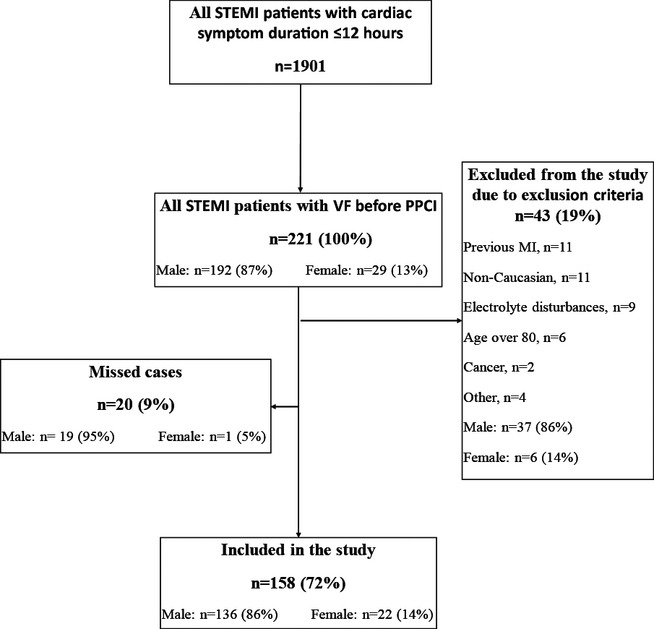
Representativeness of the control population to all STEMI patients at 2 centers: Flow diagram demonstrating screening and inclusion of STEMI at 2 of the 4 centers during the 8‐month enrollment of the controls. STEMI indicates ST‐segment elevation myocardial infarction.
Statistical Methods
Medians or proportions of baseline and presenting characteristics were computed for cases and controls, and significance of associations was tested using the Wilcoxon rank‐sum test for continuous variables and the χ2 test or Fisher exact test (as appropriate) for categorical variables. A 2‐tailed P value ≤0.05 was considered statistically significant. A logistic regression model was constructed to identify clinical risk factors that preceded STEMI and were associated with subsequent VF (Tables 1 and 2). Because the majority of presenting characteristics (Table 3) such as creatine kinase‐MB level, left ventricular ejection fraction, 12‐lead ECG, time from onset of symptoms to PPCI, postprocedural thrombolysis in myocardial infarction flow, and Killip class were recorded after STEMI with VF and could have been influenced by the VF arrest, we chose not to include these variables in the logistic regression model. We assumed that infarct location and preprocedural thrombolysis in myocardial infarction flow would not be influenced by VF and/or prolonged hypoxia; therefore, these variables were considered in the model building. Because infarct location and culprit vessel are highly correlated, we chose to include only infarct location in the logistic regression model.
Missing data for the baseline characteristics and variables measured in advance of STEMI with VF were <2% except for educational level (3%), healthcare contact prior to STEMI (4%), and syncope or presyncope (15%). A variable with missing data >4% (syncope or presyncope) was not included in the logistic regression model. For the remaining variables, only patients with complete information were used in univariable and multivariable analyses. Age as a continuous variable was fairly normally distributed but was categorized for easier interpretation. Alcohol intake and smoking in pack‐years was not normally distributed (right skewed), even after log transformation, and thus both variables were categorized. Regarding outliers, if the patients reported high units of alcohol intake per week or smoking in pack‐years, we contacted the patients or relatives to verify the reported values, and these verified outliers are included in the analysis for all patients.
An initial multivariable model was built by including any covariate with P<0.20 on the univariate test. Because age, sex, family history of sudden death, smoking, and preinfarction angina were previously associated with VF,6–8,13 these variables were included regardless of univariate P value. After building the initial model, we sequentially added covariates that did not meet P<0.20 on univariate testing. A covariate was retained if it was significant or changed the coefficient of a risk factor by >10% and was excluded if it changed the standard error by >10%. Adjusted odds ratios (ORs) and accompanying 95% confidence intervals (CIs) were computed to determine the effect of each variable on the risk of VF. The Hosmer‐Lemeshow goodness‐of‐fit test (by forming 10 groups) was used to evaluate the final model, and area under the receiver operating characteristic curve for the logistic regression model was used to measure prediction accuracy.
Tests for linear trend across alcohol intake categories were performed by assigning the median value to each category and modeling this as a continuous variable in separate logistic regression models. To test for a curvilinear association, a quadratic term (average intake squared) was added to the linear term in a separate model. We also examined the possibility of a nonlinear relationship between alcohol and VF risk nonparametrically using restricted cubic spline transformations with 4 knots (Figure 3). All analyses were performed using the Stata software package version 12.0 (StataCorp).
Figure 3.
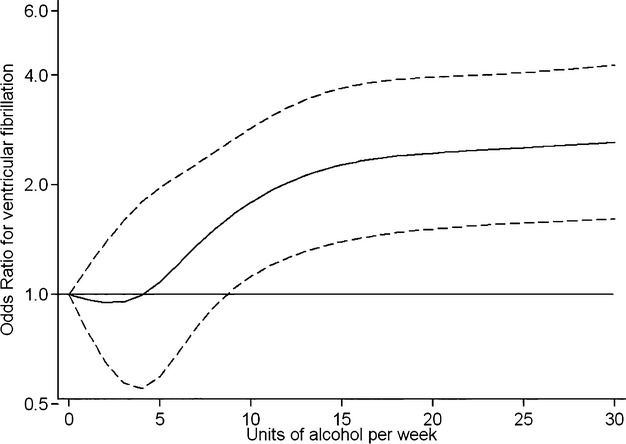
Restricted cubic spline for alcohol consumption. Multivariate odds ratio of ventricular fibrillation before percutaneous coronary intervention as a function of alcohol intake in units per week. Data are fitted by a restricted cubic spline logistic regression model and controlled for age, sex, preinfarction angina, infarct location, preprocedural thrombolysis in myocardial infarction flow, atrial fibrillation, family history of sudden death, statins before ST‐segment elevation myocardial infarction, hypercholesterolemia, hypertension, and smoking. The 95% CIs are indicated by dashed lines.
Results
Consecutive STEMI Registries
At the largest center, 1901 consecutive patients with acute STEMI due to symptoms and/or ECG changes were admitted to Rigshospitalet during the 2‐year enrollment period (Figure 1). VF occurred before PCI in 221 patients (11.6%) (Figure 1). This number represents all STEMI patients who underwent angiography and subsequent PPCI including patients of nonwhite ethnicity and those with prior CAD or MI. Of these 221 VF patients, 43 (19%) were excluded based on exclusion criteria, 20 (9%) who potentially could have been enrolled in the study were missed, and 158 (72%) were included in the study. Compared with the VF cases included in the study, the missed‐case group did not differ with respect to age (P=0.400) or sex (P=0.500).
During the 8‐month period during which controls were enrolled in the study, 664 STEMI patients with symptom duration ≤12 hours were treated with PPCI at 2 of the 4 centers (Figure 2). Of these patients, 126 (19%) were not enrolled in the study because of admission during weekends or holidays or expedited discharge to local hospitals. The majority (283 patients, 43%) were excluded based on the exclusion criteria, and 255 (38%) were included. Compared with the controls included in the study, the missed‐control group (19%) did not differ with respect to age (P=0.400) or sex (P=0.900).
Case–Control Series
In the case–control series performed at all 4 PCI centers, 219 case patients with VF and 441 control patients without VF in the setting of STEMI were included. The baseline characteristics are shown in Table 1. In the case group, 82 (37%) patients who underwent angiography were unconscious at arrival at the hospital, and the informed consent was signed by the next kin. VF occurred out‐of‐hospital in 83% of the cases, and the remainder (17%) occurred on arrival at the hospital (Table 1). Eighty VF patients had STEMI on ECG and developed VF afterward. In the rest of the cases (n=139), the STEMI ECG was recorded after the VF arrest. In‐hospital death occurred in 17% (n=38) of the cases and 1% (n=3) of the controls (P<0.001).
Compared with the control group, patients in the case group were more likely to be younger, male, have a history of atrial fibrillation (AF) or hypercholesterolemia, and a family history of sudden death. Furthermore, the proportion on statin therapy was higher in the case group compared with the control group, likely due to the higher degree of hypercholesterolemia in the case group (Table 1). Median levels of average weekly alcohol intake were higher in the case group (7 units per week) as compared with the control group (3 units per week) without VF (P=0.001). A history of angina prior to STEMI was more likely in the control group compared with the case group, but no difference was observed in self‐reported contact with the healthcare system due to cardiac symptoms within a year prior to STEMI (Table 2).
The angiographic and other presenting characteristics at the time of STEMI according to case and control status are outlined in Table 3. Patients with VF had shorter times from symptom onset to PPCI, lower left ventricular ejection fraction, larger infarct size (based on maximal creatine kinase‐MB peak), and higher Killip class and were more likely to have anterior infarctions with left anterior descending artery occlusions. Regarding the index ECG at the time of STEMI, the case group was more likely to have left or right bundle branch block and higher baseline ST‐segment deviation (Table 3).
The results of univariate and multivariate logistic regression analysis are summarized in Table 4. Patients who were aged <60 years had 1.75‐fold elevated odds of experiencing VF with STEMI compared with those aged ≥60 years. When analyzed as a continuous variable, the OR for each 10 years of age was 0.73 (95% CI 0.59 to 0.88). Prior history of AF, family history of sudden death, use of statins, and alcohol intake >7 units per week (up to 1 drink per day) were positively associated with VF after multivariable adjustment (Table 4). For alcohol intake, the test for linear trend across categories was significant (P<0.001), and patients who reported drinking >15 units of alcohol per week were at the highest odds of developing VF (OR 3.30; 95% CI 1.80 to 5.90) compared with nondrinkers.
Table 4.
Univariate and Multivariate Analysis of Risk Factors of Ventricular Fibrillation
| Variable | Contrast | Univariate | Multivariate* | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| Younger age | Aged <60 years vs ≥60 years | 1.38 | 0.99 to 1.90 | 0.050 | 1.75 | 1.20 to 2.60 | 0.005 |
| Male sex | Male vs female | 1.90 | 1.21 to 2.95 | 0.005 | 1.65 | 0.98 to 2.75 | 0.060 |
| Alcohol | |||||||
| 1 to 7 units/week | Nondrinkers | 1.30 | 0.83 to 2.05 | 0.300 | 1.30 | 0.80 to 2.20 | 0.300 |
| 8 to 14 units/week | Nondrinkers | 1.94 | 1.12 to 3.37 | 0.020 | 2.30 | 1.20 to 4.20 | 0.008 |
| ≥15 units/week | Nondrinkers | 3.25 | 1.93 to 5.50 | <0.001 | 3.30 | 1.80 to 5.90 | <0.001 |
| Atrial fibrillation | Yes vs no | 2.94 | 1.29 to 6.73 | 0.010 | 2.80 | 1.10 to 7.30 | 0.040 |
| Preinfarction angina | Yes vs no | 0.53 | 0.38 to 0.73 | <0.001 | 0.46 | 0.32 to 0.67 | <0.001 |
| FH of sudden death | Yes vs no | 1.80 | 1.27 to 2.56 | 0.001 | 1.60 | 1.10 to 2.40 | 0.010 |
| Infarct location | Anterior vs nonanterior | 1.55 | 1.10 to 2.10 | 0.009 | 2.10 | 1.40 to 3.00 | <0.001 |
| TIMI flow before PPCI | TIMI flow grade 0 vs grade 1 to 3 | 1.35 | 0.97 to 1.90 | 0.070 | 1.65 | 1.14 to 2.40 | 0.008 |
| Statins before STEMI | Yes vs no | 2.05 | 1.34 to 3.15 | 0.001 | 2.10 | 1.15 to 4.0 | 0.020 |
| Hypercholesterolemia | Yes vs no | 1.40 | 1.00 to 1.95 | 0.050 | 0.90 | 0.55 to 1.50 | 0.800 |
| Hypertension | Yes vs no | 1.39 | 0.99 to 1.94 | 0.050 | 1.20 | 0.79 to 1.85 | 0.400 |
| Smoking | |||||||
| Past | Past vs never | 1.48 | 0.90 to 2.40 | 0.100 | 1.18 | 0.68 to 2.00 | 0.600 |
| Current | Current vs never | 1.30 | 0.83 to 2.00 | 0.300 | 1.10 | 0.66 to 1.85 | 0.700 |
| Study site | 1.00 | 0.87 to 1.12 | 0.900 | 1.00 | 0.87 to 1.15 | 1.000 | |
FH indicates family history; OR, odds ratio; PPCI, primary percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
219 cases and 441 controls were included from the 4 centers.
200 cases and 431 controls from the 4 centers included in the full multivariable model. Because the ratio of cases to controls did differ at each of the sites, we added a variable study site to the multivariable model to control for any unmeasured differences between cases and controls enrolled at each of the 4 centers.
Preinfarction angina was inversely associated with VF (OR 0.46; 95% CI 0.32 to 0.67) after multivariable adjustment. The included presenting characteristics of anterior infarct and preprocedural thrombolysis in myocardial infarction flow grade 0 were also associated with VF. Other standard cardiovascular risk factors including hypertension, hypercholesterolemia, and smoking (categorically and continuously) were not significantly associated with VF after multivariable adjustment. Although highly significant in the univariate analysis, male sex was no longer significant in the multivariate model after controlling for alcohol intake (P=0.06). The area under the receiver operating characteristic curve for the final logistic regression model was 0.730 and the Hosmer‐Lemeshow goodness‐of‐fit test was P=0.370, indicating acceptable risk accuracy and model, respectively.
We further examined the relationship between alcohol intake and VF in separate multivariable models controlling for the above covariates by adding a quadratic term to the linear term for alcohol intake. These models suggested a possible curvilinear component (Pquadratic=0.048). We further tested for departure from linearity using the raw continuous value for alcohol intake in a restricted cubic spline model (Figure 3). In this model, there was a nonlinear relationship between alcohol intake and VF (Pnonlinear trend=0.0003). The shape of the curve is consistent with an increased VF risk at >7 units per week with leveling off of the curve at intakes of alcohol >14 units per week (Figure 3).
Discussion
In this nationwide study of patients with first STEMI who survived to undergo angiography, we identified several patient characteristics associated with significantly higher odds of experiencing VF before PPCI after controlling for cardiovascular risk factors, infarct location, and thrombolysis in myocardial infarction flow. These independent risk factors include age of <60 years, family history of sudden death, absence of preinfarction angina, use of statins, history of AF, and alcohol intake >7 units per week. Traditional CAD risk factors such as smoking, diabetes, hypertension, and hypercholesterolemia did not predict risk. These data, along with those from prior studies,13–15 suggest that certain patients may be particularly vulnerable to the proarrhythmic effects of ischemia and more likely to present with VF or SCD as their manifestation of CAD. In our series of patients, 11.6% of patients with STEMI transferred for PPCI experience VF prior to undergoing the procedure. Consequently, primary prevention of VF in STEMI patients is of the utmost importance, and our study suggests that several factors may help identify high‐risk patients and, in the case of alcohol, could potentially be modified to lower risk.
To our knowledge, our study is the first to examine the relationship between alcohol consumption and risk of VF in the setting of STEMI. Higher intake of alcohol has been linearly associated with lower risks of MI,25 whereas the relationship with SCD is U‐shaped, with lower risks observed only at low to moderate levels of alcohol consumption (2 to 6 drinks per week)16–17 and higher risks observed with heavy consumption (>6 drinks per day).18 These divergent relationships could be explained if the favorable effects of alcohol on atherosclerosis and thrombosis are offset by potential proarrhythmic effects at higher levels of intake. Our study supports this hypothesis and suggests that the proarrhythmic properties of alcohol may be observed even at fairly moderate levels of intake. Because both cases and controls in our study had STEMI, we were able to examine the relationship of alcohol to VF independent of its association with MI. Patients who reported drinking 8 to 14 units of alcohol per week had 2.3‐fold significantly higher odds of VF in the setting of STEMI. This risk continued to increase but to a lesser degree at higher levels of intake, with patients consuming >15 drinks being at a 3.3‐fold increased VF risk.
Moderate levels of alcohol intake have also been linked to incident AF,26–27 and a prior history of AF was found to be a risk factor for VF (OR 2.8) before PPCI in this population of STEMI patients, even after controlling for alcohol intake. Our study adds to the growing literature regarding the link between AF and ventricular arrhythmias and SCD. Population‐based studies have previously associated history of AF with SCD,28 and patients with AF appear to derive a greater benefit from primary prevention implantable cardioverter‐defibrillators.29–31 Recently, a large population‐based case–control study found similar elevations in the risk of VF arrest associated with a preceding diagnosis of AF.32 AF at initial presentation in the setting of acute MI has also been associated with increased risk of in‐hospital VF33 and total mortality.34 Our results suggest that AF patients may have a lower threshold for VF in the setting of MI, and this could account for the increased risk of SCD in population‐based studies. Heritable factors and cardiac channelopathies have been identified for VF and AF35; therefore, further research regarding joint pathways affecting cardiac electrical function in the atrium and ventricle is needed to further our understanding regarding the link between AF and VF.
Our study also supports prior work showing that a genetic predisposition for VF and/or SCD exists in the setting of MI.13,36 These studies showed that a family history of sudden death among parents or siblings increased the risk of VF or SCD with an OR of 2.7 for VF13 and 1.6 for SCD.36 These findings, in combination with the results of the present study (OR 1.6 for VF), lend support for the hypothesis that a portion of the familial aggregation of VF and SCD37 is distinct from that for atherosclerosis. Factors that directly predispose to fatal arrhythmia may be responsible, providing a rationale for further research on genetic determinants of VF in the STEMI population.
Whether age and sex are associated with VF is not clear. In our data, age was paradoxically associated with VF, with higher risks among those aged <60 years. This association could be due, at least in part, to higher resuscitation and survival rates for out‐of‐hospital VF in younger persons; however, a recent study also showed age of <60 years to be significantly associated with in‐hospital VF in the setting of MI.33 Given the high lethality of VF, patients with a genetic and/or physiologic predisposition to VF in the setting of MI may be less likely to survive to an older age. Men also tended to have a higher risk of VF in the setting of STEMI in our population, consistent with the higher incidence of SCD among men in several studies;3 however, after controlling for alcohol intake, the association became nonsignificant.
Apart from age, other standard cardiovascular risk factors were not associated with VF in our population of STEMI patients after multivariable adjustment. These results are largely in agreement with the AGNES study, which also examined risk factors for VF before PPCI, except the latter study found an inverse association between hypercholesterolemia and VF among 275 cases and 325 controls with an OR of 0.64 (P=0.027).13 We found a positive association between statin therapy and VF in the adjusted regression analysis that is difficult to interpret in this observational study, given the potential for confounding by indication. All patients on statins before STEMI with VF were diagnosed with hypercholesterolemia, and treatment with a statin may be a proxy for higher cholesterol levels and/or higher cardiovascular risk profile.
Preinfarction angina was inversely associated with VF (OR 0.46) in our study and in a small cohort of 72 consecutive out‐of‐hospital VF patients with MI (OR 0.40) and in a meta‐analysis8,15 but not in the AGNES study.13 Preinfarction angina may act as a clinical surrogate for ischemic preconditioning, which recently has been demonstrated to reduce infarct size in STEMI patients.38 Furthermore, animal studies have shown that preconditioning protects against VF during acute coronary occlusion.39–40 A completely occluded artery is also associated with a higher risk of VF in STEMI patients both prior to PPCI in the present study and during and after catheterization in another.10 Our study also presents supporting data to suggest that an anterior location of the MI and/or a left anterior descending culprit artery occlusion may also elevate VF risk.13–14,33 Although anterior MI location may be a proxy for infarct size, which is difficult to accurately measure in the setting of resuscitated VF arrest, studies controlling for infarct size still found an anterior location to be associated with VF.13
Strengths and Limitations
Patients who died outside of hospitals are not included in the present study; therefore, our results may not be generalizable to STEMI patients who do not survive to reach the hospital and who do not undergo angiography. Even though we had permission to include patients before informed consent, it was not possible to include all patients (Figures 1 and 2). The ratio of cases and controls enrolled in the study differed across the centers, and different enrollment patterns and patient populations across centers could have influenced our analysis; however, the baseline characteristics of case and control patients across centers were similar with respect to all variables besides the proportion with previous hypercholesterolemia in the case group (P=0.032), and control for center did not affect our results. There is also the potential for recall bias, which is a limitation in any case–control study in which the exposure is ascertained after the event. Baseline characteristics and especially symptoms prior to STEMI with VF could have been underreported in the case group because of retrograde amnesia or overrepresented due to recall bias; therefore, all potential sources were used to retrieve complete data through the national electronic health records and contacting of the next of kin and the patients after discharge. Recall bias with respect to family history could have been present in both groups; nevertheless, we were able to replicate the association of family history of sudden death to VF shown in previous studies.13,36 With respect to our findings on alcohol intake, we did not collect information on drinking pattern or recent withdrawal from alcohol and thus were unable to analyze how these factors might influence VF risk. We also did not ask about diet (eg, coffee intake) or illegal drug use, such as cocaine, that could increase the risk of VF. Finally, our results in this population with white, European ancestry may not be generalizable to other populations.
Conclusion
Several easily assessed risk factors were found to be associated with a subsequent higher risk of VF in STEMI. Novel risk factor with potential clinical implications in this population include alcohol intake of >7 units per week. This study also identified and confirmed younger age, prior history of AF, family history of sudden death, and absence of preinfarction angina as risk factors associated with VF. Because AF patients and those with a family history of sudden death are at higher risk of sudden death during an acute MI due to VF, targeting these patients for aggressive primary prevention of MI may be warranted. Our data also raise the possibility that alcohol intake might affect the risk of VF in the setting of STEMI. Furthermore, because younger patients and those without preceding angina appear to be at higher risk for VF in the setting of STEMI, these data could be useful when counseling younger, apparently healthy persons regarding the importance of CAD and lifestyle risk‐factor modification to prevent MI and sudden death.
Sources of Funding
This study was supported by the University of Copenhagen, the Danish National Research Foundation, the John and Birthe Meyer Foundation, The Research Foundation of the Heart Centre Rigshospitalet, Bikuben Scholar‐Danmark‐Amerika Fonden and Fulbright Commission.
Disclosures
Dr Javad Jabbari is an employee of LEO Pharma A/S, Denmark.
Acknowledgments
The author group would like to thank the patients and also the research coordinators Charlotte Schmidt Skov, Pauline Gøgsig Johansen, and Karin Møller Pedersen for their enormous help with the enrollment of the patients and with the data collection.
References
- 1.Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel‐Smoller S, Wong ND, Wylie‐Rosett J. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010; 121:948-954. [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ. Anatomic features in victims of sudden coronary death. Coronary artery pathology. Circulation. 1992; 85:I19-I24. [PubMed] [Google Scholar]
- 3.Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012; 125:620-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risgaard B, Nielsen JB, Jabbari R, Haunsø S, Holst AG, Winkel BG, Tfelt‐Hansen J. Prior myocardial infarction in the young: predisposes to a high relative risk but low absolute risk of a sudden cardiac death. Europace. 2013; 15:48-54. [DOI] [PubMed] [Google Scholar]
- 5.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001; 345:1473-1482. [DOI] [PubMed] [Google Scholar]
- 6.Volpi A, Cavalli A, Santoro L, Negri E. Incidence and prognosis of early primary ventricular fibrillation in acute myocardial infarction–results of the gruppo italiano per lo studio della sopravvivenza nell'infarto miocardico (GISSI‐2) database. Am J Cardiol. 1998; 82:265-271. [DOI] [PubMed] [Google Scholar]
- 7.Al‐Khatib SM, Granger CB, Huang Y, Lee KL, Califf RM, Simoons ML, Armstrong PW, Van de Werf F, White HD, Simes RJ, Moliterno DJ, Topol EJ, Harrington RA. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST‐segment elevation: incidence, predictors, and outcomes. Circulation. 2002; 106:309-312. [DOI] [PubMed] [Google Scholar]
- 8.Gheeraert PJ, De Buyzere ML, Taeymans YM, Gillebert TC, Henriques JP, De Backer G, De Bacquer D. Risk factors for primary ventricular fibrillation during acute myocardial infarction: a systematic review and meta‐analysis. Eur Heart J. 2006; 27:2499-2510. [DOI] [PubMed] [Google Scholar]
- 9.Naruse Y, Tada H, Harimura Y, Hayashi M, Noguchi Y, Sato A, Yoshida K, Sekiguchi Y, Aonuma K. Early repolarization is an independent predictor of occurrences of ventricular fibrillation in the very early phase of acute myocardial infarction. Circ Arrhythm Electrophysiol. 2012; 5:506-513. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RH, Starr AZ, Lopes RD, Hochman JS, Widimsky P, Pieper KS, Armstrong PW, Granger CB. Incidence of and outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA. 2009; 301:1779-1789. [DOI] [PubMed] [Google Scholar]
- 11.Clements‐Jewery H, Andrag E, Hearse DJ, Curtis MJ. Complex adrenergic and inflammatory mechanisms contribute to phase 2 ventricular arrhythmias in anaesthetized rats. Br J Pharmacol. 2009; 156:444-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrag E, Curtis MJ. Feasibility of targeting ischaemia‐related ventricular arrhythmias by mimicry of endogenous protection by endocannabinoids. Br J Pharmacol. 2013; 169:1840-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekker LRC, Bezzina CR, Henriques JPS, Tanck MW, Koch KT, Alings MW, Arnold AER, de Boer M‐J, Gorgels APM, Michels HR, Verkerk A, Verheugt FWA, Zijlstra F, Wilde AAM. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case‐control study in acute myocardial infarction patients. Circulation. 2006; 114:1140-1145. [DOI] [PubMed] [Google Scholar]
- 14.Gheeraert PJ, Henriques JP, De Buyzere ML, Voet J, Calle P, Taeymans Y, Zijlstra F. Out‐of‐hospital ventricular fibrillation in patients with acute myocardial infarction: coronary angiographic determinants. J Am Coll Cardiol. 2000; 35:144-150. [DOI] [PubMed] [Google Scholar]
- 15.Gheeraert PJ, Henriques JP, De Buyzere ML, De Pauw M, Taeymans Y, Zijlstra F. Preinfarction angina protects against out‐of‐hospital ventricular fibrillation in patients with acute occlusion of the left coronary artery. J Am Coll Cardiol. 2001; 38:1369-1374. [DOI] [PubMed] [Google Scholar]
- 16.Albert CM, Manson JE, Cook NR, Ajani UA, Gaziano JM, Hennekens CH. Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation. 1999; 100:944-950. [DOI] [PubMed] [Google Scholar]
- 17.Chiuve SE, Rimm EB, Mukamal KJ, Rexrode KM, Stampfer MJ, Manson JE, Albert CM. Light‐to‐moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm. 2010; 7:1374-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wannamethee G, Shaper AG. Alcohol and sudden cardiac death. Br Heart J. 1992; 68:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta RH, Harjai KJ, Grines L, Stone GW, Boura J, Cox D, O'Neill W, Grines CL. Sustained ventricular tachycardia or fibrillation in the cardiac catheterization laboratory among patients receiving primary percutaneous coronary intervention: incidence, predictors, and outcomes. J Am Coll Cardiol. 2004; 43:1765-1772. [DOI] [PubMed] [Google Scholar]
- 20.Zehender M, Utzolino S, Furtwängler A, Kasper W, Meinertz T, Just H. Time course and interrelation of reperfusion‐induced ST changes and ventricular arrhythmias in acute myocardial infarction. Am J Cardiol. 1991; 68:1138-1142. [DOI] [PubMed] [Google Scholar]
- 21.Gressin V, Louvard Y, Pezzano M, Lardoux H. Holter recording of ventricular arrhythmias during intravenous thrombolysis for acute myocardial infarction. Am J Cardiol. 1992; 69:152-159. [DOI] [PubMed] [Google Scholar]
- 22.Buckingham TA, Devine JE, Redd RM, Kennedy HL. Reperfusion arrhythmias during coronary reperfusion therapy in man. Clinical and angiographic correlations. Chest. 1986; 90:346-351. [DOI] [PubMed] [Google Scholar]
- 23.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011; 39:30-33. [DOI] [PubMed] [Google Scholar]
- 24.Winkel BG, Holst AG, Theilade J, Kristensen IB, Thomsen JL, Ottesen GL, Bundgaard H, Svendsen JH, Haunsø S, Tfelt‐Hansen J. Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur Heart J. 2011; 32:983-990. [DOI] [PubMed] [Google Scholar]
- 25.Walsh CR, Larson MG, Evans JC, Djousse L, Ellison RC, Vasan RS, Levy D. Alcohol consumption and risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2002; 136:181-191. [DOI] [PubMed] [Google Scholar]
- 26.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005; 112:1736-1742. [DOI] [PubMed] [Google Scholar]
- 27.Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, Anasako Y, Nishigaki Y, Yachi Y, Iida KT, Ohashi Y, Yamada N, Sone H. Alcohol consumption and risk of atrial fibrillation: a meta‐analysis. J Am Coll Cardiol. 2011; 57:427-436. [DOI] [PubMed] [Google Scholar]
- 28.Chen LY, Sotoodehnia N, Buzkova P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: the Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. JAMA Intern Med. 2013; 173:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long‐term (8‐year) benefit of the implantable cardioverter‐defibrillator. J Am Coll Cardiol. 2012; 59:2075-2079. [DOI] [PubMed] [Google Scholar]
- 30.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter‐defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008; 51:288-296. [DOI] [PubMed] [Google Scholar]
- 31.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007; 50:1150-1157. [DOI] [PubMed] [Google Scholar]
- 32.Bardai A, Blom MT, van Hoeijen DA, van Deutekom HW, Brouwer HJ, Tan HL. Atrial fibrillation is an independent risk factor for ventricular fibrillation: a large‐scale population‐based case‐control study. Circ Arrhythm Electrophysiol. 2014. 10.1161/CIRCEP.114.002094 [DOI] [PubMed] [Google Scholar]
- 33.Bougouin W, Marijon E, Puymirat E, Defaye P, Celermajer DS, Le Heuzey JY, Boveda S, Kacet S, Mabo P, Barnay C, Da Costa A, Deharo JC, Daubert JC, Ferrieres J, Simon T, Danchin N. Incidence of sudden cardiac death after ventricular fibrillation complicating acute myocardial infarction: a 5‐year cause‐of‐death analysis of the FAST‐MI 2005 registry. Eur Heart J. 2014; 35:116-122. [DOI] [PubMed] [Google Scholar]
- 34.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, Roger VL. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011; 123:2094-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsman RF, Tan HL, Bezzina CR. Genetics of sudden cardiac death caused by ventricular arrhythmias. Nat Rev Cardiol. 2013; 11:96-111. [DOI] [PubMed] [Google Scholar]
- 36.Kaikkonen KS, Kortelainen ML, Linna E, Huikuri HV. Family history and the risk of sudden cardiac death as a manifestation of an acute coronary event. Circulation. 2006; 114:1462-1467. [DOI] [PubMed] [Google Scholar]
- 37.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999; 99:1978-1983. [DOI] [PubMed] [Google Scholar]
- 38.Reiter R, Henry TD, Traverse JH. Preinfarction angina reduces infarct size in ST‐elevation myocardial infarction treated with percutaneous coronary intervention. Circ Cardiovasc Interv. 2013; 6:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagar JM, Hale SL, Kloner RA. Effect of preconditioning ischemia on reperfusion arrhythmias after coronary artery occlusion and reperfusion in the rat. Circ Res. 1991; 68:61-68. [DOI] [PubMed] [Google Scholar]
- 40.Shiki K, Hearse DJ. Preconditioning of ischemic myocardium: reperfusion‐induced arrhythmias. Am J Physiol. 1987; 253:H1470-H1476. [DOI] [PubMed] [Google Scholar]


